Introduction
Hepatocellular carcinoma (HCC) is the fifth most
common cancer and the third most common cause of cancer-related
death (1). Although surgical
resection offers the best prognosis for long-term survival, most of
HCC patients are not suitable for surgical resection because at the
time of diagnosis the tumor may be too large or has expanded into
nearby major blood vessels or metastasized (2,3).
Therefore, chemotherapy continues to be one of the major
non-surgical therapeutic approaches for patients with advanced HCC.
However, because of the inherently chemotherapy-resistant nature of
HCC and the unacceptable adverse effects of most currently-used
chemotherapeutic agents, the systemic cytotoxic chemotherapy is not
always effective at improving patient survival (4). Thus, developing novel anti-cancer
therapeutic agents is urgently needed. Recently, natural products
have received great interest as they have relatively few
side-effects compared to modern chemotherapeutics and have long
been used clinically to treat various diseases including cancer
(5–8). Rubus aleaefolius Poir
(Rubus L.) is a major genus of the Rosaceae, widely
distributed all over the world. This herb is generally used as a
folk medicine to treat various types of hepatitis in southern part
of Fujian Province, China. Previously, we reported that the
ethylacetate and then-butanol fractions of Rubus aleaefolius
Poir displays in vivo hepatoprotective effects in carbon
tetrachloride-induced acute liver injury mouse model (9). In addition, modern pharmacological
studies have proposed that Rubus aleaefolius Poir contains
antitumor activity (10,11). However, the precise mechanisms of
its potential tumoricidal activity remain unclear.
Apoptosis eliminates redundant or damaged cells and
hence is crucial for maintaining tissue homeostasis. Disturbed
regulation of apoptosis contributes to a variety of diseases,
including autoimmunity, neurodegeneration and cancer (12–14).
Mitochondria play an important role in apoptotic death, which is
highly regulated by Bcl-2 family proteins including both
anti-apoptotic members (such as Bcl-2 and Bcl-XL) and pro-apoptotic
members (such as Bax and Bak). It has been demonstrated that after
activation, the pro-apoptotic Bax or Bak is sufficient to induce
mitochondrial outer membrane permeabilization (MOMP) (15–17),
releasing pro-apoptotic proteins such as cytochrome c and
Diablo/Smac that eventually lead to the activation of
aspartate-directed cysteine proteases (caspases) and nucleases,
resulting in destruction of the cell (18–20).
The anti-apoptotic Bcl-2 protein protects cells from apoptosis
probably by interacting with Bax and inhibiting Bax-mediated MOMP
(17,21–23).
The sensitivity of cells to apoptotic stimuli can depend on the
ratio of pro- and anti-apoptotic Bcl-2 proteins. Alteration of the
ratio by aberrant expression of these proteins impairs the normal
apoptotic program contributing to cancer development (24,25).
Therefore, promoting cell apoptosis has been suggested as a
promising strategy for the development of anticancer drugs. Using
mouse xenograft model and hepatocellular carcinoma cell line, in
the present study we evaluated the antitumor effect of total
alkaloids of Rubus aleaefolius Poir (TARAP) in vivo
and in vitro, and investigated the possible molecular
mechanisms mediating its biological activity.
Materials and methods
Materials and reagents
Dulbecco’s modified Eagle’s medium (DMEM), fetal
bovine serum (FBS), penicillin-streptomycin, Trypsin-EDTA, TRIzol
reagent and
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl-benzimidazol-carbocyanine
iodide (JC-1), and caspase-3 and -9 colorimetric protease assays
were purchased from Invitrogen (Carlsbad, CA, USA). SuperScript II
reverse transcriptase was obtained from Promega (Madison, WI, USA).
Antibodies for Bax and Bcl-2 were obtained from Santa Cruz
Biotechnology Inc. (Santa Cruz, CA, USA), a fluorescein
isothiocyanate (FITC)-conjugated Annexin V apoptosis detection kit
was obtained from Becton-Dickinson (San Jose, CA, USA). All other
chemicals, unless otherwise stated, were obtained from Sigma
Chemicals (St. Louis, MO, USA).
Preparation and content of total
alkaloids of Rubus aleaefolius Poir (TARAP)
The preparation of TARAP was performed as described
(26). The roots of Rubus
alceifolius Poir were collected from Anxi of Fujian Province,
identified and authenticated by experts in our University, and the
alkaloids were extracted. The herb powder (1 g) was extracted with
50 ml chloroform:methanol:ammonia solution (15:4:3) for 2 h in an
ice bath, sonicated for 30 min, brought to room temperature and
filtered. The filtered solution was collected and dessicated. The
resultant residue was dissolved by 2 ml of 2% sulfuric acid
solution and filtered. The filter paper and residue were re-washed
with 2 ml of 2% sulfuric acid solution and with buffer solution (pH
3.6). Buffer was then added to make a final volume of 50 ml and the
solution saved for future use. Acid dye colorimetry was used to
measure total alkaloid content. Total alkaloid content was 0.81 mg
of alkaloid per gram of initial herb powder.
Cell culture
Human hepatocellular carcinoma cell HepG2 cells were
obtained from American Type Culture Collection (ATCC, Manassas, VA,
USA). The cells were grown in DMEM containing 10% (v/v) FBS, and
100 U/ml penicillin and 100 μg/ml streptomycin in a 37°C
humidified incubator with 5% CO2. The cells were
subcultured at 80–90% confluency.
In vivo tumor xenograft study
HepG2 cells were grown in culture and then detached
by trypsinization, washed and resuspended in serum-free DMEM.
Six-week-old athymic BALB/c nu/nu male mice were given an s.c.
injection of 4×106 HepG2 cells mixed with Matrigel (1:1)
in the right flank to initiate tumor growth. After 7 days of
xenograft implantation when tumor size reached 3 mm in diameter,
mice were randomly divided into two groups and gavaged with the
following: i) control group (n=10), physiological saline (PS); and
ii) TARAP group (n=10), 3 g/kg/d dose of TARAP in PS. All
treatments were given 5 days a week for 21 days. Body weight and
diet consumption were recorded twice weekly throughout the study.
Tumor sizes were measured twice weekly and volume was calculated as
reported recently (27). At the
end of experiment, tumors were excised and weighed, and part of
tumor was fixed in buffered formalin and the remaining was stored
at −80°C for molecular analyses.
Evaluation of cell viability by MTT
assay
Cell viability was assessed by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
colorimetric assay. HepG2 cells were seeded into 96-well plates at
a density of 1×105 cells/ml in 0.1 ml medium. The cells
were treated with various concentrations of TARAP for 24, 48 and 72
h. Treatment with 0.5% DMSO was included as vehicle control. At the
end of the treatment, 10 μl MTT [5 mg/ml in
phosphate-buffered saline (PBS)] were added to each well, and the
samples were incubated for an additional 4 h at 37°C. The
purple-blue MTT formazan precipitate was dissolved in 100 μl
DMSO. The absorbance was measured at 570 nm using an ELISA reader
(BioTek, Model ELX800, Winooski, VT, USA).
Observation of morphologic changes
HepG2 cells were seeded into 6-well plates at a
density of 2×105 cells/well in 2 ml medium. The cells
were treated with various concentrations of TARAP for 48 h. Cell
morphology was observed using a phase-contrast microscope (Olympus,
Japan). The photographs were taken at a magnification of ×200.
Detection of apoptosis by TUNEL
The TUNEL reaction was carried out after treatment
with TARAP as previously described (28). Briefly, sequential 4 μm
tissue sections were adhered to silane-coated slides and allowed to
dry at room temperature (RT). Subsequently, sections were
deparaffinized and rehydrated. Protein digestion was done by
incubating tissue sections in 20 mg/ml proteinase K (Worthington
Co., Lakewood, CO, USA) for 15 min at RT. Endogenous peroxidase was
inactivated with 2% H2O2 in distilled water
(dH2O) for 5 min, RT. The labelling mixture containing biotinylated
dUTP in TdT enzyme buffer was added to sections and incubated at
37°C in an humified chamber for 1 h. After stopping the enzymatic
reaction, sections were rinsed with PBS, covered with
anti-digoxigenin peroxidase conjugate and incubated for 30 min at
RT in an humified chamber. Then, sections were incubated in TBS
with 0.05% diaminobenzidine (DAB) plus 3%
H2O2 until colour development was achieved.
Finally, sections were washed, counterstained in haematoxylin,
dehydrated and mounted with DPX (Panreac SA, Barcelona, Spain), and
as a negative control active TdT buffer was replaced by the kit
equilibration buffer.
Detection of apoptosis by flow cytometry
analysis with Annexin V/PI staining
After incubation with various concentrations of
TARAP, apoptosis of HepG2 cells was determined by flow cytometry
analysis using a fluorescence-activated cell sorting (FACS)Calibur
(Becton-Dickinson) and Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) kit. Staining was performed according
to the manufacturer’s instructions. The percentage of cells in
early apoptosis was calculated by Annexin V-positivity and
PI-negativity, while the percentage of cells in late apoptosis was
calculated by Annexin V-positivity and PI-positivity.
Measurement of mitochondrial membrane
potential (ΔΨm) by flow cytometric analysis with JC-1 staining
JC-1 is a cationic dye that exhibits
potential-dependent accumulation in mitochondria, indicated by a
fluorescence emission shift from green to red, which thus can be
used as an indicator of mitochondrial potential. In this
experiment, 1×106 treated HepG2 cells were resuspended
after trypsinization in 1 ml of medium and incubated with 10
μg/ml of JC-1 at 37°C, 5% CO2, for 30 min. Both
red and green fluorescence emissions were analyzed by flow
cytometry after JC-1 staining.
Analysis of caspase activation
The activities of caspase-3 and -9 were determined
by a colorimetric assay using the caspase-3 and -9 activation kits,
following the manufacturer’s instructions. Briefly, after treatment
with various concentrations of TARAP for 48 h, HepG2 cells were
lysed with the lysis buffer provided by the manufacturer, for 30
min on ice. The lysed cells were centrifuged at 16,000 × g for 10
min. The protein concentration of the clarified supernatant was
determined and 100 μg of the protein were incubated with 50
μl of the colorimetric tetrapeptides, Asp-Glu-Val-Asp
(DEAD)-p-nitroaniline (pNA) (specific substrate of caspase-3) or
Leu-Glu-His-Asp (LEHD)-pNA (specific substrate of caspase-9) at
37°C in the dark for 2 h. Samples were read at 405 nm in an ELISA
plate reader (BioTek, Model ELX800). The data were normalized to
the activity of the caspases in control cells (treated with 0.5%
DMSO vehicle) and are presented as ‘fold of control’.
RNA extraction and RT-PCR analysis
The expression of Bax and Bcl-2 genes were detected
by RT-PCR. Total RNA was isolated with TRIzol Reagent.
Oligo(dT)-primed RNA (1 μg) was reverse-transcribed with
SuperScript II reverse transcriptase (Promega) according to the
manufacturer’s instructions. The obtained cDNA was used to
determine the mRNA amount of Bcl-2 or Bax by PCR. GAPDH was used as
an internal control. The sequences of the primers used for
amplification of Bcl-2, Bax and GAPDH transcripts are as follows:
Bcl-2 forward: 5′-CAG CTG CAC CTG ACG CCC TT-3 and reverse: 5′-GCC
TCC GTT ATC CTG GAT CC-3′; Bax forward: 5′-TGC TTC AGG GTT TCA TCC
AGG-3′ and reverse: 5′-TGG CAA AGT AGA AAA GGG CGA-3′; GAPDH
forward: 5′-GT CAT CCA TGA CAA CTT TGG-3′ and reverse: 5′-GA GCT
TGA CAA AGT GGT CGT-3′.
Immunohistochemistry analysis
Immunohistochemical staining for Bcl-2 and Bax was
performed as previously (29). The
sections were deparaffinised in xylene and hydrated through graded
alcohols. Antigen unmasking was performed using heat treatment in a
microwave oven at 750 W for 7 min in a container with 10 mM sodium
citrate buffer, pH 6.0. Sections were allowed to cool in the buffer
at room temperature for 30 min and were rinsed in deionised water
three times for 2 min each. The endogenous peroxidase activity was
blocked with 3% (v/v) hydrogen peroxide for 10 min. The sections
were incubated with 1% bovine serum albumin in order to decrease
non-specific staining and reduce endogenous peroxidase activity.
The sections were then incubated with Bax or Bcl-2 antibody (all in
1:200 dilution, Santa Cruz Biotechnology Inc.) at 4°C overnight
using a staining chamber. Primary antibodies were diluted (1:100)
in PBS. After rinsing three times in PBS, sections were incubated
in biotinylated goat anti-rabbit IgG (Boshide, Wuhan, China)
followed by avidin-biotin-peroxidase complex (Vector).
Immunostaining was visualized by incubation in 3,3-diaminobenzidine
(DAB) as a chromogen. Sections were counterstained with
haematoxylin. The Bax and Bcl-2 positive immunostainings were
evaluated by the use of Nikon Eclipse 50i microscope (×40
objective). The evaluation of Bax and Bcl-2 expression was analysed
in 6 different fields and the mean percentage of Bax or Bcl-2
positive staining was evaluated.
Statistical analysis
All data are the means of three determinations. The
data were analyzed using the SPSS package for Windows (Version
11.5). Statistical analysis of the data was performed with
Student’s t-test and ANOVA. Differences with P<0.05 were
considered statistically significant.
Results
TARAP inhibited hepatocellular carcinoma
growth in vivo and in vitro
The anticancer activity of TARAP in vivo was
determined via examining tumor volume and weight in hepatocellular
carcinoma (HCC) xenograft mice, whereas its side-effects were
determined by measuring the body weight change. As shown in
Fig. 1A, tumor volume per mouse
was 1,900±167 mm3 or 1,370±98 mm3 in control
or TARAP treated group, respectively, accounting for a 28% decrease
in tumor volume (P<0.01). Consistently, TARAP treatment caused
39% decrease in tumor weight (P<0.01) compared with control
(0.97±0.21 g or 0.59±0.16 g per mouse in control or TARAP-treated
group). In contrast, TARAP treatment did not affect body weight
gain. These findings together demonstrated the in vivo
antitumor efficacy of TARAP against human HCC tumor xenograft in
nude mice without any apparent sign of toxicity. To evaluate the
in vitro antitumor activity of TARAP, we performed MTT assay
to examine its effect on the viability of human hepatocellular
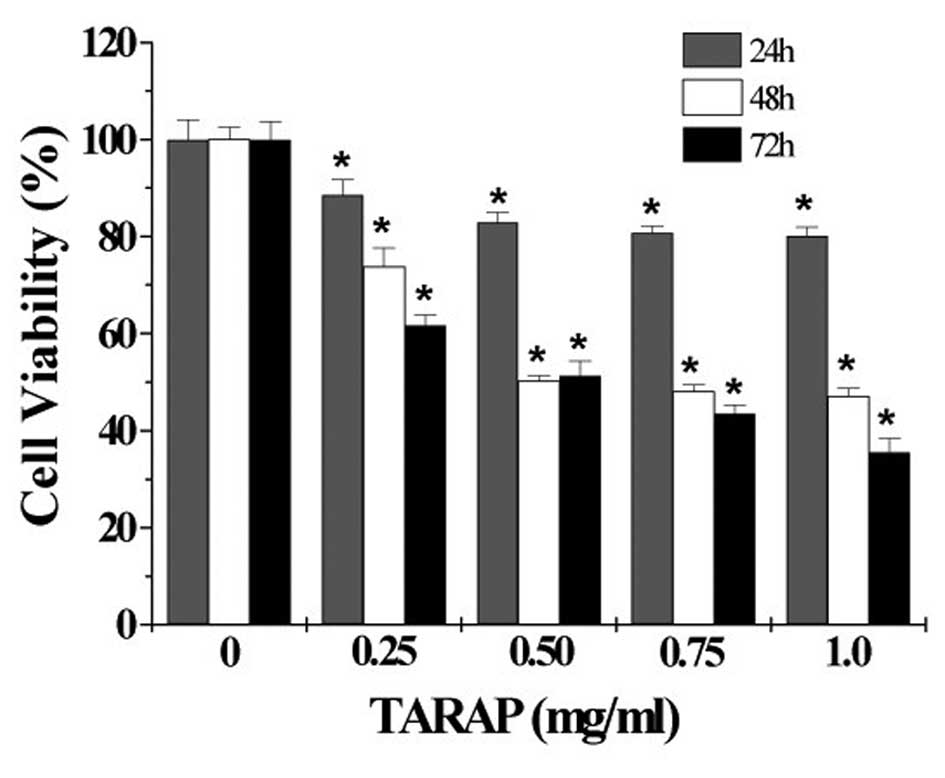
carcinoma HepG2 cells. As shown in Fig. 2, treatment with 0.25–1.0 mg/ml of
TARAP for 24, 48 or 72 h, respectively, reduced cell viability by
11–20, 26–53 or 39–65%, compared to untreated control cells
(P<0.01), indicating that TARAP inhibits the growth of HepG2
cells in a dose- and time-dependent manner. To further verify these
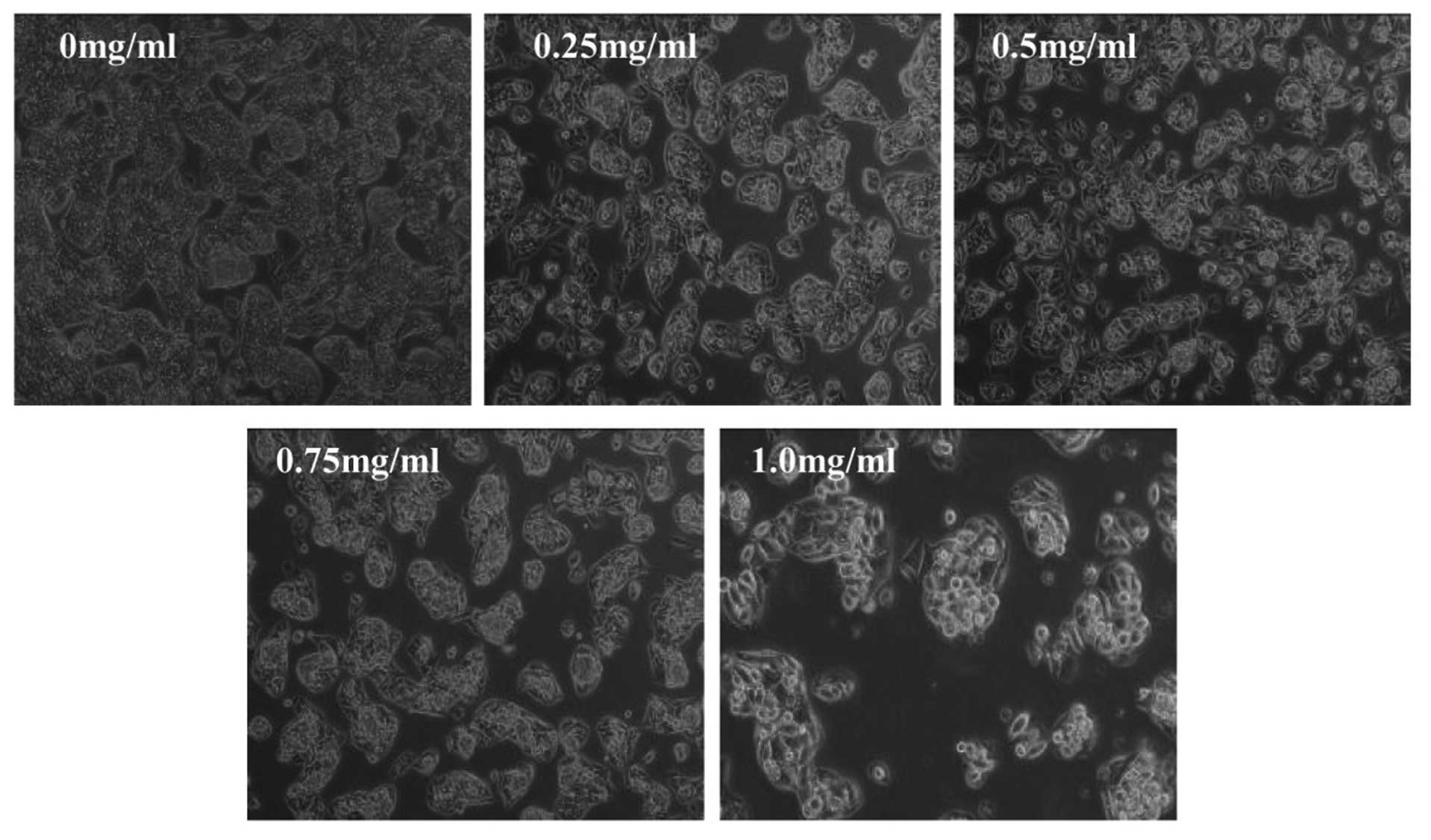
results, we evaluated the effect of TARAP on HepG2 cell morphology
that represents the healthy status of cells in culture. As shown in
Fig. 3, untreated HepG2 cells
appeared as densely packed and disorganized multilayers, whereas,
many of the TARAP-treated cells were rounded, shrunken and detached
from adjacent cells adhering to the plate or floating in the
medium. Taken together, these data demonstrate that TARAP inhibits
the growth of HepG2 cells. Taken together, it is suggested that
TARAP inhibits hepatocellular carcinoma growth both in vivo
and in vitro, without apparent adverse effects.
TARAP induces hepatocellular carcinoma
cell apoptosis in vivo and in vitro
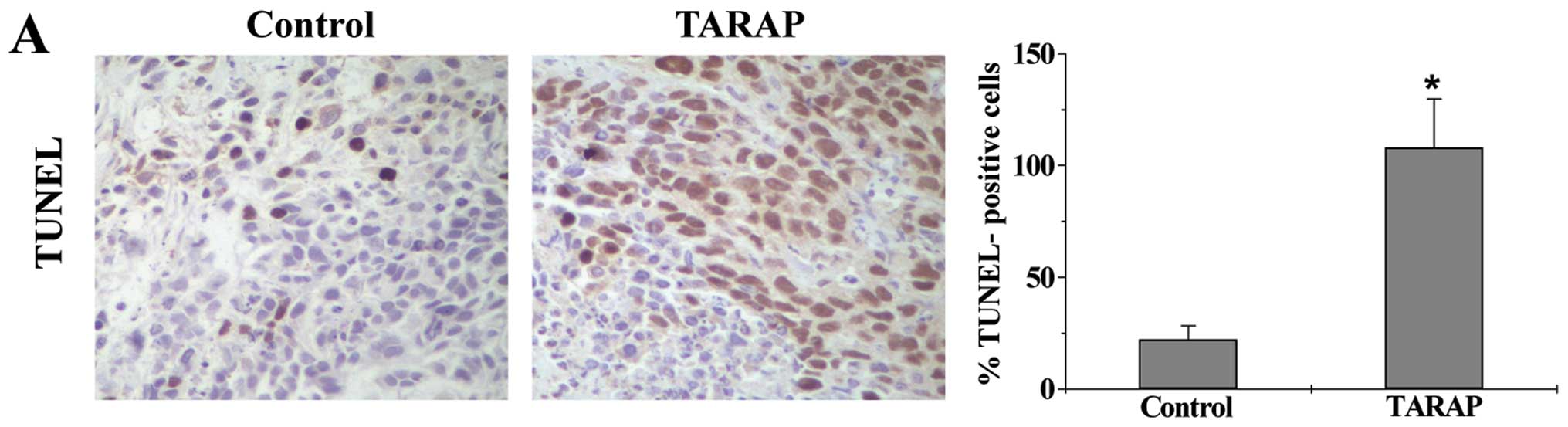
Cell apoptosis in HCC tumor tissues was determined
via immunohistochemical (IHC) staining for TUNEL. As shown in
Fig. 4A, the percentage of
TUNEL-positive cells in control or TARAP-treated mouse group was
22.23±6.13 or 83.83±12.24%, respectively, indicating that TARAP
treatment significantly induced cell apoptosis in HCC tumors. The
apoptosis of HepG2 cells was evaluated by FACS analysis with
Annexin V/PI staining. As shown in Fig. 4B and C, the percentage of cells
undergoing either early apoptosis or late apoptosis following
treatment with 0, 0.25, 0.5, 0.75 and 1.0 mg/ml of TARAP was
9.18±1.32, 17.5±3.41, 21.92±3.49, 33.1±3.31 and 54.72±8.76%,
respectively (P<0.05,). These data together demonstrate that
TARAP promotes hepatocellular carcinoma cell apoptosis both in
vivo and in vitro.
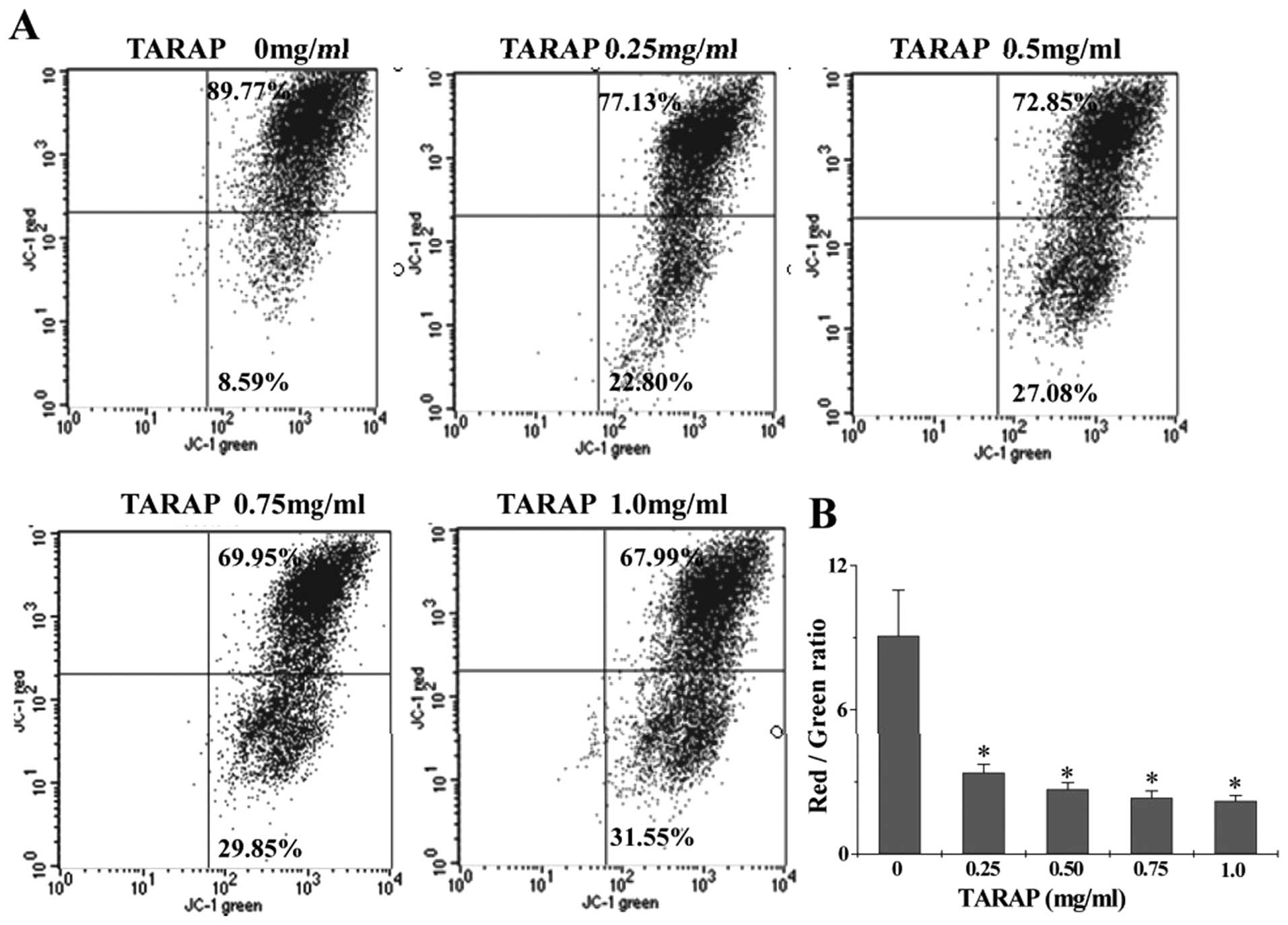
TARAP induces loss of mitochondrial
potential (ΔΨm) and the activation of caspase-9 and -3 in HepG2
cells
The effect of TARAP on the change of mitochondrial
membrane potential in HepG2 cells was examined via JC-1 staining
followed by FACS analysis. The membrane-permeant JC-1 dye displays
potential-dependent accumulation in mitochondria, indicated by a
fluorescence emission shift from green (∼525 nm) to red (∼590 nm).
Therefore, collapse of mitochondrial potentail during apoptosis is
indicated by a decrease in the ratio of red/green fluorescence
intensity. As shown in Fig. 5,
after treatment with 0, 0.25, 0.5, 0.75 and 1.0 mg/ml of TARAP the
JC-1 red/green fluorescent ratio in HepG2 cells was 9.08±1.896,
3.38±0.348, 2.69±0.275, 2.34±0.282 and 2.21±0.226, respectively,
suggesting that TARAP dose-dependently induces the loss of
mitochondrial membrane potential in hepatocellular carcinoma cells.
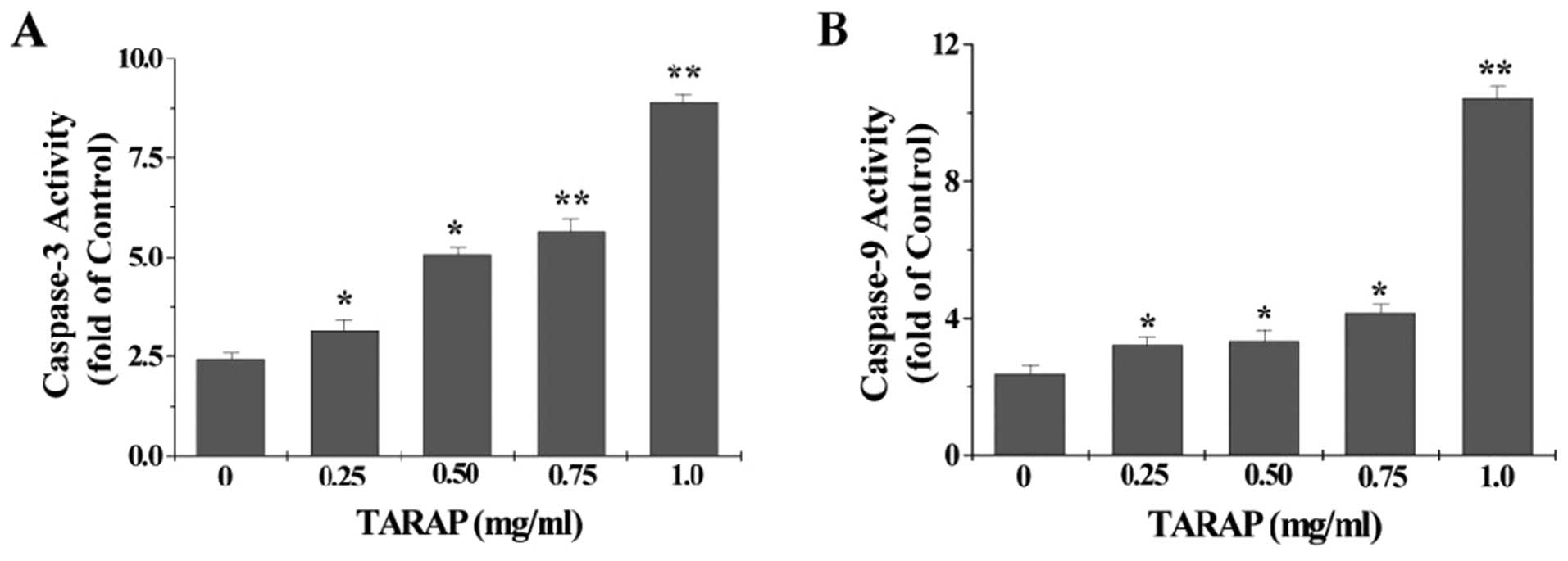
To identify the downstream effectors in the apoptotic signaling
pathway, the activation of caspase-9 and caspase-3 was examined by
a colorimetric assay using specific chromophores, DEVD-pNA
(specific substrate of caspase-3) and LEHD-pNA (specific substrate
of caspase-9). As showed in Fig.
6, TARAP treatment significantly and dose-dependently induced
activation of both caspase-9 and caspase-3 in HepG2 cells
(P<0.05 vs. untreated control cells).
TARAP upregulates the ratio of
pro-apoptotic Bax to anti-apoptotic Bcl-2 in HCC xenograft
mice
To further investigate the mechanism of TARAP’s
pro-apoptotic activity, we performed RT-PCR and IHC analyses to,
respectively, determined the mRNA or protein expression of Bcl-2
and Bax in HCC mice. As shown in Fig.
7A, TARAP treatment significantly reduced the mRNA expression
of anti-apoptotic Bcl-2 in HCC tumors, whereas that of
pro-apoptotic Bax was significantly increased after TARAP
treatment. Consistently, results of IHC assay showed that the
protein expression patterns of Bcl-2 and Bax were similar to their
respective mRNA levels. The percentage of Bcl-2- or Bax-positive
cells in control group was 45.6±2.67 or 51.67±12.37%, whereas that
in TARAP-treated mice was 22.5±5.73 or 86±20.36% (Fig. 7B). Collectively, it is suggested
that TARAP promotes hepatocellular carcinoma cell apoptosis via
increasing the pro-apoptotic Bax/Bcl-2 ratio.
Discussion
Cancer cells are characterized by a reduction in
cell apoptosis (12), which
contributes to drug resistance of tumor cells and thus becomes a
major obstacle for the successful management of patients with
malignant tumors. Moreover, many currently used anticancer agents
contain potent cytotoxicity against normal cells. Drug resistance
and adverse effects limits the effectiveness of cancer
chemotherapies (30). Therefore,
developing novel anticancer agents is urgently needed. Rubus
aleaefolius Poir is a natural plant which is typically used to
treat various types of hepatitis in Southern part of Fujian
Province, China. Recently, Rubus aleaefolius Poir has been
reported to possess antitumor activity (10,11).
Therefore, before Rubus aleaefolius Poir can be developed as
an anticancer agent, its antitumor activity and underlying
molecular mechanism should first be elucidated. Using mouse
xenograft model and hepatocellular carcinoma cell line, in the
present study we found that the total alkaloids of Rubus
aleaefolius Poir (TARAP) inhibited cancer growth both in
vivo and in vitro, without apparent sign of toxicity. In
addition, the inhibitory role of TARAP in cancer growth was due to
its pro-apoptotic activity.
The mitochondrion-dependent pathway is the most
common apoptotic pathway in vertebrate animal cells. Mitochondrial
outer membrane permeabilization (MOMP) is a key commitment step in
the induction of cellular apoptosis, since it is the point of
convergence for a large variety of intracellular apoptotic
signaling pathways leading to the release of many apoptogenic
proteins from the mitochondrial intermembrane space. During the
process of MOMP, the electrochemical gradient across the
mitochondrial membrane collapses. Therefore, the loss of
mitochondrial membrane potential is a hallmark for apoptosis. Our
data clearly showed that TARAP treatment led to a collapse of
mitochondrial membrane potential. Caspases, represented by a family
of cysteine proteases, are the key proteins that modulate the
apoptotic response. Caspase-3 is a key executioner of apoptosis,
which is activated by an initiator caspase such as caspase-9 during
mitochondrion-mediated apoptosis. In this study, we found that
TARAP induced the activation of caspase-9 and caspase-3 in
hepatocellular carcinoma HepG2 cells in a dose-dependent manner.
Thus, TARAP induces HepG2 cell death through activation of
mitochondrion-dependent pathway.
Members of the Bcl-2 family such as Bax and Bcl-2
proteins have been found to be the most prominent actors in
controlling the release of cytochrome c and in the
mitochondria-mediated apoptosis pathway (31). The pro-apoptotic Bax translocates
to the mitochondria and integrates into the outer mitochondrial
membrane, where it induces MOMP resulting in the release of
cytochrome c(16,32). In contrast, anti-apoptotic protein
Bcl-2 prevents this process by preserving mitochondrial integrity.
Therefore, the ratio of Bax to Bcl-2 is critical for determining
the fate of cells and higher Bcl-2-to-Bax ratio by aberrant
expression of the proteins commonly found in various cancers. In
this study, we found that TARAP treatment enhanced Bax expression
but reduced Bcl-2 expression in tumors of HCC mice, indicating that
TARAP induces mitochondrion-dependent apoptosis through increasing
the pro-apoptotic Bax/Bcl-2 ratio.
In conclusion, here for the first time we
demonstrate that the total alkaloids of Rubus aleaefolius
Poir inhibits hepatocellular carcinoma growth both in
vivo and in vitro via promoting the
mitochondrion-dependent apoptosis of cancer cells, which is
mediated by the regulation of Bcl-2 family members. Our findings
suggest that Rubus aleaefolius Poir may be a potential novel
therapeutic agent for cancer treatment.
Acknowledgements
This study was supported by the Nature
Science Foundation of Fujian Province of China (no. 2010J01191 and
no. 2010J01194); and the project was sponsored by Medical
Originality Foundation of Fujian Province of China (no.
2009-CX-18).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Estimating the world cancer burden: Globocan 2000. Int J Cancer.
94:153–156. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Levin B and Amos C: Therapy of
unresectable hepatocellular carcinoma. N Engl J Med. 332:1294–1296.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
El-Serag HB, Marrero JA, Rudolph L and
Reddy KR: Diagnosis and treatment of hepatocellular carcinoma. J
Gastroenterol. 134:1752–1763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thomas M: Molecular targeted therapy for
hepatocellular carcinoma. J Gastroenterol. 44:136–141. 2009.
View Article : Google Scholar
|
|
5
|
Newman DJ, Cragg GM and Snader KM: The
influence of natural products upon drug discovery. Nat Prod Rep.
17:215–234. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gordaliza M: Natural products as leads to
anticancer drugs. Clin Transl Oncol. 9:767–776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang W and Eisenbrand G: Chinese Drugs of
Plant Origin: Chemistry, Pharmacology and Use in Traditional and
Modern Medicine. Springer-Verlag; Berlin: 1992, View Article : Google Scholar
|
|
8
|
Huang KC: The Pharmacology of Chinese
Herbs. 2nd edition. CRC Press; Boca Raton, FL: 1999
|
|
9
|
Hong ZF: Hepatoprotective effects of
Rubus aleaefolius Poir and identification of its active
constituents. J Hepatol. 129:267–272. 2010.
|
|
10
|
Xue H, Aziz RM and Sun N: Inhibition of
cellular transformation by berry extracts. Carcinogenesis.
22:351–356. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee JH: Activity of crude extract of
Rubus crataegifolius roots as a potent apoptosis inducer and
DNA to topoisomerase 1 inhibitor. Arch Pharm Res. 23:338–343.
2000.
|
|
12
|
Adams JM and Cory S: The Bcl-2 apoptotic
switch in cancer development and therapy. Oncogene. 26:1324–1337.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borner C: Bcl-2 family members integrators
of survival and death. Biochim Biophys Acta. 1644:71–72. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cory S and Adams JM: The Bcl-2 family:
regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hsu YT, Wolter K and Youle RJ: Cytosol to
membrane redistribution of members of the Bcl-2 family during
apoptosis. Proc Natl Acad Sci USA. 94:3668–3672. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wolter KG: Movement of Bax from the
cytosol to mitochondria. J Cell Biol. 139:1281–1292. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang J, Liu X, Bhalla K, Kim CN, Ibrado
AM, Cai J, Peng TI, Jones DP and Wang X: Prevention of apoptosis by
Bcl-2: release of cytochrome c from mitochondria blocked. Science.
275:1129–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Antonsson B, Montessuit S, Lauper S, Eskes
R and Martinou JC: Bax oligomerization is required for
channel-forming activity in liposomes and to trigger cytochrome c
release from mitochondria. Biochem J. 345:271–278. 2000. View Article : Google Scholar
|
|
19
|
Jürgensmeier JM, Xie Z, Deveraux Q,
Ellerby L, Bredesen D and Reed JC: Bax directly induces release of
cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA.
95:4997–5002. 1998.PubMed/NCBI
|
|
20
|
Kluck RM, Bossy-Wetzel E, Green DR and
Newmeyer DD: The release of cytochrome c from mitochondria: a
primary site for Bcl-2 regulation of apoptosis. Science.
275:1132–1136. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gross A, McDonnell JM and Korsmeyer SJ:
Bcl-2 family members and the mitochondria in apoptosis. Genes Dev.
13:1899–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thomenius MJ, Wang NS, Reineks EZ, Wang Z
and Distelhorst CW: Bcl-2 on the endoplasmic reticulum regulates
Bax activity by binding to BH3-only proteins. J Biol Chem.
278:6243–6250. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Antonsson B, Conti F and Ciavatta A:
Inhibition of Bax channel-forming activity by Bcl-2. Science.
277:370–372. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Youle RJ and Strasser A: The BCL-2 protein
family: opposing activities that mediate cell death. Nat Rev Mol
Cell Biol. 9:47–59. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yip KW and Reed JC: Bcl-2 family proteins
and cancer. Oncogene. 27:6398–6406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin JM, Zhao JY, Li TJ, Zhou JH, Hu J and
Hong ZF: Hepatoprotection in a rat model of acute liver damage
through inhibition of CY2E1 activity by total alkaloids extracted
from Rubus alceifolius Poir. Int J Toxicol. 30:237–243.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh RP, Dhanalakshmi S, Tyagi AK, Chan
DCF, Agarwal C and Agarwal R: Dietary feeding of silibinin inhibits
advance human prostate carcinoma growth in athymic nude mice, and
increases plasma insulin-like growth factor-binding protein-3
levels. Cancer Res. 62:3063–3069. 2002.
|
|
28
|
Resendes AR, Majó N, Segalés J, Espadamala
J, Mateu E, Chianini F, Nofrarías M and Domingo M: Apoptosis in
normal lymphoid organs from healthy normal, conventional pigs at
different ages detected by TUNEL and cleaved caspase-3
immunohistochemistry in paraffin-embedded tissues. Vet Immunol
Immunopathol. 99:203–213. 2004. View Article : Google Scholar
|
|
29
|
Yang JX, Wang YL, Bao Y and Guo J: The
total flavones from Semen cuscutae reverse the reduction of
testosterone level and the expression of androgen receptor gene in
kidney-yang deficient mice. J Ethnopharmacol. 119:166–171.
2008.
|
|
30
|
Boose G and Stopper H: Genotoxicity of
several clinically used topoisomerase II inhibitors. Toxicol Lett.
116:7–16. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Borner C: The Bcl-2 protein family:
sensors and checkpoints for life-or-death decisions. Mol Immunol.
39:615–647. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ow YP, Green DR, Hao Z and Mak TW:
Cytochrome c: functions beyond respiration. Nat Rev Mol Cell Biol.
9:532–542. 2008. View
Article : Google Scholar : PubMed/NCBI
|