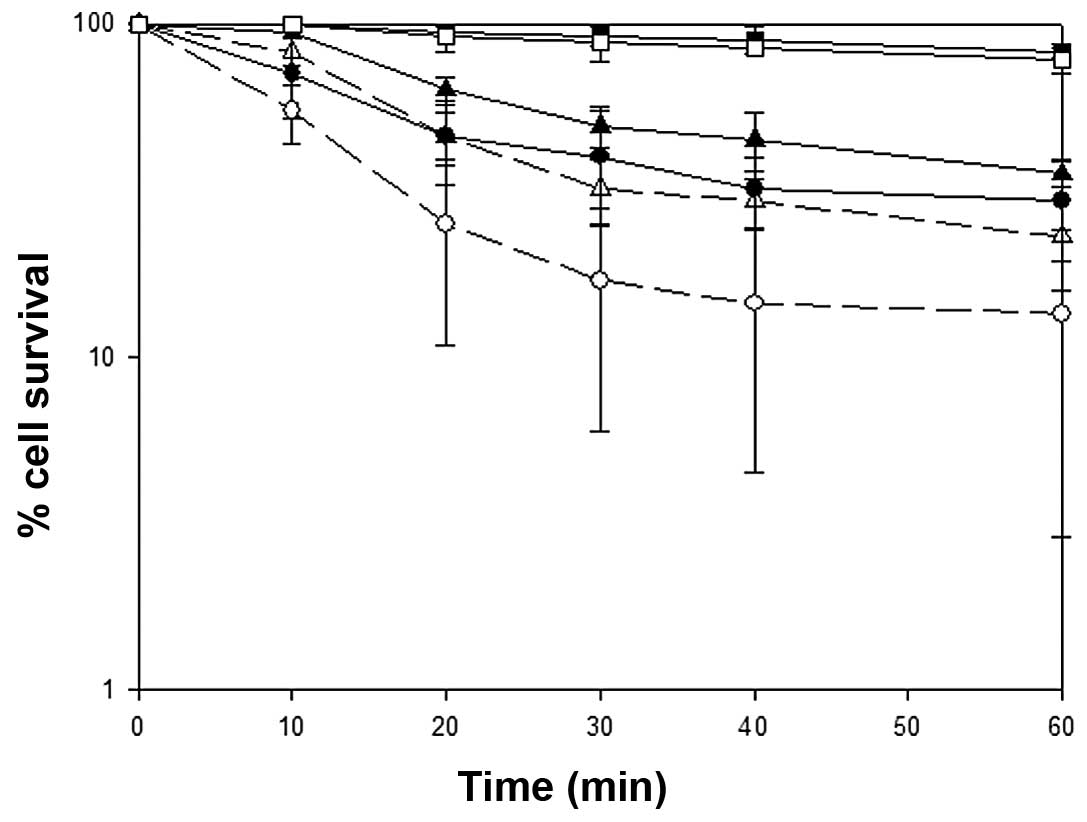
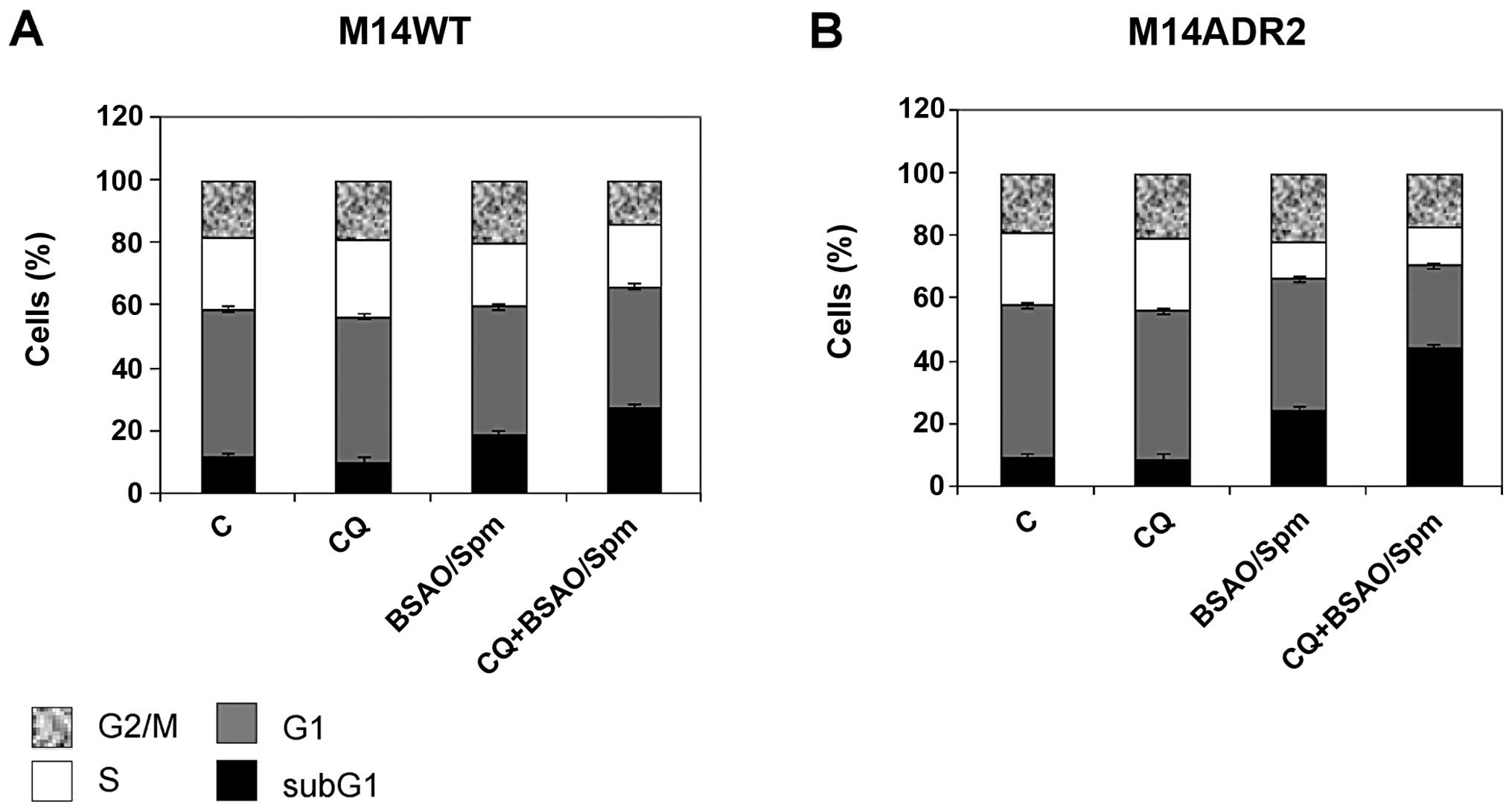
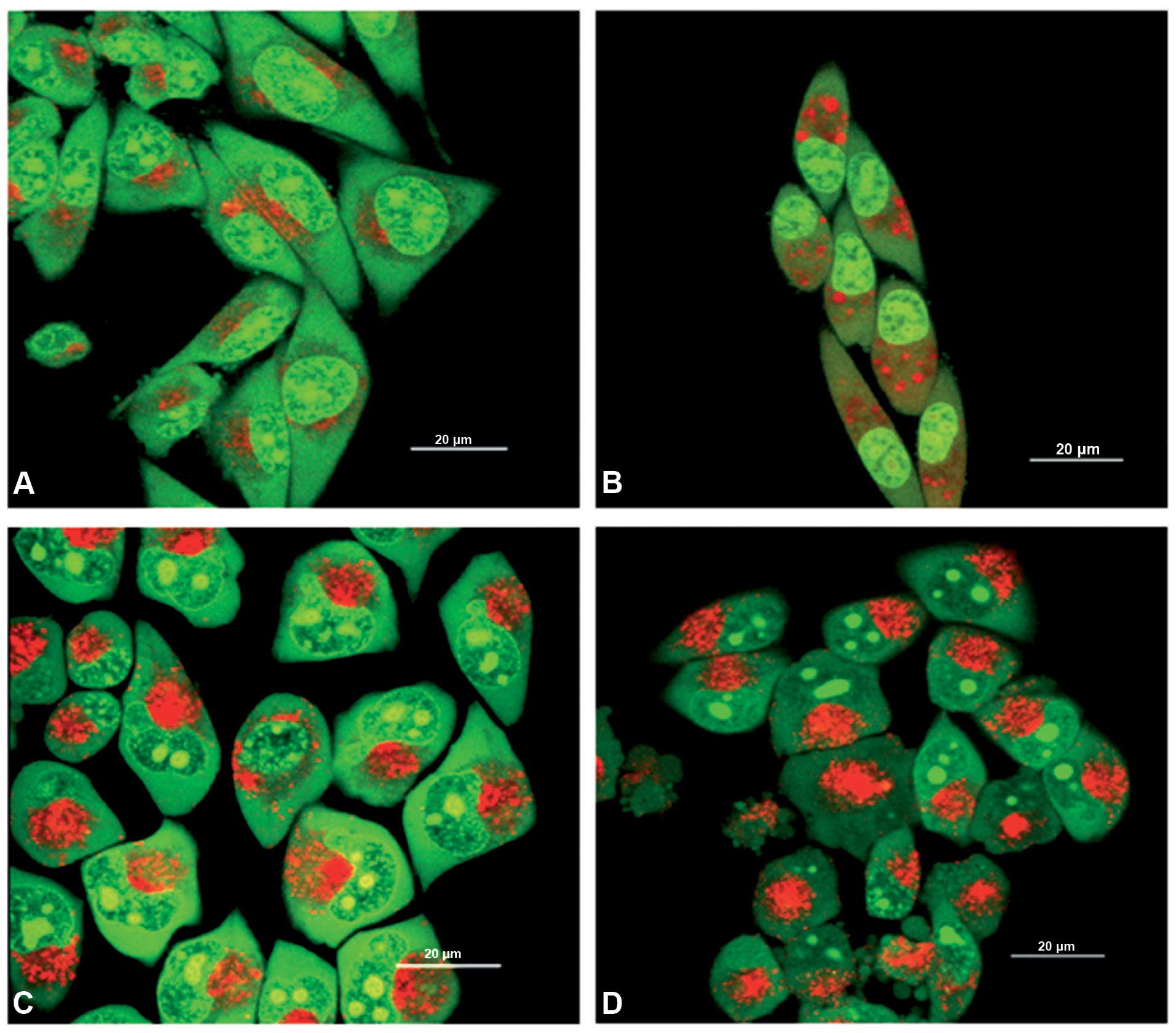
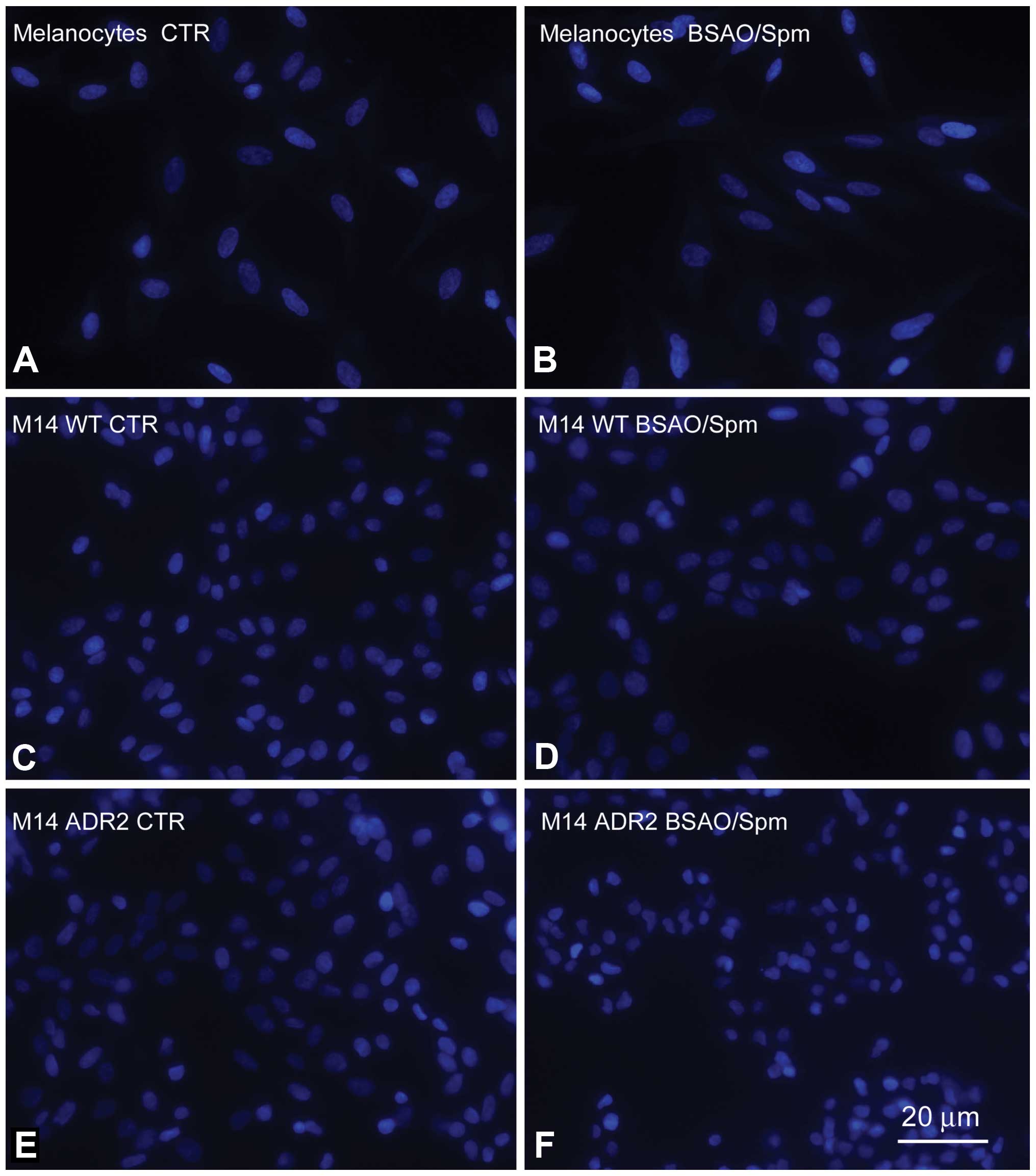
|
1
|
La Porta CA: Mechanism of drug sensitivity
and resistance in melanoma. Curr Cancer Drug Targets. 3:391–397.
2009.PubMed/NCBI
|
|
2
|
Setia N, Abbas O, Sousa Y, Garb JL and
Mahalingam M: Profiling of ABC transporters ABCB5, ABCF2 and
nestin-positive stem cells in nevi, in situ and invasive melanoma.
Mod Pathol. 25:1169–1175. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Davies MA: The role of the PI3K-AKT
pathway in melanoma. Cancer J. 18:142–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nikolaous VA, Stratigos AJ, Flaherty KT,
et al: Melanoma: new insights and new therapies. J Invest Dermatol.
132:854–863. 2012. View Article : Google Scholar
|
|
5
|
Goodwin AC, Jadallah S, Toubaji A,
Lecksell K, Hicks JL, Kowaski J, Bova GS, De Marzo AM, Netto GJ and
Casero RA Jr: Increased spermine oxidase expression in human
prostate cancer and prostatic intraepithelial neoplasia tissues.
Prostate. 68:766–772. 2008. View Article : Google Scholar
|
|
6
|
Simoneau AR, Gerner EW, Nagle R, Ziogas A,
Fujikawa-Brooks S, Yerushalmi H, Ahlering TE, Lieberman R, Mclaren
CE, Anton-Culver H and Meyskens FL Jr: The effect of
difluoromethylornithine on decreasing prostate size and polyamines
in men: results of a year-long phase IIb randomized
placebo-controlled chemoprevention trial. Cancer Epidemiol
Biomarkers Prev. 17:292–299. 2008.
|
|
7
|
Gerner EW and Meyskens FL: Polyamines and
cancer: old molecules, new understanding. Nat Rev Cancer.
4:782–792. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Agostinelli E, Belli F, Molinari A,
Condello M, Palmigiani P, Dalla Vedova L, Marra M, Seiler N and
Arancia G: Toxicity of enzymatic oxidation products of spermine to
human melanoma cells (M14): sensitization by heat and MDL 72527.
Biochim Biophis Acta. 1763:1040–1050. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Agostinelli E, Condello M, Molinari A,
Tempera G, Viceconte N and Arancia G: Cytotoxicity of spermine
oxidation products to multidrug resistant melanoma cells (M14
ADR2): sensitisation by MDL 72527, a lysosomotropic compound. Int J
Oncol. 35:485–498. 2009. View Article : Google Scholar
|
|
10
|
Marra M, Agostinelli E, Tempera G,
Lombardi A, Meo G, Budillon A, Abbruzzese A, Giuberti G and
Caraglia M: Anticancer drugs and hyperthermia enhance cytotoxicity
induced by polyamine enzymatic oxidation products. Amino Acids.
33:273–281. 2007. View Article : Google Scholar
|
|
11
|
Marra M, Lombardi A, Agostinelli E,
Giuberti G, Zappavigna S, Tempera G, Vitale G, Bifulco M,
Abbruzzese A and Caraglia M: Bovine serum amine oxidase and spm
potentiate docetaxel and interferon-alpha effects in inducing
apoptosis on human cancer cells through the generation of oxidative
stress. BBA-Mol Cell Res. 1783:2269–2278. 2008.
|
|
12
|
Agostinelli E, Dalla Vedova L, Belli F,
Condello M, Arancia G and Seiler N: Sensitization of human colon
adenocarcinoma cells (LoVo) to reactive oxygen species by
lysosomotropic compounds. Int J Oncol. 29:947–955. 2006.PubMed/NCBI
|
|
13
|
Dai H, Kramer DL, Yang C, Murti KG, Porter
CW and Cleveland JL: The polyamine oxidase inactivator MDL-72527
selectively induces apoptosis in transformed hematopoietic cells
through lysosomotropic effects. Cancer Res. 59:4944–4954. 1999.
|
|
14
|
Iyamu E, Perdew H and Woods G: Growth
inhibitory and differentiation effect of chloroquine and its
analogue on human leukemic cells potentiate fetal hemoglobin
production by targeting the polyamine pathway. Biochem Pharmacol.
77:1021–1029. 2009. View Article : Google Scholar
|
|
15
|
Turini P, Sabatini S, Befani O, Chimenti
F, Casanova C, Riccio P and Mondovi B: Purification of serum amine
oxidase. Anal Biochem. 125:294–298. 1982. View Article : Google Scholar
|
|
16
|
Janes SM, Mu D, Wemmer D, Smith AJ, Kaur
S, Maltby D, Burlingam AL and Klinman JP: A new redox cofactor in
eukaryotic enzymes: 6-Hydroxydopa at the active site of bovine
serum amine oxidase. Science. 248:981–987. 1990. View Article : Google Scholar
|
|
17
|
Molinari A, Toccaceli L, Calcabrini A,
Diociaiuti M, Cianfriglia M and Arancia G: Induction of
P-glycoprotein expression on the plasma membrane of human melanoma
cells. Anticancer Res. 20:2691–2696. 2000.PubMed/NCBI
|
|
18
|
Molinari A, Calcabrini A, Crateri P and
Arancia G: Interaction of anthracyclin with cytoskeletal components
of cultured carcinoma cells (CG5). Exp Mol Pathol. 53:11–33. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Engeland M, Nieland LJ, Ramaekers FC,
Schutte B and Reutelingsperger CP: Annexin V-affinity assay: a
review on an apoptosis detection system based on phosphatidylserine
exposure. Cytometry. 31:1–9. 1998.PubMed/NCBI
|
|
20
|
Nicoletti I, Migliorati G, Pagliacci MC,
Grignani F and Riccardi C: A rapid and simple method for measuring
thymocyte apoptosis by propidium iodide staining and flow
cytometry. J Immunol Methods. 139:271–279. 1991. View Article : Google Scholar
|
|
21
|
Sharmin S, Sakata K, Kashiwagi K, Ueda S,
Iwasaki S, Shirahata A and Igarashi K: Polyamine cytotoxicity in
the presence of bovine serum amine oxidase. Biochem Biophys Res
Commun. 282:228–235. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Calcabrini A, Arancia G, Marra M, Crateri
P, Befani O, Martone A and Agostinelli E: Enzymatic oxidation
products of spermine induce greater cytotoxic effects on human
multidrug-resistant colon carcinoma cells (LoVo) than on their
wild-type counterparts. Int J Cancer. 99:43–52. 2002. View Article : Google Scholar
|
|
23
|
Agostinelli E and Seiler N:
Non-irradiation-derived reactive oxygen species (ROS) and cancer.
Therapeutic implications. Amino Acids. 31:341–355. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Agostinelli E and Seiler N: Lysosomotropic
compounds and spermine enzymatic oxidation products in cancer
therapy (review). Int J Oncol. 31:473–484. 2007.PubMed/NCBI
|
|
25
|
Agostinelli E, Przybytkowskj E, Mondovì B
and Averill-Bates DA: Heat enhancement of cytotoxicity induced by
oxidation products of spermine in Chinese hamster ovary cells.
Biochem Pharmacol. 72:36–42. 1994.PubMed/NCBI
|
|
26
|
Averill-Bates DA, Ke Q, Tanel A, Roy J,
Fortier G and Agostinelli E: Mechanism of cell death induced by
spermine and amine oxidase in mouse melanoma cells. Int J Oncol.
32:79–88. 2008.PubMed/NCBI
|
|
27
|
Lord-Fontaine S, Agostinelli E,
Przybytkowskj E and Averill-Bates DA: Amine oxidase, spermine, and
hyperthermia induce cytotoxicity in P-glycoprotein overexpressing
multidrug resistant Chinese hamster ovary cells. Biochem Cell Biol.
79:165–175. 2001. View Article : Google Scholar
|
|
28
|
Averill-Bates DA, Cherif A, Agostinelli E,
Tanel A and Fortier G: Anti-tumoral effect of native and
immobilized bovine serum amine oxidase in a mouse melanoma model.
Biochem Pharmacol. 69:1693–1704. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Demers N, Agostinelli E, Averill-Bates DA
and Fortier G: Immobilization of native and poly(ethylene
glycol)-treated (‘PEGylated’) bovine serum amine oxidase into a
biocompatible hydrogel. Biotechnol Appl Biochem. 33:201–207.
2001.PubMed/NCBI
|
|
30
|
Gerner EW and Meyskens Fl Jr: Combination
chemoprevention for colon cancer targeting polyamine synthesis and
inflammation. Clin Cancer Res. 15:758–761. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park BC, Park SH, Paek SH, et al:
Chloroquine-induced nitric oxide increase and cell death is
dependent on cellular GSH depletion in A172 human glioblastoma
cells. Toxicol Lett. 178:52–60. 2008. View Article : Google Scholar
|
|
32
|
Jiang P, Zhao Y, Shi W, et al: Cell growth
inhibition, G2/M cell cycle arrest, and apoptosis induced by
Chloroquine in human breast cancer cell line Bcap-37. Cell Physiol
Biochem. 22:431–440. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kimura T, Yoshitsugu T, Atsushi T, et al:
Chloroquine in cancer therapy: a double-edged sword of autophagy.
Cancer Res. 73:3–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Michaud M, Martins I, Sukkurwala AQ,
Adjemian S, Ma Y, Pellegatti P, et al: Autophagy-dependent
anticancer immuneresponses induced by chemotherapeutic agents in
mice. Science. 334:1573–1577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Amaravadi RK and Thompson CB: The roles of
therapy-induced autophagy and necrosis in cancer treatment. Clin
Cancer Res. 13:7271–7279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tasdemir E, Galluzzi L, Maiuri MC, Criollo
A, Vitale I, Hangen E, Modjtahedi N and Kroemer G: Methods for
assessing autophagy and autophagic cell death. Methods Mol Biol.
445:29–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vezmar M and Georges E: Reversal of
MRP-mediated doxorubicin resistance with quinoline-based drugs.
Biochem Pharmacol. 59:1245–1252. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Solomon VR and Lee H: Chloroquine and its
analogs: a new promise of an old drug for effective and safe cancer
therapies. Eur J Pharmacol. 625:220–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Homewood CA, Warhurst DC, Peters W and
Baggaley VC: Lysosomes, pH and the anti-malarial action of
chloroquine. Nature. 235:50–52. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Slater AF: Chloroquine: mechanism of drug
action and resistance in Plasmodium falciparum. Pharmacol Ther.
57:203–235. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Agostinelli E, Tempera G, Dalla Vedova L,
Condello M and Arancia G: MDL 72527 and spermine oxidation products
induce a lysosomotropic effect and mitochondrial alterations on
tumor cells. Biochem Soc Trans. 35:343–348. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guicciardi ME, Leist M and Gores GJ:
Lysosomes in cell death. Oncogene. 23:2881–2890. 2004. View Article : Google Scholar
|
|
43
|
Zhao M, Antunes F, Eaton JW and Brunk UT:
Lysosomal enzymes promote mitochondrial oxidant production,
cytochrome c release and apoptosis. Eur J Biochem. 270:13778–13786.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu Z, Li W and Brunk UT: 3-Aminopropanal
is a lysosomotropic aldehyde that causes oxidative stress and
apoptosis by rupturing lysosomes. APMIS. 111:643–652. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zdolsek J, Zhang H, Roberg K and Brunk U:
HO-mediated damage to lysosomal membranes of J-774 cells. Free
Radic Res Commun. 18:71–85. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gottesman MM and Pastan I: Biochemistry of
multidrug resistance mediated by the multidrug transporter. Ann Rev
Biochem. 62:385–342. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Arancia G, Calcabrini A, Marra M, Crateri
P, Artico M, Martone A, Martelli F and Agostinelli E: Mitochondrial
alterations induced by serum amine oxidase and spermine on human
multidrug resistant tumor cells. Amino Acids. 26:273–282. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Fehrenbacher N, Gyrd-Hansen M, Poulsen B,
Felbor U, Kallunki T, Boes M, Weber E, Leist M and Jäättelä M:
Sensitization to the lysosomal cell death pathway upon
immortalization and transformation. Cancer Res. 64:5301–5310. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fehrenbacher N and Jäättelä M: Lysosomes
as targets for cancer therapy. Cancer Res. 65:2993–2995.
2005.PubMed/NCBI
|
|
50
|
Savarino A, Lucia MB, Giordano F and Cauda
R: Risks and benefits of chloroquine use in anticancer strategies.
Lancet Oncol. 7:792–793. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Briceño E, Reyes S and Sotelo J: Therapy
of glioblastoma multiforme improved by the antimutagenic
chloroquine. Neurosurg Focus. 14:e32003.PubMed/NCBI
|