Introduction
Correct staging of prostate cancer is an unmet
clinical need. Conventional anatomical imaging modalities (CT and
MRI) tend to understage prostate cancer due to poor sensitivity to
soft tissue metastases. Currently, a significant number of patients
with extraprostatic disease undergo non-curative surgery due to
false negative diagnoses (1). The
utility of PET or PET/CT using 18F-FDG is limited in
this arena; prostate cancer glucose utilization is low, and FDG
uptake is insufficient in up to 81% of primary prostate cancers
(2,3). Other metabolic PET tracers, such as
11C- or 18F-labeled choline or
11C-acetate, have shown some promising results in the
clinic. However, elevated radiolabeled choline uptake was detected
not only in malignant but also in hyperplastic prostate tissues.
Histological evaluation suggests an alarmingly high false-positive
rate for 11C-choline (4). Sensitivity of
11C-acetate-based PET is suboptimal in patients with low
PSA values (5). Thus, accurate
staging of prostate cancer using PET appears to require more
research and development.
An alternative approach to visualization of prostate
cancer is radionuclide targeting of antigens expressed in prostate
cancer cells, e.g. ‘free’ prostate specific antigen, fPSA (6), prostate stem cell antigen, PSCA
(7) or prostate specific membrane
antigen, PSMA (8). Recently it was
shown in a preclinical study (9)
that radiolabeled monoclonal antibody (mAb) 5A10 recognizing an
epitope adjacent to the catalytic cleft of PSA, can selectively
target fPSA in androgen receptor positive xenografts. However,
decrease of androgen receptor expression in castrate refractory
patients could be a challenge in clinical setting.
Expression of PSMA is low in normal prostate tissue,
but it increases in prostate cancer and is significantly
upregulated as tumors dedifferentiate into higher grade,
androgen-resistant and metastatic lesions (10). Recently, it was proposed that PSMA
over-expression on circulating cancer cells could serve as a
biomarker in castration resistance prostate cancers (11). High-affinity small-molecule
inhibitors of PSMA labeled with 123/131I and
99mTc for SPECT and with 68Ga for PET imaging
modalities demonstrated high accumulation in small metastases in
men and are under evaluation in international clinical trials
(12). Currently, PSMA is used to
image prostate cancer via 111In-labeled capromab
pendetide (ProstaScint), which is approved for clinical use in the
United States. In a pivotal study, ProstaScint demonstrated better
sensitivity (63%) in detection of lymph node metastases than CT
(4%) or MRI (15%) (13). The use
of SPECT/CT for imaging increased the sensitivity of
111In-capromab pendetide further. Nonetheless, further
improving the sensitivity of prostate cancer imaging is desirable
(10).
A possible way to increase the sensitivity of
radionuclide imaging using monoclonal antibodies is the use of PET
instead of SPECT (14). PET
provides better resolution (and, accordingly, lower influence of
partial volume effect) and better registration efficiency, which
improves imaging quality. Feasibility of antibody-mediated PET
imaging (immunoPET) has been demonstrated in a number of
preclinical studies using monoclonal antibodies targeting several
tumor-associated antigens (6,15–20).
Preliminary clinical data concerning immunoPET imaging of
HER2-expressing breast cancer (21) and renal cell carcinoma (22) are very encouraging. This gives good
support to the hypothesis that the use of PET in combination with
capromab labeled with an appropriate positron-emitting label would
improve the sensitivity of PSMA imaging and therefore the quality
of prostate cancer staging.
Clearance of intact monoclonal antibodies from the
circulation is relatively slow. To provide sufficient clearance and
increase contrast, imaging is usually performed four to five days
after the injection of 111In-capromab (10). A positron-emitting radionuclide
with a sufficient half-life is required as a label for capromab.
Two nuclides, 89Zr (half-life 78.4 h) and
124I (half-life 100.3 h), are potential options. It has
been shown that 89Zr (as a radiometal) is most suitable
for monoclonal antibodies that are internalized upon binding to
their antigens. After internalization of an antibody and its
degradation in lysosomes, 89Zr is trapped
intracellularly, e.g., Perk et al (17). This increases the retention of
radioactivity by the tumor(s). A downside of this property is that
89Zr is efficiently retained by healthy organs that
catabolize antibodies, mainly liver and spleen (17). In addition to this, there is an
appreciable accumulation of 89Zr in bone (17,21).
Indeed, labeling with 89Zr of two anti-PSMA monoclonal
antibodies (mAbs) targeting the intra- (7E11, capromab) and extra-
(J591) cellular domains of PSMA was recently reported (24,32). In a murine xenograft model, both
mAbs demonstrated high and specific tumor uptake (up to 40% ID/g at
4 days pi) but also high liver, spleen and bone uptake. The
radiocatabolites of 124I-labeled internalized antibodies
‘leak’ from cancer cells after internalization, which reduces tumor
accumulation of radioiodine in comparison with radiometals.
However, the retention of 124I radioactivity in liver,
spleen and bones is lower than that of 89Zr (17).
Capromab binds to an intracellular portion of PSMA
(25). In fact, the antibody works
by targeting necrotic cells in the tumors and does not undergo
internalization and proteolytic lysosomal degradation after
binding. We hypothesized that the use of 124I would be
possible for capromab-mediated immunoPET because tumor retention
should not be dependent on residualizing properties of a radiometal
label but accumulation in normal tissues might be lower for
radioiodine.
The goals of the study were to select a method for
radio-iodination that preserves the specificity of binding to PSMA
after labeling and to test whether the use of radioiodine reduces
the uptake of radioactivity in healthy organs in comparison with
the use of radiometals.
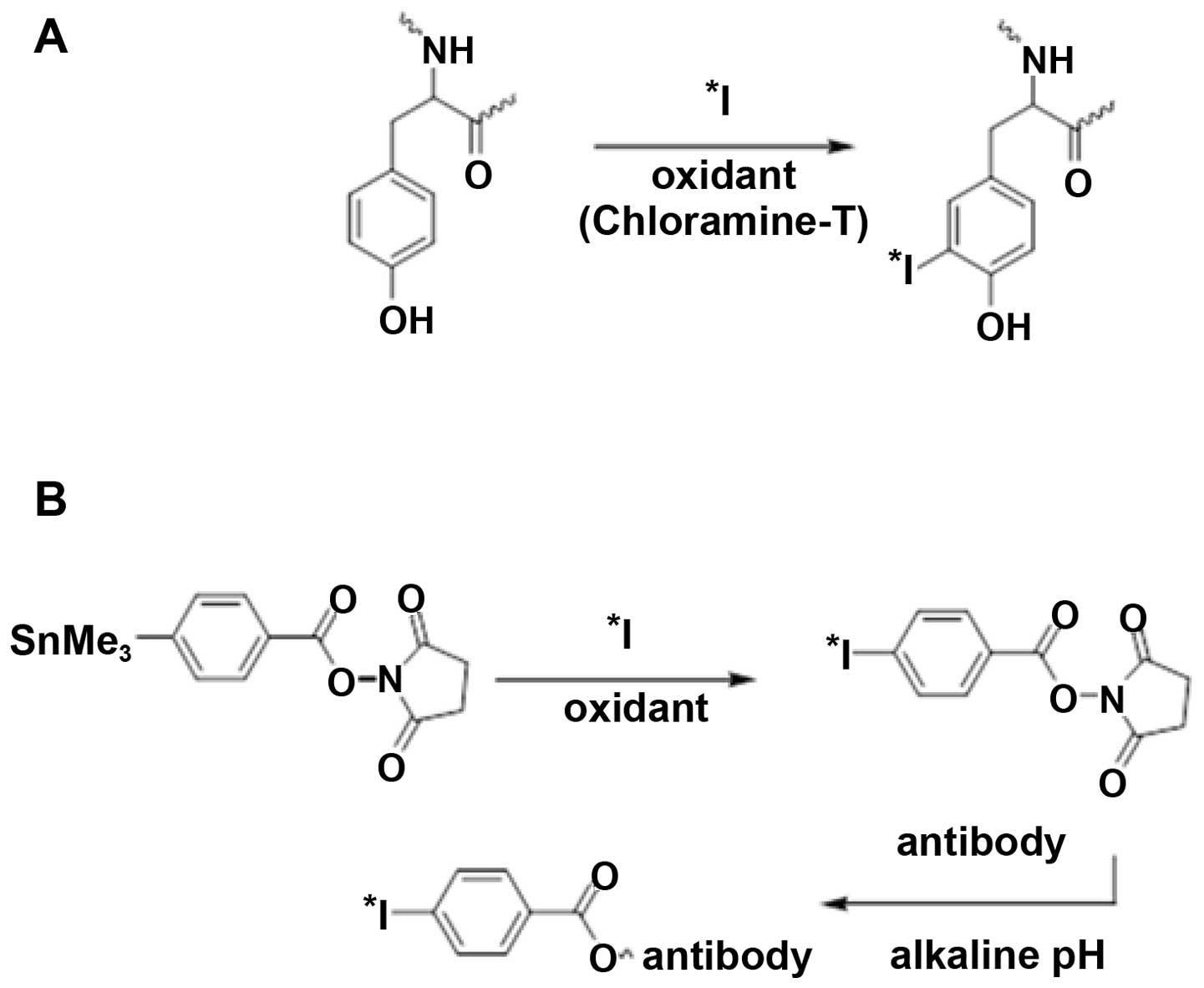
Two approaches (Fig.
1) to the radioiodination of capromab were selected: direct
iodination using chloramine-T and indirect radioiodination using
N-succinimidyl-p-(trimethylstannyl)-benzoate.
Direct radioiodination using chloramine-T was
evaluated because it is a simple and robust method. However, the
use of chloramine-T may destroy the binding specificity of some
antibodies due to iodination of tyrosines in the binding site or by
disruption of critical disulfide bonds in an antibody (26). For this reason, a milder indirect
radioiodination using
N-succinimidyl-p-(trimethylstannyl)-benzoate was
evaluated as an alternative. The iodine isotope 125I
(half-life 60 days) was used in vitro and in biodistribution
studies as a convenient surrogate for 124I (half-life
78.4 h).
Materials and methods
Materials
The monoclonal antibody capromab pendetide
(ProstaScint®) was kindly provided by EUSA Pharma as a
commercial kit (0.5 mg/ml, PBS, pH 6.0).
N-succinimidyl-p-(trimethylstannyl)-benzoate has been
synthesized in our laboratories according to the method described
by Koziorowski et al (27).
Chloramine-T and sodium metabisulfite were from Sigma-Aldrich (St.
Louis, MO).
Buffers, such as 0.1 M phosphate-buffered saline
(PBS), pH 7.5, and 0.07 M sodium borate, pH 9.3, were prepared
using common methods from chemicals supplied by Merck (Darmstadt,
Germany). High-quality Milli-Q water (resistance higher than 18 MΩ
cm) was used for preparing solutions. [111In]-indium
chloride was purchased from Covidien (Mansfield, MA),
[125I]-sodium iodide from Perkin-Elmer (Waltham, MA),
and [124I]-sodium iodide from IBA Molecular (Albany,
NY). NAP-5 size-exclusion columns were from GE Healthcare (Little
Chalfont, UK).
The radioactivity was measured using an automated
gamma-counter with a 3-inch NaI(Tl) detector (1480 Wizard; Wallac
Oy, Turku, Finland). In the dual-isotope biodistribution
experiments, 125I radioactivity was measured in the
energy window from 6 to 60 keV, and 111In was measured
from 100 to 450 keV. The data were corrected for dead time, spill
over and background. Evaluation of purity was performed using
150–771 Dark Green Tec-Control Chromatography strips (Biodex
Medical Systems, Shirley, NY), and distribution of radioactivity
along the ITLC strips was measured on a Cyclone™ Storage Phosphor
System (further referred to as Phosphor Imager) and analyzed using
the OptiQuant™ image analysis software. MicroPET-CT and
microSPECT-CT studies were performed in a Triumph™ Trimodality
system (GammaMedica Inc, Salem, NH), an integrated SPECT/PET/CT
platform optimized for small animals in pre-clinical applications.
Image co-registration and analysis was performed in PMOD v3.13
(PMOD Technologies Ltd, Zurich, Switzerland).
The PSMA-expressing cell line LNCaP (prostate cancer
lymph node metastasis) and PSMA-negative cell line PC3 (prostate
cancer bone metastasis), both from ATCC (LGC Promochem, Borås,
Sweden), were used in this study. Cells were cultured in RPMI
medium (Flow Irvine, Lake Forest, CA). The medium was supplemented
with 10% fetal calf serum (Sigma-Aldrich), 2 mM L-glutamine and
PEST (100 IU/ml penicillin and 100 μg/ml streptomycin), all
from Biokrom AG (Berlin, Germany). For the LNCaP cell line, media
was additionally supplemented with Na-pyruvate and HEPES (Biokrom
AG).
Labeling chemistry
Labeling of capromab pendetide with 111In
was performed according to the manufacturer’s instructions. The
purity of the conjugate (designated as 111In-capromab)
was determined using ITLC.
For indirect radioiodination, the buffer provided in
the commercial kit was changed to 0.07 M sodium borate, pH 9.3, by
ultrafiltration using Centricon 30. For labeling, 125I
solution (6 μl, 15 MBq) was diluted with 10 μl 0.1%
acetic acid/water. A solution of
N-succinimidyl-p-(trimethylstannyl)-benzoate (5
μl, 1 mg/ml in 5% acetic acid/methanol) was added, and the
reaction was initiated by adding 10 μl of chloramine-T
solution (4 mg/ml in water). After 5 min of incubation at room
temperature, the reaction was quenched by the addition of sodium
metabisulfite (10 μl, 6 mg/ml in water), and capromab
pendetide (500 μg in 129 μl 0.07 M sodium borate, pH
9.3) was then added. The mixture was incubated at room temperature
for 60 min. The conjugate (designated 125I-PIB-capromab)
was purified on a NAP-5 column. The purity of the conjugate was
determined using ITLC eluted with 80% acetone in water.
For direct iodination, the buffer provided in the
commercial kit was changed to 0.1 M PBS, pH 7.5, using a NAP-5
column. For labeling, 125I solution (10 μl, 23
MBq) was mixed with 40 μg capromab in 160 μl PBS. The
reaction was initiated by adding 10 μl of chloramine-T
solution (2 mg/ml in PBS). After 2 min of incubation at room
temperature, the reaction was quenched by the addition of sodium
metabisulfite (10 μl, 4 mg/ml in PBS). The conjugate
(designated as 125I-capromab) was purified as described
above.
For labeling of capromab with iodine-124, 20
μl of [124I]-sodium iodide stock solution was
mixed with 4 μl sodium iodide (50 μM in water), and
10 min later 40 μg capromab in 160 μl PBS (prepared
as described above) was added. The reaction was performed and the
product purified as described above.
In vitro specificity test
PSMA-expressing LNCaP cells were seeded at
106 cells per dish in RPMI media or in media designed to
induce membrane permeability (Eagle’s minimum essential medium,
M8167) (25). Experiments were
performed 24 h later. All in vitro experiments were
performed in triplicate.
The cells were washed with PBS and the labeled
conjugates added to the cell dishes at concentrations of 10 nM. To
saturate the binding sites, a blocking amount of non-labeled
capromab (100-fold excess) was added to one set of dishes 15 min
before the addition of radiolabeled capromab. Cells were incubated
for 2 h at 37°C. Thereafter, the media was collected; cells were
washed once with PBS and detached by treatment with trypsin-EDTA
(Biokrom AG). Detached cells were re-suspended and collected. The
radioactivity in the samples was measured.
Measurement of immunoreactive
fraction
Immunoreactive fraction of radiolabeled conjugates
was measured according Lindmo et al (28) using cell membranes of LNCaP cells.
For unspecific binding cell membranes of PC-3 cells (PSMA negative)
were used. The binding assay was set up using 10 nM concentration
of labeled antibody and performed at 4°C. Serial dilution of cell
membrane suspension in PBS, starting at 107 cells/ml
lyzed by Polytron PT 3000 (Kinematica AB, Bohemia, NY), was used.
After incubation cell membranes were pelleted and half of the
supernatant volume was transferred to Eppendorf tubes. The
radioactivity of the samples was measured and the cell associated
radioactivity was calculated as Acells =
(Apellet + media −
Amedia)/(Apellet + media +
Amedia). The unspecific binding to membranes of
PC-3 cells for each data point was subtracted. The data were
plotted as double inverse plot of the applied radioactivity
(Apellet + media + Amedia) over
the specifically bound radioactivity as a function of the inverse
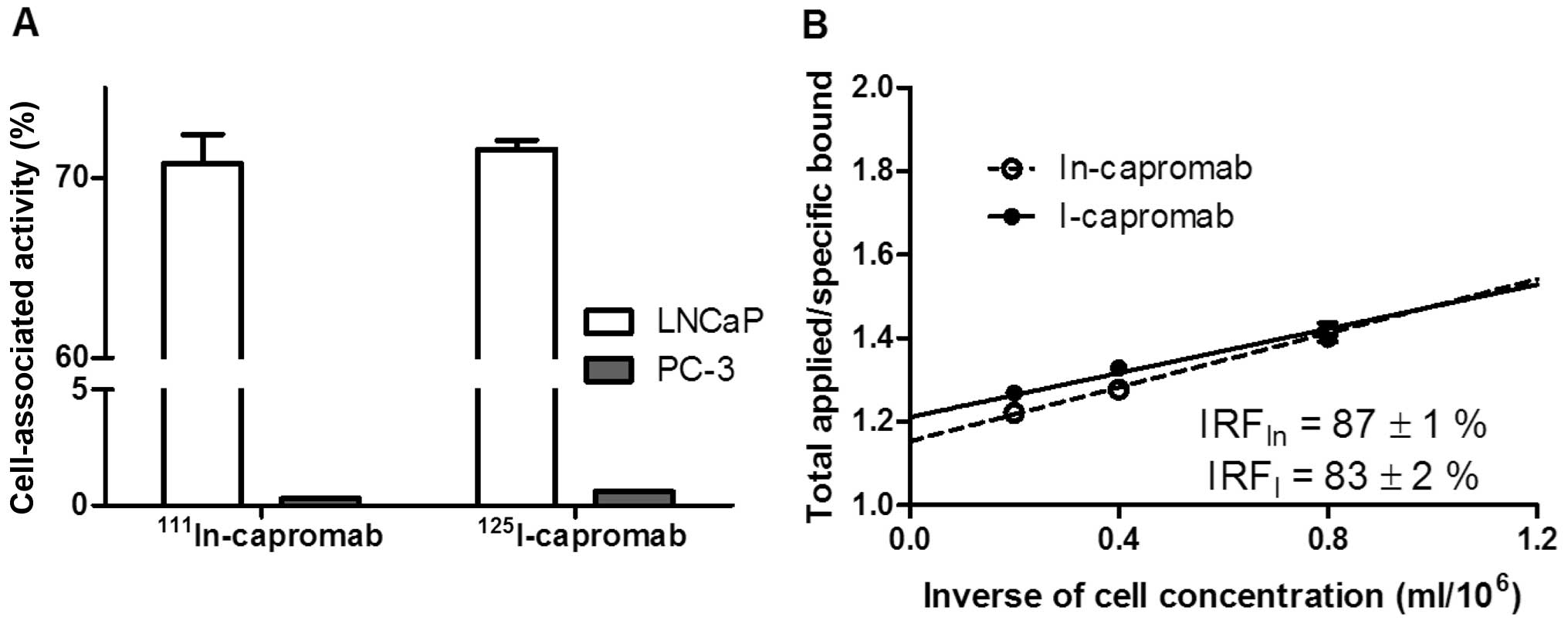
cell concentration (ml/million cells), Fig. 3B. The immunoreactive fraction (IRF)
was calculated as IRF = 1/y(x=0).
Biodistribution experiments
All animal experiments were planned and performed in
accordance with national legislation on laboratory animal
protection. The studies were approved by the Local Ethics Committee
for Animal Research. The drinking water for the mice was
supplemented with potassium iodide (1%) to prevent accumulation of
radioiodine in organs expressing the Na/I symporter (thyroid,
stomach, salivary gland, etc.). In comparative biodistribution
studies, non-tumor-bearing male NMRI mice were used. In tumor
targeting and imaging experiments, male BALB/c nu/nu mice were
used. The tumors were grafted by subcutaneous injection of
6×106 LNCaP cells (Matrigel™/medium, 1/1) or
5×106 PC3 cells onto the right hind legs and were
allowed to develop for 3 weeks. In all biodistribution studies, the
mice (four animals per group) were intravenously injected (tail
vein) with a mixture of 111In-capromab (20 kBq) and
125I-capromab (10 kBq) in 100 μl PBS each. The
total injected antibody dose was 3 μg per animal. All
injections were tolerated well.
In NMRI mice, the biodistribution of
125I/111In-capromab was measured at 4 h and
1, 2 and 3 days pi. At pre-determined time points, sedated animals
were euthanized by heart puncture. Samples were collected from
blood, heart, lung, liver, spleen, pancreas, stomach, colon,
kidney, salivary glands, thyroid, muscle and bone. The intestines
along with their content and the carcasses were also collected. The
organs and tissue samples were weighed and their radioactivity was
measured. A whole gamma-spectrum of each sample was recorded. The
organ uptake values are expressed as per cent of injected dose per
gram of tissue (% ID/g), except for the thyroid, intestines and the
remaining carcass where values are expressed as % ID per whole
sample. A paired t-test was used to determine a significant
difference (p<0.05) between the distributions of 125I
and 111In.
Tumor targeting of capromab (labeled with iodine and
indium) was compared in a dual isotope study in LNCaP xenografted
male mice at 1, 3, 5 and 7 days pi using the protocol described
above. The targeting specificity of radiolabeled capromab was
measured in PC3 xenografted mice (PSMA negative model) 3 days
pi.
Imaging studies
Two tumor-bearing mice were injected with either
124I-capromab (10 MBq) or 111In-capromab (25
MBq) in 100 μl PBS at 10 μg of protein per animal. At
five days pi, the animals were euthanized. The urinary bladder was
excised post-mortem to improve image quality. Static whole body
tomographical examinations were then performed by either microPET
[field-of-view (FOV), 7 cm] or microSPECT (FOV, 8 cm; 75A10
collimators, acquisition over 200–250 keV, 32 projections). The
remaining amount of tracer in each animal at the time of imaging
was 2.75 MBq (124I-capromab) and 5.5 MBq
(111In-capromab). Each animal was examined by CT for
anatomical correlation following the PET/SPECT examination.
Results
Labeling chemistry
As expected, radiolabeling using 111In
was efficient (yield over 99%, specific radioactivity 2.5
MBq/μg), and the conjugate did not require any additional
purification. Direct radioiodination was more efficient (yield 71%,
specific radioactivity 0.41 MBq/μg) than indirect
radioiodination (yield 56%, specific radioactivity 0.017
MBq/μg) for iodine-125. The direct labeling with iodine-124
provided 93% yield (specific radioactivity 1.25 MBq/μg). A
simple purification of radioiodinated capromab using a disposable
NAP-5 column provided purity of over 99% for all used methods and
radioisotopes.
In vitro specificity test and
immunoreactive fraction
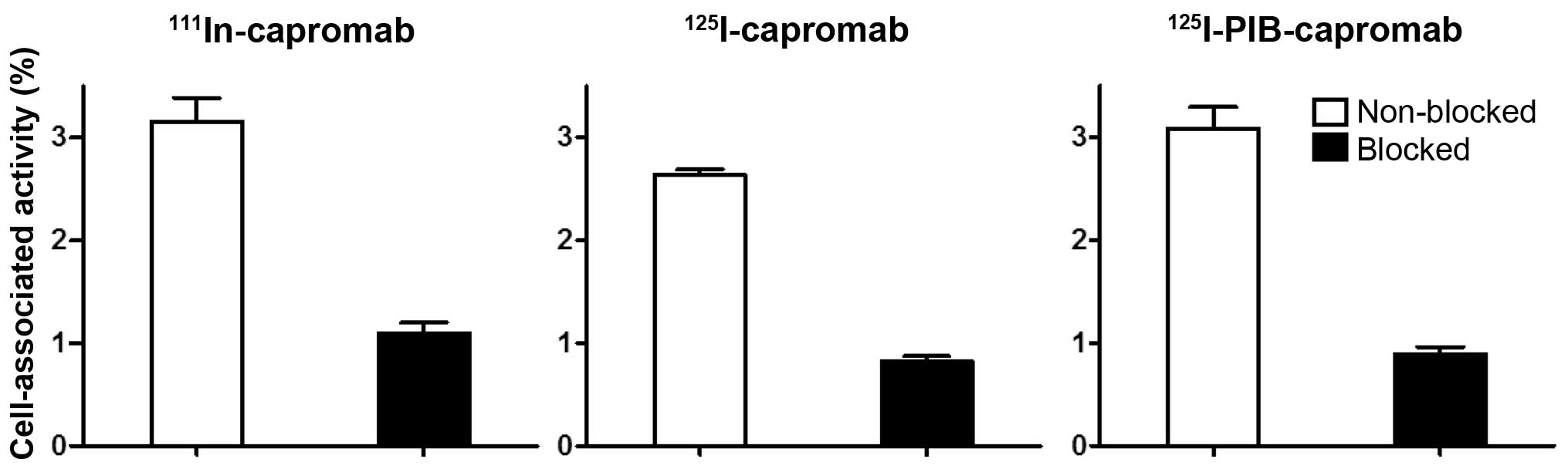
The specificity tests demonstrated that the binding
of 111In-capromab, 125I-capromab, and
125I-PIB-capromab conjugates to PSMA-expressing cells
was specific because saturation of the binding sites by
pre-incubation with non-labeled capromab significantly decreased
the binding of the radiolabeled conjugates (p<0.0001 for all
experiments) (Fig. 2).
Unspecific binding of 111In- and
125I-capromab (Fig. 3A)
to membranes of PSMA negative PC-3 cells was 0.4 and 0.8% from
binding to membranes from equal amount of PSMA-positive LNCaP
cells, respectively. Immunoreactive fraction after labeling was
retained (Fig. 3B) and was in the
same range for indium-111 (87±1%) and iodine-125 (83±2%) labeled
antibody.
In vivo experiments
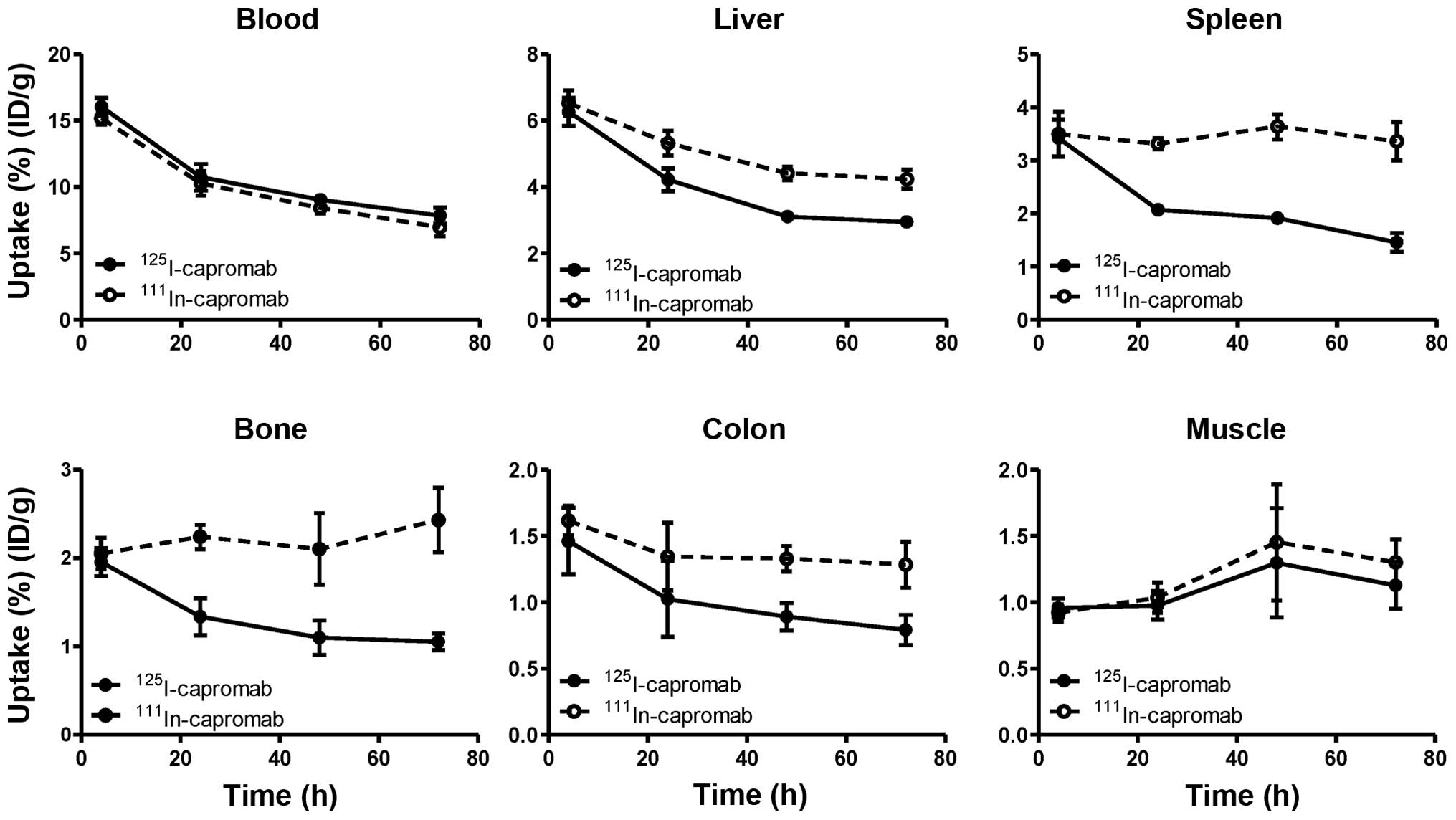
Data regarding the biodistribution of the
radiolabeled antibodies in NMRI male mice are presented in Fig. 4 and Table I. The results of the
biodistribution experiments suggest that the use of the radioiodine
label for capromab results in less accumulation of radioactivity in
a number of organs in comparison with the radiometal label. In the
lower abdomen, the accumulation of 125I was 1.6-fold
lower in colon and 2.3-fold lower in bone in comparison with
111In. The difference for muscle was not as pronounced,
but the accumulation of radioiodine was 12% lower, and the
difference was significant (p<0.005).
 | Table I.Comparative biodistribution of
125I-capromab and 111In-capromab in male NMRI
mice after intravenous injection. |
Table I.
Comparative biodistribution of
125I-capromab and 111In-capromab in male NMRI
mice after intravenous injection.
| 4 h
| 24 h
| 48 h
| 72 h
|
|---|
|
125I-capromab |
111In-capromab |
125I-capromab |
111In-capromab |
125I-capromab |
111In-capromab |
125I-capromab |
111In-capromab |
|---|
| Uptake, %ID/g | | | | | | | | |
| Blood | 16.0±0.7a | 15.2±0.5 | 10.7±1.0a | 10.3±0.9 | 9.0±0.5a | 8.4±0.4 | 7.8±0.6a | 6.9±0.7 |
| Heart | 4.3±0.4 | 4.3±0.3 | 2.4±0.3 | 2.7±0.3a | 2.53±0.05 | 2.8±0.1a | 2.09±0.47 | 2.4±0.5a |
| Lung | 5.0±0.6a | 4.6±0.5 | 4.2±0.8 | 4.2±0.6 | 3.6±0.5 | 3.9±0.7 | 3.1±0.3 | 3.3±0.3a |
| Liver | 6.3±0.4 | 6.5±0.4a | 4.2±0.3 | 5.3±0.4a | 3.1±0.1 | 4.4±0.2a | 2.9±0.2 | 4.2±0.3a |
| Spleen | 3.4±0.3 | 3.5±0.4 | 2.07±0.05 | 3.3±0.1a | 1.9±0.1 | 3.6±0.2a | 1.5±0.2 | 3.4±0.4a |
| Pancreas | 1.5±0.4 | 1.5±0.4 | 1.4±0.2 | 1.6±0.3a | 1.04±0.09 | 1.4±0.2a | 1.06±0.09 | 1.5±0.2a |
| Stomach | 1.6±0.2a | 1.3±0.2 | 0.9±0.1 | 1.1±0.1a | 0.91±0.03 | 1.2±0.1a | 0.85±0.12 | 1.3±0.2a |
| Colon | 1.5±0.3 | 1.6±0.1 | 1.0±0.3 | 1.3±0.3a | 0.9±0.1 | 1.3±0.1a | 0.8±0.1 | 1.3±0.2a |
| Kidney | 4.7±0.4 | 5.3±0.3a | 2.5±0.3 | 5.7±0.3a | 2.3±0.1 | 7.6±0.7a | 1.8±0.6 | 8.1±0.6a |
| Muscle | 0.96±0.07 | 0.92±0.07 | 0.98±0.11 | 1.0±0.1a | 1.30±0.41 | 1.5±0.4a | 1.13±0.18 | 1.3±0.2a |
| Bone | 2.0±0.2 | 2.1±0.2a | 1.3±0.2 | 2.2±0.1a | 1.1±0.2 | 2.1±0.4a | 1.05±0.09 | 2.4±0.4a |
| Thyroid | 0.06±0.002 | 0.06±0.01 | 0.03±0.01 | 0.054±0.009a | 0.08±0.10 | 0.09±0.07 | 0.05±0.03 | 0.09±0.04 |
| GI tract | 3.8±0.6 | 3.9±0.7 | 2.4±0.6 | 3.3±0.7a | 2.1±0.4 | 3.0±0.5a | 1.8±0.4 | 3.3±0.5a |
| Carcass | 28±3 | 30±3a | 33.9±0.9 | 40±1a | 31.9±0.5 | 40.1±0.6a | 30±2 | 40±2a |
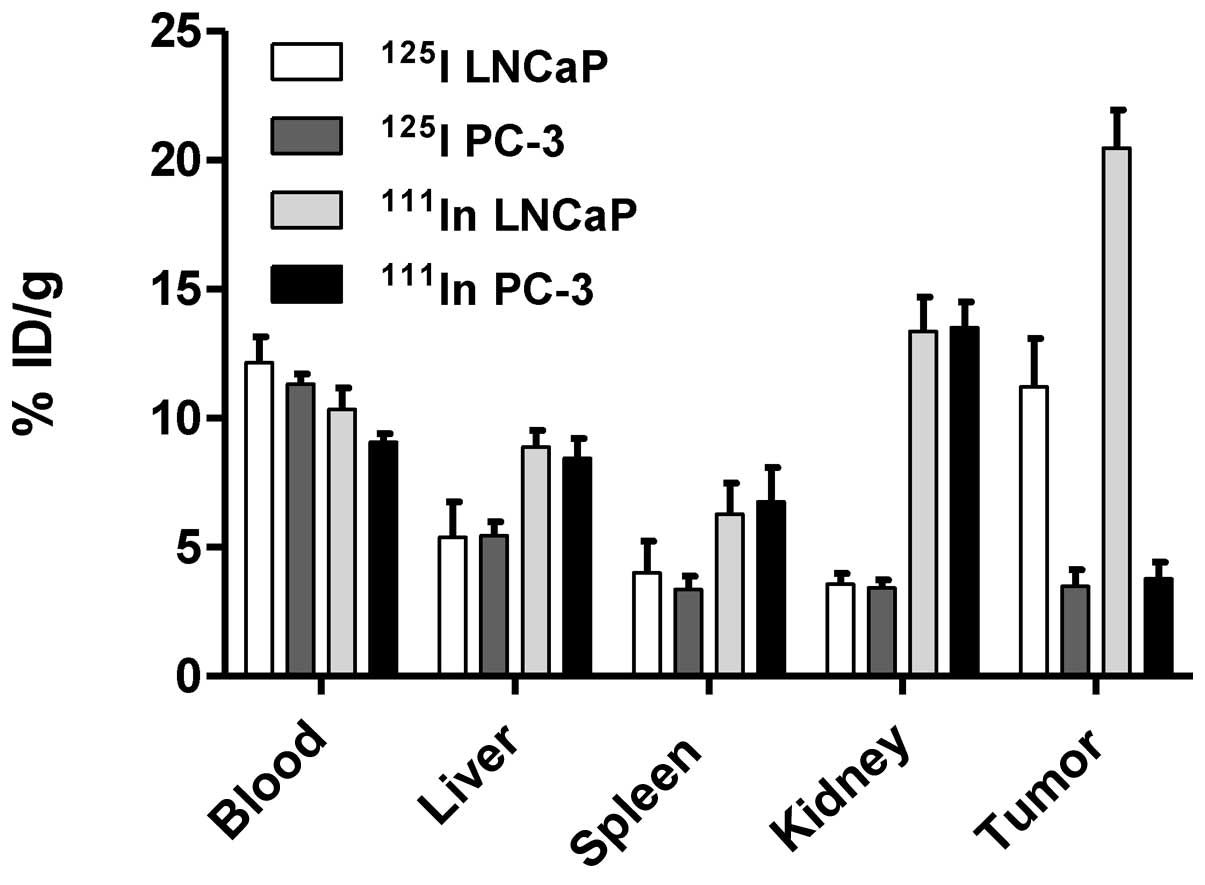
In vivo targeting specificity was confirmed
in comparison of tumor uptake of radiolabeled capromab in PSMA
positive (LNCaP) and negative (PC3) xenografts 3 days pi (Fig. 5). Significantly lower radioactivity
uptake was found in PC3 xenografts in comparison with LNCaP
xenografts for both 111In-capromab and
125I-capromab (p<0.01). There was no significant
difference in the uptake by normal organs in mice bearing PC3 and
LNCaP xenografts.
Data representing the comparative biodistribution
over time in Balb/c nu/nu male mice bearing LNCaP xenografts are
presented in Fig. 6 and Table II. In agreement with the
biodistribution of radiolabeled capromab in NMRI mice, the
concentration of radioactivity in the blood was significantly
higher for iodine-125 at all studied time points. Conversely, the
accumulation of radioactivity in tumors was lower for the iodinated
antibody. In the excretory organs (liver, kidneys, gastrointestinal
tract) and organs of low abdomen (colon), the concentration of
radioactivity was significantly higher for
111In-capromab. The whole body clearance was more rapid
for 125I-capromab.
 | Table II.Tumor targeting and comparative
biodistribution of 125I-capromab and
111In-capromab in male Balb/c nu/nu mice bearing LNCaP
xenografts after intravenous injection. |
Table II.
Tumor targeting and comparative
biodistribution of 125I-capromab and
111In-capromab in male Balb/c nu/nu mice bearing LNCaP
xenografts after intravenous injection.
| 24 h
| 72 h
| 120 h
| 168 h
|
|---|
|
125I-capromab | 111In-
capromab |
125I-capromab | 111In-
capromab |
125I-capromab | 111In-
capromab |
125I-capromab | 111In-
capromab |
|---|
| Uptake, % ID/g | | | | | | | | |
| Blood | 18.1±0.3a | 15.1±0.3 | 12±2a | 10±2 | 10±3a | 7±2 | 9.2±0.7a | 6.5±0.5 |
| Heart | 4.7±0.6a | 4.0±0.6 | 2.8±0.7a | 2.6±0.7 | 2.5±0.5a | 2.2±0.5 | 2.4±0.2a | 2.1±0.2 |
| Lung | 10±2a | 8±1 | 6±2 | 6±1 | 5±1 | 5.7±0.8 | 4.5±0.7 | 4.6±0.5 |
| Liver | 9±2 | 14±1 | 5±3 | 9±1a | 3.5±0.7 | 10±2a | 3.6±0.5 | 9±2a |
| Spleen | 5.6±0.9 | 8±1a | 4±2 | 6±2a | 3±2 | 8±2a | 3.5±0.4 | 6.6±0.6a |
| Pancreas | 2.0±0.2a | 1.8±0.2 | 1.7±0.4 | 1.8±0.4a | 1.4±0.5 | 1.7±0.4a | 1.1±0.2 | 1.4±0.2a |
| Stomach | 2.6±0.2a | 1.7±0.2 | 2.0±0.3a | 1.49±0.07 | 1.7±0.3 | 1.4±0.2 | 1.4±0.1 | 1.26±0.10 |
| Colon | 2.36±0.06 | 2.3±0.2 | 1.4±0.5 | 1.8±0.6a | 1.6±0.5 | 2.1±0.5a | 1.4±0.2 | 1.9±0.1a |
| Kidney | 5.1±0.1 | 11.0±0.8a | 3.6±0.8 | 13±3a | 2.6±0.4 | 14±2a | 2.5±0.4 | 15±2a |
| Tumor | 11±2 | 15±4 | 11±4 | 20±3a | 13±8 | 21±9a | 6±3 | 12±8a |
| Muscle | 2.3±0.2a | 1.9±0.2 | 1.5±0.4 | 1.5±0.2 | 1.6±0.5 | 1.4±0.4 | 1.3±0.2 | 1.4±0.2 |
| Bone | 2.8±0.1 | 3.3±0.2 | 3±1 | 3.5±0.6 | 3±1 | 3.6±0.5 | 2.3±0.6 | 3.0±0.7 |
| Thyroid | 0.06±0.02 | 0.05±0.02 | 0.05±0.02 | 0.04±0.01 | 0.04±0.01 | 0.03±0.01 | 0.03±0.01 | 0.02±0.01 |
| GI tract | 3.0±0.3 | 3.5±0.5a | 2.0±0.3 | 2.8±0.6a | 1.4±0.4 | 2.4±0.5a | 1.4±0.2 | 2.7±0.4a |
| Carcass | 38±2 | 39.2±0.7 | 27±5 | 32±3a | 22±5 | 28±5a | 20±3 | 28±2a |
| Tumor-to-organ
ratio | | | | | | | | |
| Blood | 0.6±0.1 | 1.0±0.2 | 0.9±0.3 | 2.0±0.3a | 2±2 | 4±2a | 0.7±0.3 | 2±1 |
| Liver | 1.4±0.6 | 1.1±0.2 | 2.4±0.8 | 2.3±0.3 | 5±3 | 2±2 | 1.6±0.8 | 1.4±0.9 |
| Spleen | 2.0±0.6 | 1.9±0.8 | 3±2 | 4±1 | 6±4 | 3±2 | 1.7±0.8 | 1.3±1.0 |
| Pancreas | 5±1 | 8±1 | 7±3 | 12±2a | 14±9 | 14±8 | 6±3 | 8±6 |
| Stomach | 4.1±0.7 | 9±1a | 6±3 | 14±2a | 10±4 | 16±8 | 4±2 | 9±6 |
| Colon | 4.6±1.0 | 7±1 | 8±3 | 12±3 | 12±7 | 11±6 | 5±2 | 6±4 |
| Kidney | 2.1±0.5 | 1.3±0.2 | 3±1a | 1.6±0.5 | 7±3 | 1.5±0.7 | 3±1a | 0.9±0.6 |
| Muscle | 4.8±0.7 | 8±2 | 8±3 | 14±2a | 13±10 | 17±12a | 5±2 | 9±6 |
| Bone | 3.9±0.7 | 4.5±0.8 | 4±1 | 6±2a | 6±2 | 6±3 | 3±1 | 4±2 |
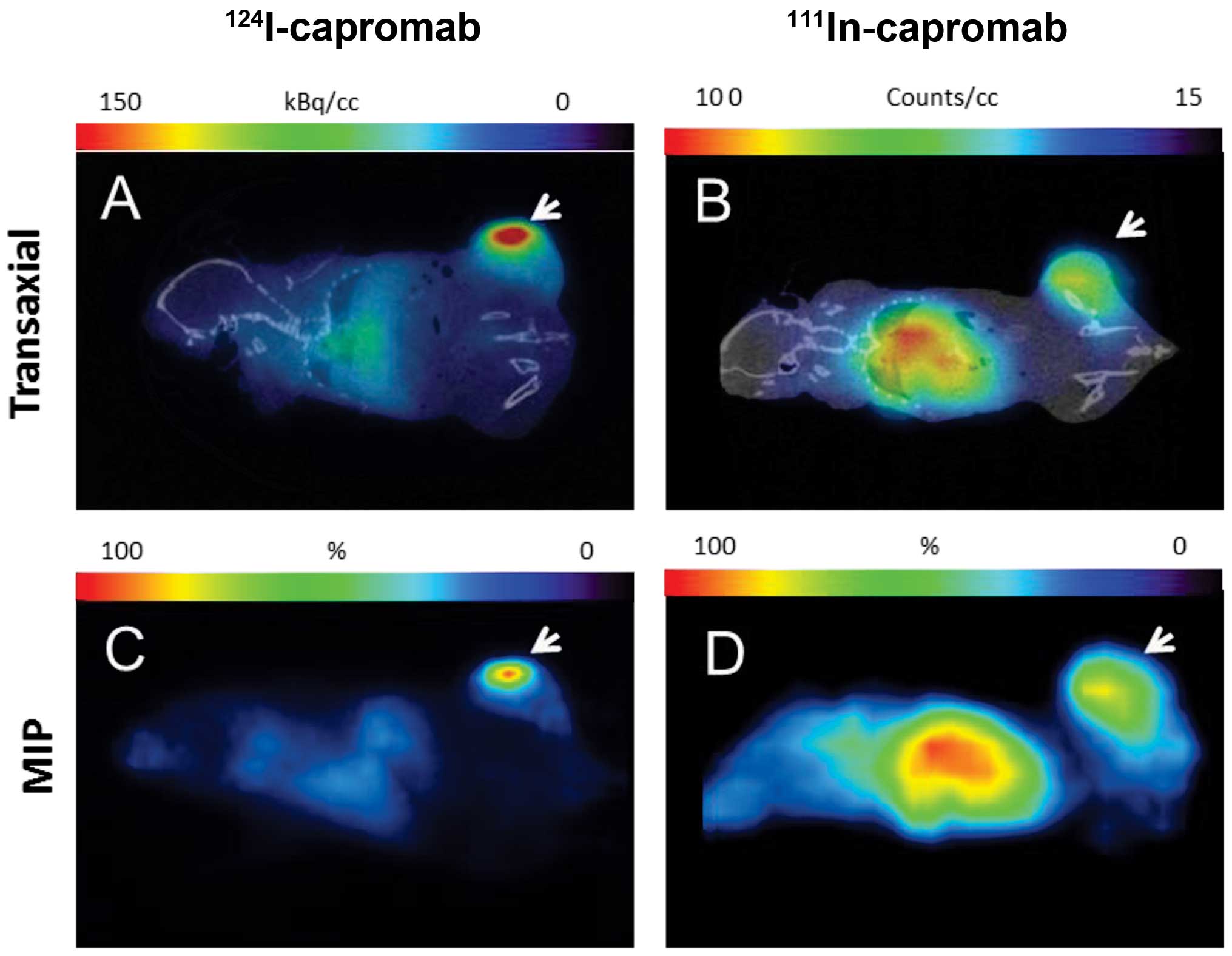
MicroPET and microSPECT images of mice bearing LNCaP
xenografts 5 days pi of 124I-capromab or
111Incapromab are presented in Fig. 7. In both cases tumors were clearly
visible. At the same time, the accumulation of radioactivity in the
heart and liver was appreciably lower for the iodinated
antibody.
Discussion
The sensitivity of radionuclide molecular imaging
depends on the contrast. The contrast is dependent on the ratio of
the radioactivity concentration in tumors to that in healthy
tissues. To obtain maximal contrast, the tumor uptake should be
increased and/or the uptake in normal tissues should be decreased
as much as possible. In this study, contrast maximization was
pursued by minimization of the accumulation in normal tissues.
Radioiodine provides the lowest level of radioactivity accumulation
in normal organs due to the non-residualizing properties of its
radiocatabolites. However, if the radioiodine is attached to an
antibody targeting an extracellular antigen present on cancer
cells, the same effect is observed in the tumor. In this case,
there is no gain in the tumor-to-non-tumor ratio, just a decrease
in the signal from tumor. However, capromab binds to the
intracellular domain of PSMA. For this reason, it can target only
necrotic cells with disrupted membranes. This property was used
recently by Ruggiero et al (23) in their study monitoring the
response to therapy of LNCaP xenografts in a murine model with
zirconium-89-labeled capromab. They clearly demonstrated that the
uptake of radioactivity into tumors irradiated from an external
source was as twice as high than that in control tumors due to
increased apoptotic and necrotic areas, resulting in cells with
disrupted cell membranes. Cellular catabolism is impossible in
apoptotic and necrotic cells, and we expected an equal tumor
accumulation of radioiodinated and radiometal-labeled capromab.
Our hypothesis was that the tumor uptake would less
likely be dependent on the residualizing properties of radio-label
due to the lack of internalization but that clearance from healthy
tissues would be improved with the non-residualizing label.
We evaluated two different radioiodination methods,
direct radioiodination on tyrosine residues and indirect
radioiodination in which the small prosthetic group was
radioiodinated and then coupled to lysine groups of capromab. Our
results show that direct iodination resulted in an antibody that
was as specific for binding to PSMA as that produced by via
indirect method. Furthermore, this labeling method did not
compromise immunoreactivity of imaging probe that means that
imaging contrast should not be decreased due to circulation of
non-immunocompetent antibody. Because direct radioiodination is
technically simpler, provides higher specific radioactivity, and
reduces the probability of user errors, directly iodinated capromab
was selected for further evaluation in vivo. Use of the
directly radioiodinated capromab is a straightforward and practical
approach for development of a tracer for prostate cancer staging
using immunoPET. The antibody is commercially available, and
clinical practice has demonstrated its safety. 124I is
also commercially available. Direct radioiodination using
chloramine-T is a very robust and reproducible method that could be
used to reliably produce large quantities of
124I-capromab. The long half-life of 124I
insures that worldwide distribution of 124I-capromab
from one or a few production facilities could be feasible. The use
of a single facility with well-trained personnel should insure a
high quality product.
Biodistribution experiments performed in NMRI mice
confirmed our hypothesis that clearance of iodinated capromab from
healthy organs of iodinated radiocatabolites is more efficient than
that of 111In-capromab (Fig. 4). Concentrations of radioactivity
in the liver, spleen and especially bone and colon tissue were
significantly higher for the indium-labeled antibody. The
concentration of radioactivity in blood was marginally but
significantly higher for radioiodine and this difference increased
over time, possibly due to the presence of radiocatabolites
(28). The uptake of radioiodine
in thyroid and stomach, tissues with expressing with
Na+/I− symporter, was blocked by adding
non-radioactive potassium iodide in the drinking water. In humans,
the use of Lugol’s solution should have similar effect. The
radioactivity uptake of radioiodine in excretory organs (kidneys
and liver) was significantly lower than uptake of radioindium
throughout the whole experiment. These data support our hypothesis
that non-residualizing properties of radioiodine should result in
lower uptake of radioactivity in normal organs.
It has been shown for several different antibodies
that uptake of 111In and 89Zr in normal
organs is very similar, with slightly higher uptake in liver and
bones for 89Zr (15,16,18).
Comparison of our data for indium-labeled capromab with recently
published data for zirconium-labeled capromab showed much higher
zirconium concentrations in lung, liver and spleen. Our data
regarding biodistribution of the antibody in normal organs and
tissues in LNCaP tumor-bearing mice were in good agreement with the
data from NMRI mice. The targeting specificity of radiolabeled
capromab was confirmed for both conjugates: radioactivity
concentrations in PSMA-negative PC3 xenografts were significantly
lower (on the level of muscle tissue) than concentrations in
PSMA-positive LNCaP xenografts. The tumor uptake and tumor to
non-tumor ratios reached maxima at 5 days pi for both labeled
conjugates. The tumor uptake of iodinated capromab was
significantly lower than that of the indium-labeled antibody,
though this difference was not as pronounced as for antibodies and
proteins that internalized after binding to extracellular domains
of antigens (29–31). For example, tumor-to-blood ratios
for 111In- and 125I-labeled trastuzumab, a
rapidly internalizing antibody, were at 3 days pi 18±7 and 1.0±0.3,
respectively (31). We speculate
that the lower radioiodine concentration in tumors might be due to
the trapping and processing of capromab by tumor-associated
macrophages because reversal of the PSMA intracellular and
extracellular domains is impossible.
Despite of the lower tumor concentration of
radioiodine, the tumor to non-tumor ratios of radioiodinated
capromab were close to that of the indium-labeled antibody
throughout the experiment. As we expected, on the day of the image
(5 days pi) tumor-to-liver, tumor-to-spleen, and tumor-to-kidney
were higher for the non-residualising radioiodine label than for
residualising radioindium one. MicroPET/CT and microSPECT/CT
imaging of PSMA in mice bearing LNCaP xenografts 5 days pi
demonstrated the superiority of the combination of the
non-residualizing radioiodine label on the non-internalizing
antibody capromab and the PET technique. Radioactivity
concentration in liver was much lower in the animal injected with
radioiodinated antibody than in the animal injected with
radioindium conjugate. LNCaP xenografts visualized with
124I-capromab obviously dominate the image, when
xenografts visualized with 111In-capromab had the same
intensity as the liver and other tissues in abdomen (where
locoregional metastases are expected).
In conclusion, direct radioiodination of capromab
provides a targeting agent that binds specifically to living
PSMA-expressing cells. Biodistribution of radioiodinated capromab
is superior to biodistribution of a radiometal-labeled counterpart
due to lower uptake into healthy tissues. Quick clearance of
radioiodine from excretory organs together with better resolution
of PET vs SPECT can provide higher contrast images of disseminated
prostate cancer and improve diagnostic accuracy.
Abbreviations:
|
PSMA
|
prostate-specific membrane antigen
|
Acknowledgements
This study was supported by grants
from the Swedish Cancer Society (Cancerfonden) and the Swedish
Research Council (Vetenskapsrådet).
References
|
1.
|
Takahashi N, Inoue T, Lee J, Yamaguchi T
and Shizukuishi K: The roles of PET and PET/CT in the diagnosis and
management of prostate cancer. Oncology. 2:226–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lawrentschuk N, Davis ID, Bolton D-M and
Scott AM: Positron emission tomography and molecular imaging of the
prostate: an update. BJU Int. 97:923–931. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Beresford MJ, Gillatt D, Benson RJ and
Ajithkumar T: A systematic review of the role of imaging before
salvage radio-therapy for post-prostatectomy biochemical
recurrence. Clin Oncol (R Coll Radiol). 22:46–55. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Schilling D, Schlemmer HP, Wagner PH, et
al: Histological verification of 11C-choline-positron
emission/computed tomography-positive lymph nodes in patients with
biochemical failure after treatment for localized prostate cancer.
BJU Int. 102:446–451. 2008.
|
|
5.
|
Vees H, Buchegger F, Albrecht S, et al:
18F-choline and/or 11C-acetate positron
emission tomography: detection of residual or progressive
subclinical disease at very low prostate-specific antigen values
(<1 ng/ml) after radical prostatectomy. BJU Int. 99:1415–1420.
2007. View Article : Google Scholar
|
|
6.
|
Lilja H, Ulmert D and Vickers AJ:
Prostate-specific antigen and prostate cancer: prediction,
detection and monitoring. Nat Rev Cancer. 8:268–278. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Raff AB, Gray A and Kast WM: Prostate stem
cell antigen: A prospective therapeutic and diagnostic target.
Cancer Lett. 277:126–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Silver DA, Pellicer I, Fair WR, Heston WD
and Cordon-Cardo C: Prostate-specific membrane antigen expression
in normal and malignant human tissues. Clin Cancer Res. 3:81–85.
1997.PubMed/NCBI
|
|
9.
|
Ulmert D, Evans MJ, Holland JP, et al:
Imaging androgen receptor signaling with a radiotracer targeting
free prostate-specific antigen. Cancer Discov. 2:320–327. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Manyak MJ: Indium-111 capromab pendetide
in the management of recurrent prostate cancer. Expert Rev
Anticancer Ther. 8:175–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Miyamoto DT, Lee RJ, Stott SL, et al:
Androgen receptor signaling in circulating tumor cells as a marker
of hormonally responsive prostate cancer. Cancer Discov.
2:995–1003. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Eder M, Eisenhut M, Babich J and Haberkorn
U: PSMA as a target for radiolabelled small molecules. Eur J Nucl
Med Mol Imaging. 40:819–823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Manya MJ, Hinkle GH, Olsen JO, et al:
Immunoscintigraphy with indium-111-capromab pendetide: evaluation
before definitive therapy in patients with prostate cancer.
Urology. 54:1058–1063. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Van Dongen GA and Vosjan MJ:
Immuno-positron emission tomography: shedding light on clinical
antibody therapy. Cancer Biother Radiopharm. 25:375–385.
2010.PubMed/NCBI
|
|
15.
|
Brouwers A, Verel I, van Eerd J, et al:
PET radioimmunoscintigraphy of renal cell cancer using
89Zr-labeled cG250 monoclonal antibody in nude rats.
Cancer Biother Radiopharm. 19:155–163. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Nagengast WB, de Vries EG, Hospers GA, et
al: In vivo VEGF imaging with radiolabeled bevacizumab in a human
ovarian tumor xenograft. J Nucl Med. 48:1313–1319. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Perk LR, Stigter-van Walsum M, Visser GW,
et al: Quantitative PET imaging of Met-expressing human cancer
xenografts with 89Zr-labelled monoclonal antibody DN30.
Eur J Nucl Med Mol Imaging. 35:1857–1867. 2008. View Article : Google Scholar
|
|
18.
|
Dijkers EC, Kosterink JG, Rademaker AP, et
al: Development and characterization of clinical-grade
89Zr-trastuzumab for HER2/neu immunoPET imaging. J Nucl
Med. 50:974–981. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Nagengast WB, de Korte MA, Oude Munnink
TH, et al: 89Zr-bevacizumab PET of early antiangiogenic
tumor response to treatment with HSP90 inhibitor NVP-AUY922. J Nucl
Med. 51:761–767. 2010. View Article : Google Scholar
|
|
20.
|
Orlova A, Wållberg H, Stone-Elander S and
Tolmachev V: On the selection of a tracer for PET imaging of
HER2-expressing tumors: direct comparison of a
124I-labeled affibody molecule and trastuzumab in a
murine xenograft model. J Nucl Med. 50:417–425. 2009. View Article : Google Scholar
|
|
21.
|
Dijkers EC, Oude Munnink TH, Kosterink JG,
et al: Biodistribution of 89Zr-trastuzumab and PET
imaging of HER2-positive lesions in patients with metastatic breast
cancer. Clin Pharmacol Ther. 87:586–592. 2010.
|
|
22.
|
Divgi CR, Pandit-Taskar N, Jungbluth AA,
et al: Preoperative characterisation of clear-cell renal carcinoma
using iodine-124-labelled antibody chimeric G250
(124I-cG250) and PET in patients with renal masses: a
phase I trial. Lancet Oncol. 8:304–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Ruggiero A, Holland JP, Hudolin T, et al:
Targeting the internal epitope of prostate-specific membrane
antigen with 89Zr-7E11 immuno-PET. J Nucl Med.
52:1608–1615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Holland JP, Divilov V, Bander NH,
Smith-Jones PM, Larson SM and Lewis JS: 89Zr-DFO-J591
for immunoPET of prostate-specific membrane antigen expression in
vivo. J Nucl Med. 51:1293–1300. 2010. View Article : Google Scholar
|
|
25.
|
Barren RJ III, Holmes EH, Boynton AL,
Misrock SL and Murphy GP: Monoclonal antibody 7E11.C5 staining of
viable LNCaP cells. Prostate. 30:65–68. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Tolmachev V and Orlova A: Influence of
labelling methods on biodistribution and imaging properties of
radiolabelled peptides for visualisation of molecular therapeutic
targets. Curr Med Chem. 17:2636–2355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Koziorowski J, Henssen C and Weinreich R:
A new convenient route to radioiodinated N-succinimidyl 3- and
4-iodobenzoate, two reagents for iodination of proteins. Appl
Radiat Isot. 49:955–959. 1998. View Article : Google Scholar
|
|
28.
|
Lindmo T, Boven E, Cuttila F, Fedorko J
and Bunn PA Jr: Determination of immunoractive fraction of
radiolabeled monoclonal antibody by linear extrapolation to binding
at infinite antigen excess. J Immunol Methods. 72:77–89. 1984.
View Article : Google Scholar
|
|
29.
|
Khaw BA, Cooney J, Edgington T and Strauss
HW: Differences in experimental tumor localization of dual-labeled
monoclonal antibody. J Nucl Med. 27:1293–1239. 1986.
|
|
30.
|
Meijs WE, Haisma HJ, Klok RP, van Gog FB,
Kievit E, Pinedo HM and Herscheid JD: Zirconium-labeled monoclonal
antibodies and their distribution in tumor-bearing nude mice. J
Nucl Med. 38:112–118. 1997.PubMed/NCBI
|
|
31.
|
Malmberg J, Sandström M, Wester K,
Tolmachev V and Orlova A: Comparative biodistribution of imaging
agents for in vivo molecular profiling of disseminated prostate
cancer in mice bearing prostate cancer xenografts: focus on
111In- and 125I-labeled anti-HER2 humanized
monoclonal trastuzumab and ABY-025 affibody. Nucl Med Biol.
38:1093–1102. 2011. View Article : Google Scholar : PubMed/NCBI
|