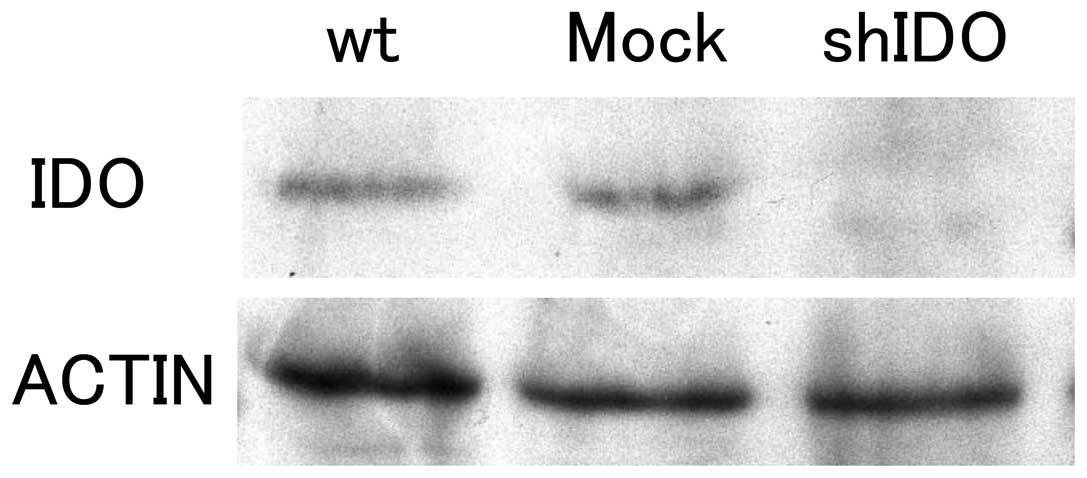
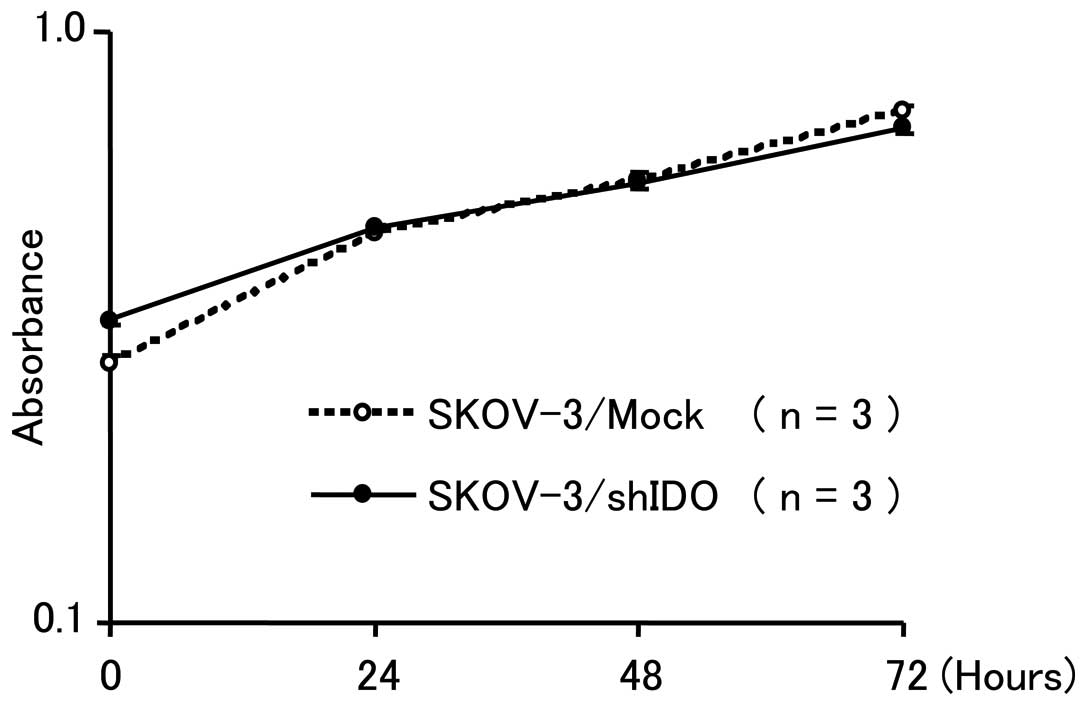
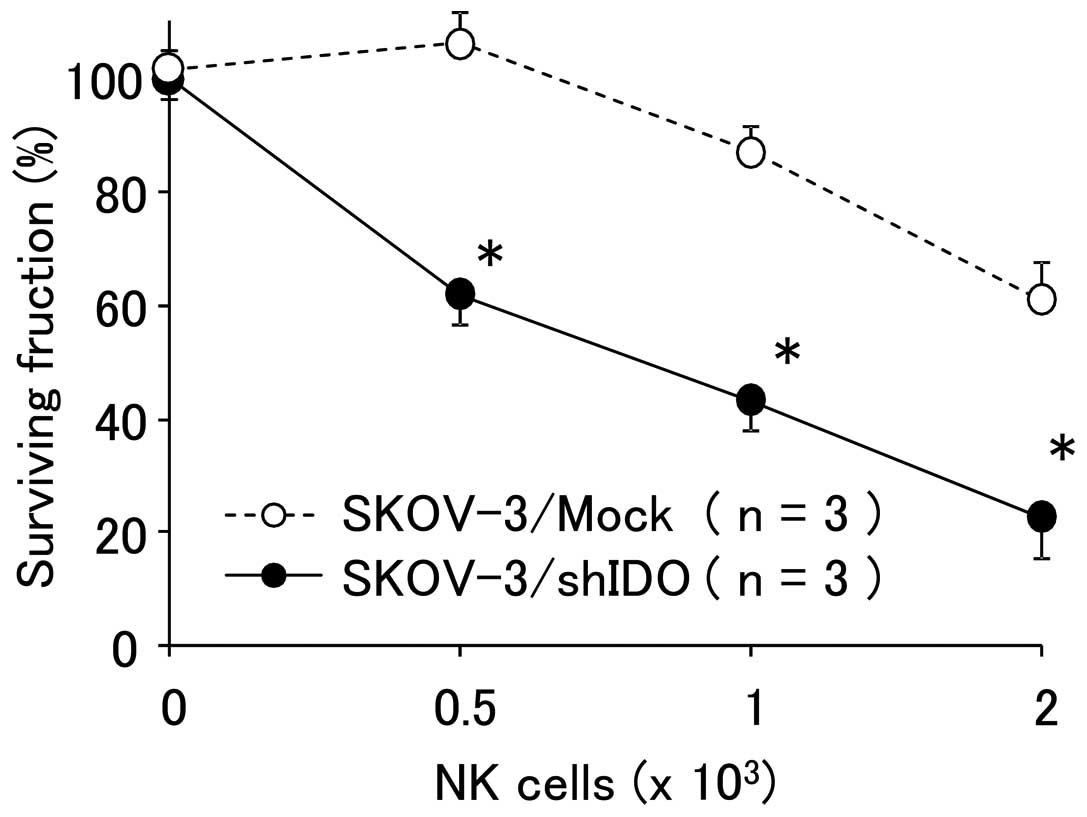
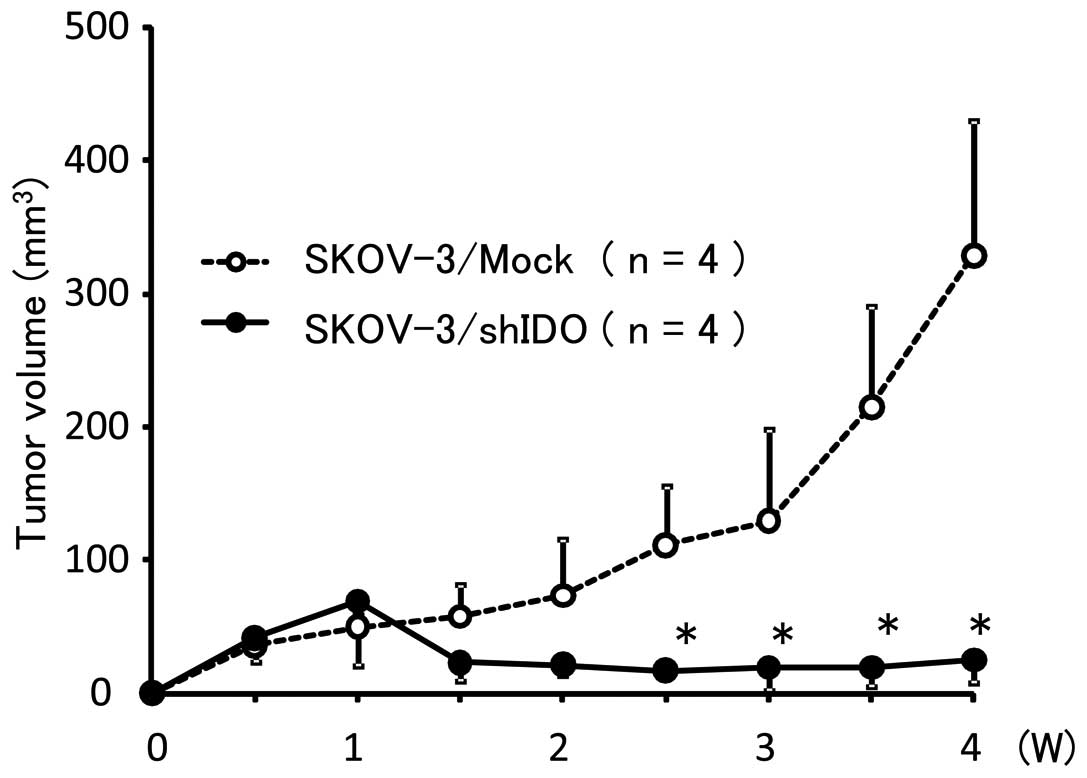
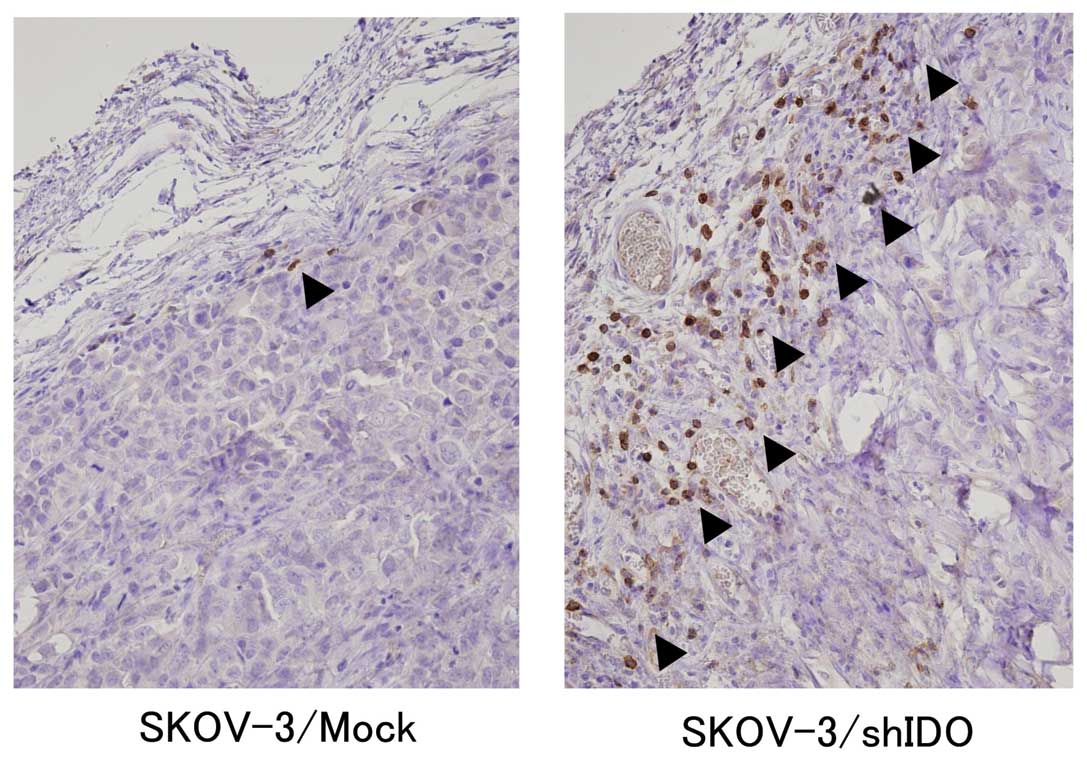
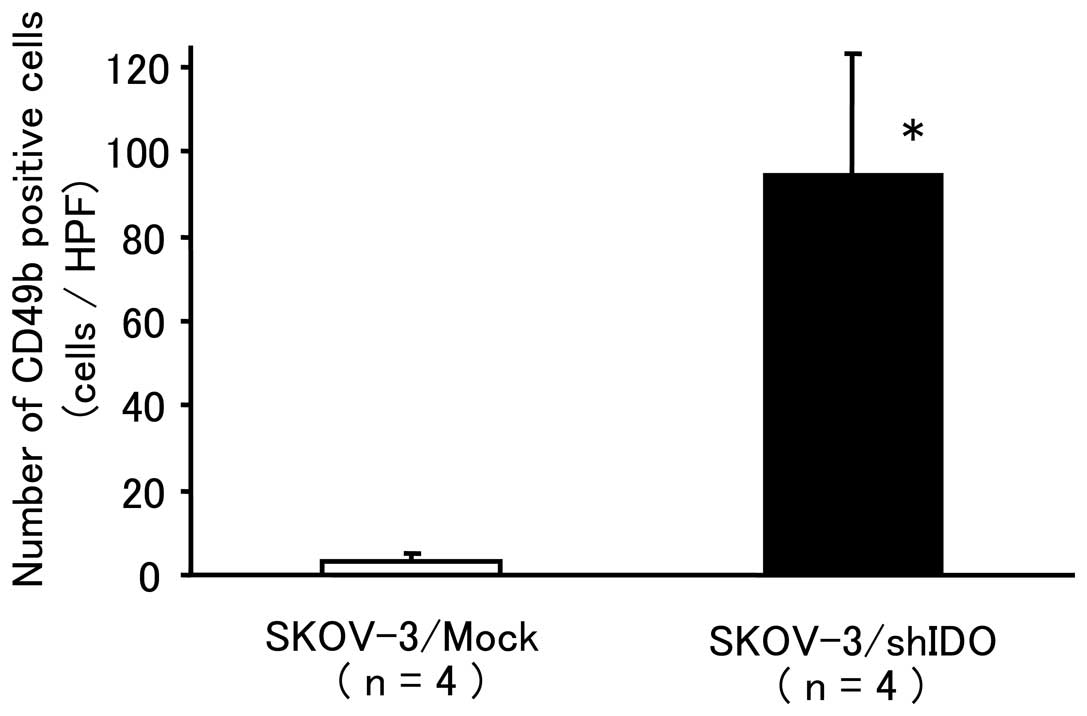
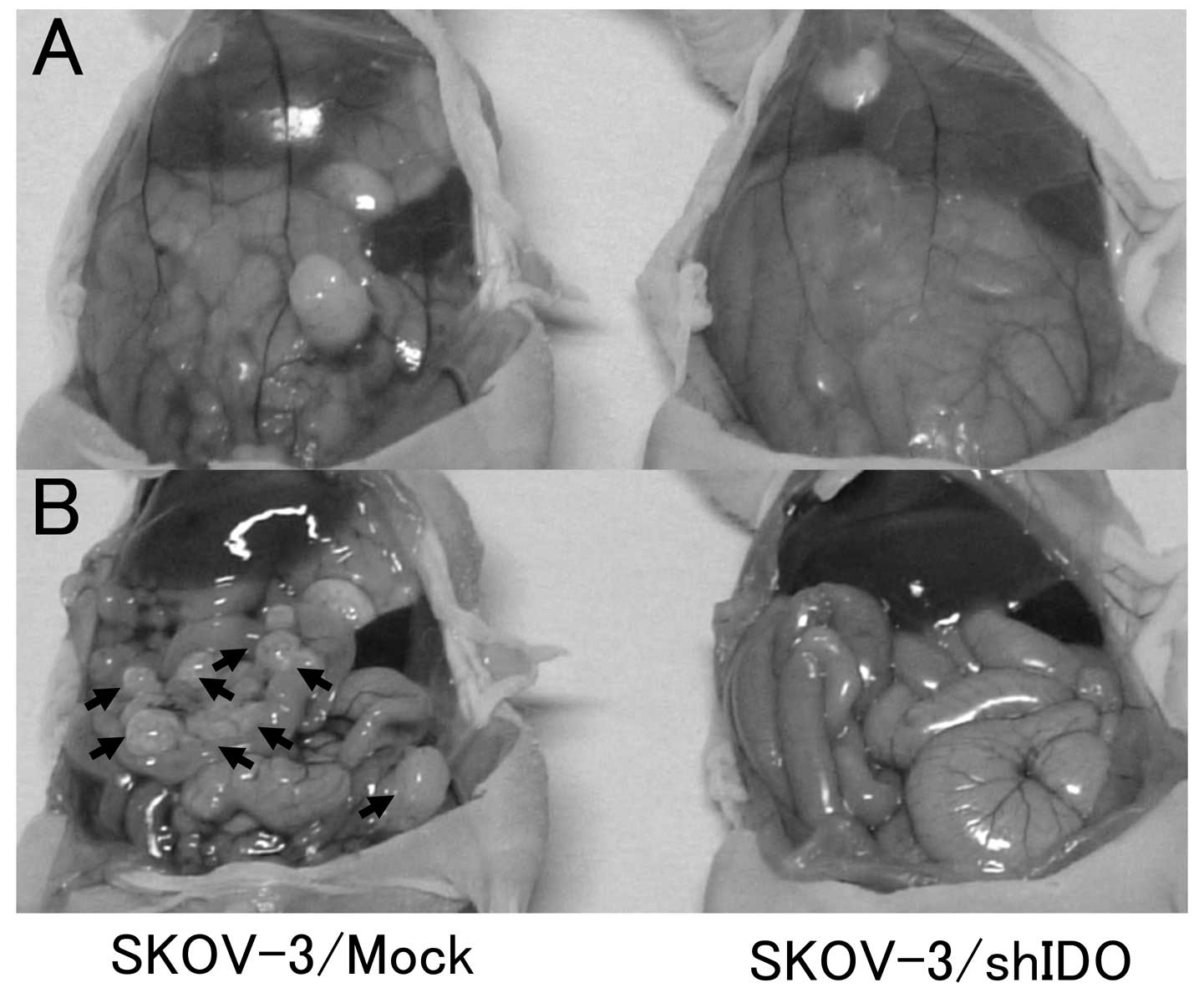
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Heintz AP: Surgery in advanced ovarian
carcinoma: is there proof to show the benefit? Eur J Surg Oncol.
14:91–99. 1988.PubMed/NCBI
|
|
3
|
McGuire WP, Hoskins WJ, Brady MF, et al:
Cyclophosphamide and cisplatin compared with paclitaxel and
cisplatin in patients with stage III and stage IV ovarian cancer. N
Engl J Med. 334:1–6. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takei Y, Suzuki M, Ohwada M, et al: A
feasibility study of paclitaxel and carboplatin therapy in Japanese
patients with epithelial ovarian cancer. Oncol Rep. 10:951–955.
2003.PubMed/NCBI
|
|
5
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: from immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schreiber RD, Old LJ and Smyth MJ: Cancer
immunoediting: integrating immunity’s roles in cancer suppression
and promotion. Science. 331:1565–1570. 2011.PubMed/NCBI
|
|
7
|
Higuchi K and Hayaishi O: Enzymic
formation of D-kynurenine from D-tryptophan. Arch Biochem Biophys.
120:397–403. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamamoto S and Hayaishi O: Tryptophan
pyrrolase of rabbit intestine. D- and L-tryptophan-cleaving enzyme
or enzymes. J Biol Chem. 242:5260–5266. 1967.PubMed/NCBI
|
|
9
|
Shimizu T, Nomiyama S, Hirata F and
Hayaishi O: Indoleamine 2,3-dioxygenase. Purification and some
properties. J Biol Chem. 253:4700–4706. 1978.PubMed/NCBI
|
|
10
|
Yoshida R, Urade Y, Tokuda M and Hayaishi
O: Induction of indoleamine 2,3-dioxygenase in mouse lung during
virus infection. Proc Natl Acad Sci USA. 76:4084–4086. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshida R and Hayaishi O: Induction of
pulmonary indoleamine 2,3-dioxygenase by intraperitoneal injection
of bacterial lipopolysaccharide. Proc Natl Acad Sci USA.
75:3998–4000. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fujigaki S, Saito K, Sekikawa K, et al:
Lipopolysaccharide induction of indoleamine 2,3-dioxygenase is
mediated dominantly by an IFN-gamma-independent mechanism. Eur J
Immunol. 31:2313–2318. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Munn DH, Zhou M, Attwood JT, et al:
Prevention of allogeneic fetal rejection by tryptophan catabolism.
Science. 281:1191–1193. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schroecksnadel K, Winkler C, Duftner C,
Wirleitner B, Schirmer M and Fuchs D: Tryptophan degradation
increases with stage in patients with rheumatoid arthritis. Clin
Rheumatol. 25:334–337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brown RR, Ozaki Y, Datta SP, Borden EC,
Sondel PM and Malone DG: Implications of interferon-induced
tryptophan catabolism in cancer, auto-immune diseases and AIDS. Adv
Exp Med Biol. 294:425–435. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Labadarios D, McKenzie DY, Dickerson JW
and Parke DV: Metabolic abnormalities of tryptophan and nicotinic
acid in patients with rheumatoid arthritis. Rheumatol Rehabil.
17:227–232. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Varga J, Yufit T and Brown RR: Inhibition
of collagenase and stromelysin gene expression by interferon-gamma
in human dermal fibroblasts is mediated in part via induction of
tryptophan degradation. J Clin Invest. 96:475–481. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mellor AL and Munn DH: IDO expression by
dendritic cells: tolerance and tryptophan catabolism. Nat Rev
Immunol. 4:762–774. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uyttenhove C, Pilotte L, Theate I, et al:
Evidence for a tumoral immune resistance mechanism based on
tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med.
9:1269–1274. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vivier E, Tomasello E, Baratin M, Walzer T
and Ugolini S: Functions of natural killer cells. Nat Immunol.
9:503–510. 2008. View
Article : Google Scholar
|
|
21
|
Lanier LL: Up on the tightrope: natural
killer cell activation and inhibition. Nat Immunol. 9:495–502.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Della Chiesa D, Carlomagno S, Frumento G,
et al: The tryptophan catabolite L-kynurenine inhibits the surface
expression of NKp46- and NKG2D-activating receptors and regulates
NK-cell function. Blood. 108:4118–4125. 2006.PubMed/NCBI
|
|
23
|
Inaba T, Ino K, Kajiyama H, et al:
Indoleamine 2,3-dioxygenase expression predicts impaired survival
of invasive cervical cancer patients treated with radical
hysterectomy. Gynecol Oncol. 117:423–428. 2010. View Article : Google Scholar
|
|
24
|
Ino K, Yoshida N, Kajiyama H, et al:
Indoleamine 2,3-dioxygenase is a novel prognostic indicator for
endometrial cancer. Br J Cancer. 95:1555–1561. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takao M, Okamoto A, Nikaido T, et al:
Increased synthesis of indoleamine-2,3-dioxygenase protein is
positively associated with impaired survival in patients with
serous-type, but not with other types of, ovarian cancer. Oncol
Rep. 17:1333–1339. 2007.
|
|
26
|
Inaba T, Ino K, Kajiyama H, et al: Role of
the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the
progression of ovarian carcinoma. Gynecol Oncol. 115:185–192. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gartel AL and Kandel ES: RNA interference
in cancer. Biomol Eng. 23:17–34. 2006. View Article : Google Scholar
|
|
28
|
Scherr M and Eder M: Gene silencing by
small regulatory RNAs in mammalian cells. Cell Cycle. 6:444–449.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hannon GJ, Chubb A, Maroney PA, Hannon G,
Altman S and Nilsen TW: Multiple cis-acting elements are required
for RNA polymerase III transcription of the gene encoding H1 RNA,
the RNA component of human RNase P. J Biol Chem. 266:22796–22799.
1991.PubMed/NCBI
|
|
30
|
Walchli S and Sioud M: Vector-based
delivery of siRNAs: in vitro and in vivo challenges. Front Biosci.
13:3488–3493. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fogh J, Wright WC and Loveless JD: Absence
of HeLa cell contamination in 169 cell lines derived from human
tumors. J Natl Cancer Inst. 58:209–214. 1977.PubMed/NCBI
|
|
32
|
Yagita M, Huang CL, Umehara H, et al: A
novel natural killer cell line (KHYG-1) from a patient with
aggressive natural killer cell leukemia carrying a p53 point
mutation. Leukemia. 14:922–930. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takikawa O, Kuroiwa T, Yamazaki F and Kido
R: Mechanism of interferon-gamma action. Characterization of
indoleamine 2,3-dioxygenase in cultured human cells induced by
interferon-gamma and evaluation of the enzyme-mediated tryptophan
degradation in its anticellular activity. J Biol Chem.
263:2041–2048. 1988.
|
|
34
|
Miyagishi M and Taira K: Strategies for
generation of an siRNA expression library directed against the
human genome. Oligonucleotides. 13:325–333. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nonaka H, Saga Y, Fujiwara H, et al:
Indoleamine 2,3-dioxygenase promotes peritoneal dissemination of
ovarian cancer through inhibition of natural killercell function
and angiogenesis promotion. Int J Oncol. 38:113–120. 2011.
|
|
36
|
Cady SG and Sono M:
1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the
oxygen analog of tryptophan), and
beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of
tryptophan) are competitive inhibitors for indoleamine
2,3-dioxygenase. Arch Biochem Biophys. 291:326–333. 1991.PubMed/NCBI
|
|
37
|
Muller AJ, DuHadaway JB, Donover PS,
Sutanto-Ward E and Prendergast GC: Inhibition of indoleamine
2,3-dioxygenase, an immunoregulatory target of the cancer
suppression gene Bin1, potentiates cancer chemotherapy. Nat Med.
11:312–319. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yen MC, Lin CC, Chen YL, et al: A novel
cancer therapy by skin delivery of indoleamine 2,3-dioxygenase
siRNA. Clin Cancer Res. 15:641–649. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shiota M, Ikeda Y and Wadhwa R: The
factors that contribute to the long-term expression of siRNA.
Nucleic Acids Symp Ser (Oxf). 2006:243–244. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Serda RE, Godin B, Blanco E, Chiappini C
and Ferrari M: Multi-stage delivery nano-particle systems for
therapeutic applications. Biochim Biophys Acta. 1810:317–329. 2011.
View Article : Google Scholar : PubMed/NCBI
|