Introduction
Uterine cancer is one of the most common
malignancies and the second leading cause of cancer-related
mortality worldwide in women. Worldwide each year 370,000 women
develop uterine cancer and 200,000 die due to the disease (1,2). The
incidence and mortality of uterine cancer in China accounts for
~1/3 of the whole world. The age distribution of this disease shows
a peak between 35 and 39 years and a second peak between 60 and 64
years, the average age at first diagnosis has decreased to 52.2
years in China (3), while the
incidence of invasive uterine cancer in China has increased in
young women within the past few years (4). Despite recent advances in combining
surgery, chemotherapy and radiotherapy no effective targeting
therapy is available for uterine cancer, unfortunately, while some
patients are eligible for curative treatment, recurrence is a
frequent issue for many patients after tumor ablation (5). Accordingly, an urgent need exists to
identify new therapeutic agents for the treatment of uterine cancer
in clinical practice.
Chinese herbs have been used widely and successfully
for centuries in treating different kinds of diseases. Natural
products occupy a very important position in the area of cancer
chemotherapy due to their excellent pharmaco logical activities and
low toxicity (6–8). C-21 steroidal glycosides is one
species of important biological active compounds widely found in
the plants of the Asclepiadaceae family, which has been
shown to effectively remove hydroxyl radicals and oxygen-free
radicals, regulating immunity, and protecting liver and gastric
cells (9–11). Caudatin, a C-21 steroidal
glycoside, is mainly isolated from the traditional Chinese medicine
‘baishouwu’, which was the root tuber of Cynanchum
auriculatum Royle ex Wight. It has been reported that the
antitumor effect of caudatin has been shown to exhibit
anti-proliferation effects against cancer cells of different
origins, including glioblastoma, lung, gastric, and liver cancer
(12–16). In our previous studies, caudatin
induced apoptosis of AGS cells or the HGC-27 cell line (14). Wang et al found that
caudatin had an inhibitory activity on the secretion of HBsAg and
HBV DNA replication (17).
Furthermore, caudatin as a prospective anti-HCC drug with the
mechanism of inhibiting cell proliferation and inducing cell
apoptosis has been reported (18).
Although evidence of antitumor effects of caudatin is expanding,
uncertainty of the mechanisms of caudatin in uterine cancer still
remains. Yet, there is no report concerning the effects of caudatin
on uterine cancer, and the underlying mechanisms are not well
documented. Up to now, the pharmacokinetics of caudatin in uterine
carcinoma model animals remains unclear.
TNFAIP1 is an immediate-early response gene of
endothelium induced by TNFα and IL-6. It may play roles in DNA
synthesis, DNA repair, cell apoptosis and human diseases (19). TNFAIP1 has been identified to be
highly expressed in Alzheimer’s disease brains and hepatitis B
virus, and is associated with diabetic nephropathy (20). Interestingly, TNFAIP1 is also
abnormally expressed in gastric cancer, breast cancer,
osteosarcoma, and cervical cancer, but little is known about its
potential function in malignant disease (21–26).
TNFAIP1 was highly expressed in normal cell lines while it was
lowly expressed in cancer cell lines (27). Indeed, TNFAIP1 elicited
proapoptotic activity, and co-expression of TNFAIP1 and RhoB
markedly increased apoptosis in HeLa cells (28). TNFAIP1 and KCTD10 suppressed the
transcriptional activities of NF-κB (29). We found that downregulation of
TNFAIP1 is correlated with enhanced tumorigenicity, enhanced
metastatic potential and poor prognosis in gastric cancer (23). Better understanding of the
mechanism of action of TNFAIP1 is crucial in the development of new
uterine tumor treatments.
The aims of this study were to determine effects of
caudatin on proliferation, migration and apoptosis in HeLa and
HEC-1A cells and to investigate its molecular mechanisms of
action.
Materials and methods
Cell culture and transfection
The human cervical carcinoma cell line (HeLa) and
endometrial carcinoma cell line (HEC-1A) were obtained from the
Cell Bank of the Chinese Academy of Sciences (China). Cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone) and
F-12 (hyclone) supplemented with 10% FBS, 100 U/ml penicillin, 100
U/ml streptomycin. All cells were grown at 37°C in a humidified
atmosphere with 5% CO2 and passaged using 0.2% (w/v)
trypsin and 0.1% (w/v) EDTA. The pCMV-Myc-TNFAIP1 and pCMV-Myc
vectors that were used in this study were purchased from Clontech.
All transfections were carried out using Lipofectamine 2000
(Invitrogen, USA), according to the manufacturer’s
instructions.
Clinical specimens
Fresh human cervical carcinoma and adjacent
non-tumor tissue samples were obtained from the Department of
Gynecologic Tumor, Hunan Cancer Hospital, Changsha, China. After
surgical resection, the fresh tissue samples were immediately
immersed in RNAlater (Ambion Inc., USA) and stored at room
temperature 3–4 h; the samples were then frozen at −80°C until RNA
extraction. The samples were classified according to World Health
Organization criteria published in 2000. All patients signed
consent forms and the study protocol was approved by the Committee
on Human Rights in Research of the Ethics Committee of the College
of Life Science, Hunan Normal University, Changsha, China.
Reagents and antibodies
Caudatin was purchased from the Shenzhen Medherb
Biotechnology Co., Ltd. (Shenzhen, China) and dissolved with 100%
dimethyl sulfoxide (DMSO) (concentration of the stock solution, 10
mmol/l). Antibodies to Bcl-2, cytochrome c (Cyt-c),
Caspase-9, Caspase-3, PARP and NFκB were obtained from Santa Cruz
Biotechnology (Santa Cruz, CA, USA). β-actin antibody was purchased
from GenScript, Inc. (Piscataway, NJ, USA), Antibody against Bax
was purchased from Proteintech (USA) and antibody against TNFAIP1
was custom made from signalway antibody (Signalway Antibody LLC,
USA).
RNA extraction and real-time PCR
analysis
Total RNA was extracted using TRIzol reagent
(Invitrogen) according to the manufacturer’s instructions. Reverse
transcription was reverse-transcribed by using the RevertAid First
Strand cDNA Synthesis kit (Thermo Scientific, USA) with 1 μg total
RNA, according to the manufacturer’s instructions. Quantitative
real-time PCR (qRT-PCR) analysis for TNFAIP1 and NFκB was performed
in triplicate with the SYBR Green PCR Master Mix (Perkin-Elmer,
Applied Biosystems) according to the manufacturer’s instructions.
RNA was used to normalize expression. The sequences of the sense
and antisense primers were as follows: 5′-GCA CTT TGG CAC CAT TTT
GA-3′ (F) and 5′-CGG TTC TGA GGG AGG GTG AT-3′ (R) for TNFAIP1;
5′-AGGAGAGGATGAAGGAGTTGTG-3′ (F) and 5′-CCAGAGTAGCCCAGTTTTTGTC-3
(R) for NFκB. 5′-CCT GTA CGC CAA CAC AGT GC-3′ (F) and 5′-ATA CTC
CTG CTT GCT GAT CC-3′ (R). β-actin data analysis was performed
using the 2−ΔΔCt method.
MTT assays
The HeLa and HEC-1A cell lines were seeded in
24-well culture plates, after attachment for 24 h, cells were
treated with 25–100 μmol/l caudatin and DMSO as a blank control or
transfected with different concentration pCMV-Myc-TNFAIP1 and
pCMV-Myc as a blank control. Twenty-four hours later, the cells
were incubated with 80 μl MTT at 37°C for another 4 h. Then the
medium was removed and the precipitated formazan was dissolved in
300 μl DMSO. After shaking for 10 min, the absorbance at 420 nm was
detected using a microplate spectrophotometer. Three wells were
assigned to each group.
Colony formation assay
We detected the effect of caudatin on proliferation
in a variety of cultured cell lines (HeLa and HEC-1A). Briefly,
6-well plates were seeded with 500 viable cells, and they were
allowed to grow for 24 h. The cells were treated with 25–100 μmol/l
caudatin and DMSO as a blank control. The cells overexpress TNFAIP1
in HEC-1A cells were transfected with different concentration
pCMV-Myc-TNFAIP1 and pCMV-Myc-negative as a blank control. The
caudatin containing medium was then removed, and the cells were
washed in PBS and incubated for an additional 15 days in complete
medium. Each treatment was performed in triplicate. The resulting
colonies were washed twice with PBS and fixed in methanol for 15
min at room temperature, followed by staining with 20% Giemsa
solution for 30 min. The colonies were counted and compared with
untreated cells. The colony formation rate was calculated with the
following formula. Plate colony formation inhibitory ratio =
(number of colonies treated with caudatin/number of cells
inoculated) × 100%.
Hoechst 33258 staining
Apoptotic morphological changes in the nuclear
chromatin of cells were detected by Hoechst 33258 (Sigma) staining.
HeLa and HEC-1A cells were seeded on sterile cover glasses and
washed with PBS and fixed with 4% paraformaldehyde for 10 min, and
then incubated with 50 μl Hoechst 33258 staining solution for 10
min. After three washes with PBS, the cells were viewed under a
fluorescence microscope (Zeiss Axioskop; Zeiss, Oberkochen,
Germany).
FACS assays
Cells were harvested at 600 g for 3 min, washed
twice in PBS at room temperature and resuspended in appropriate
PBS. Then, cells were resuspended in 300 μl 1X FACS banding buffer
containing Annexin V and propidium iodide and analyzed using a FACS
flow cytometer (BD Biosciences). A total of 10,000 cells were
counted for each sample.
Spheroid assay
For formation of spheroids, cell culture was
supplemented with 20 ng/ml b-FGF (Invitrogen) 10 ml per 500 ml of
50X B27 supplement (Invitrogen) EGF 20 ng/ml (Invitrogen) and
antibiotic and antimycotic solution. HeLa cells were seeded at low
densities (5,000 cells/ml) in cell culture bottle. The cells were
treated with increasing concentrations of caudatin (0–100 μmol/l).
After one week the spheroids were photographed.
Wound-healing assay
HeLa cells and HEC-1A cells were seeded on 6-well
plates and grown to 100% confluence. Wounds were created by
scraping the monolayer of cells with a sterile pipette tip, washed
with PBS to remove the floating cells and incubated with fresh
medium in the presence and absence of 25–100 μmol/l caudatin. The
images of scratched area were captured using a Leica DMR microscope
equipped with DC300F digital camera immediately after wounding and
at 24, 48 and 72 h after application of caudatin. The images were
compared to estimate the effects of caudatin on wound healing.
Western blot analysis
Treated cells were harvested at the indicated points
and lysed in RIPA buffer containing a protease inhibitor and
phosphatase inhibitor cocktail. After 3-freeze-thaw cycles in
liquid nitrogen, the resulting cell lysates were cleared by
centrifugation at 12,000 × g for 10 min at 4°C, and the proteins
were separated by 10% SDS-PAGE. After electrophoresis, the proteins
were transferred to polyvinylidene difluorideplus membranes. The
membranes were blocked with 50 g/l nonfat milk in TBS washing
buffer for 30 min, and then incubated with the indicated primary
antibodies at 1:1,000 (Bcl-2, Bax, Cyt-c, Caspase-9,
Caspase-3, PARP, TNFAIP1, NF-κB, and β-actin) for 3 h or overnight
at 4°C. Then, the membranes were incubated with a 1:2,000 dilution
of the proper ALP-conjugated secondary antibody for 1 h at room
temperature. After three washes with TTBS (TBS, 0.05% Tween-20),
the protein signals were visualized using an ECL Plus kit,
according to the manufacturer’s instructions. The experiments were
repeated at least three times, with protein extracts harvested
independently. Densitometry was performed using ImageJ
software.
In vivo tumor growth assay
To investigate the tumorigenicity of HeLa cell line,
five-week old female BALB/c nude mice were purchased from the SJA
Lab Animal (Changsha, China), and allowed to acclimatise for 1
week. The HeLa cells (1×106) were resuspended in 0.1 ml
Dulbecco’s modified Eagle’s medium and inoculated subcutaneously.
Seven days after inoculation, when tumors became palpable, mice
were subdivided into two groups of 5 animals each being the tumor
volumes equally distributed between the two groups. One group of
mice was treated daily with 100 mg/kg caudatin prepared in a
solution at mixture of 60% DMSO and 40% alcohol, administered by
intraperitoneal injection. The control group received only an
injection of same amount of mixture of 60% DMSO and 40% alcohol.
The mice were sacrificed and tumors were removed and weighed after
the two weeks of caudatin treatment. Primary tumor volumes were
calculated with the formula: v = length × (width)2/2.
All experiments with animals were approved by a local animal
committee for ethics.
Statistical analysis
All data are presented as means ± standard deviation
from at least three separate experiments. The differences among
groups were analyzed using the double-sided Student’s t-test, and
statistical significance was determined at a P-value of
<0.05.
Results
Caudatin reduces viability and
clonogenicity of uterine cancer cells
We first quantitatively analyzed the effect of
caudatin on cell proliferation in HeLa cells and HEC-1A cells by
MTT assay. Treatment with caudatin at the dose of 25–100 μmol/l
inhibited cell viability in dose- and time-dependent manner. As
shown in Fig. 1A, HeLa cell line
viability decreased by 46.06% with 100 μmol/l caudatin after 24 h,
and decreased by 50.13% after 36 h. The IC50 values for
caudatin in HeLa cells at 12, 24, and 36 h were 86.73, 65.85 and
61.60 μmol/l, respectively. HEC-1A cell line viability decrease by
32.1% with 100 μmol/l caudatin after 24 h, and decreased by 50%
after 36 h. The IC50 values for caudatin in HEC-1A cells
at 12, 24 and 36 h were 121.72, 97.49 and 70.70 μmol/l,
respectively.
To determine the long-term effect of caudatin
treatment, we also tested the effects of caudatin on tumor cell
clonogenicity in HeLa and HEC-1A cells (Fig. 1B). Cells were treated with
different concentrations of caudatin for 24 h, and then the cells
were allowed to grow in normal media. Treatment with caudatin
markedly inhibited colony formation, resulting in a decrease by
70.24% with 100 μmol/l caudatin after 24 h at HeLa and decrease by
54.24% with 100 μmol/l caudatin after 24 h at HEC-1A cells.
Notably, the HeLa cells presented a higher sensitivity than the
HEC-1A cells. These results indicate that caudatin inhibits the
growth of HeLa and HEC-1A cells in a dose- and time-dependent
manner.
Caudatin treatment induces apoptosis in
HeLa and HEC-1A cells
The induction of apoptosis is considered as one of
the possible mechanisms of inhibition of cancer development.
Caudatin is known to induce apoptosis in variety of cancer cells,
which is considered to be an important mechanism for their
antitumor activity and prevention of carcinogenesis (13,14).
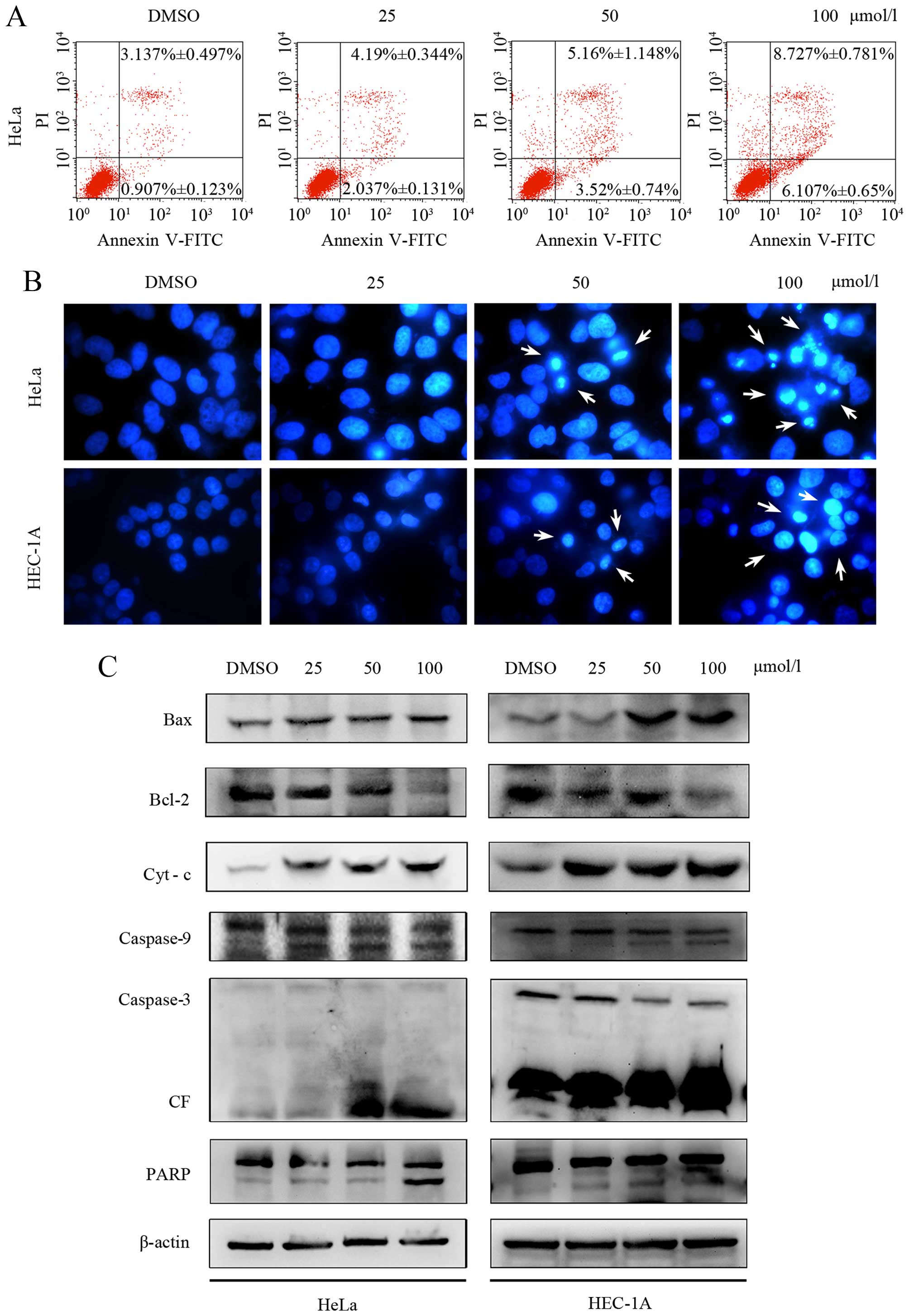
Annexin V-FITC/PI double-labeled flow cytometry was used to assess
the ratio of apoptotic HeLa cells following caudatin treatment. The
total apoptosis ratio was the sum of the early apoptotic and late
apoptotic ratios. The apoptosis rates for HeLa cells treated with
different concentrations (25,50 and 100 μmol/l) of caudatin for 24
h were 6.23±0.48, 8.68±1.89% and 14.83±1.43%, respectively, which
were significantly higher than that of the control group
(4.04±0.62%) (as shown in Fig.
2A). Moreover, Hoechst 33258 staining revealed typical
morphological changes, such as the formation of apoptotic bodies,
after 24-h treatment with different concentrations (25–100 μmol/l)
of caudatin, whereas the control cells did not show
apoptosis-related morphological changes (Fig. 2B). Normal nuclei were identified as
having non-condensed chromatin dispersed over the entire nucleus,
and apoptotic nuclei were identified as having condensed chromatin
that was contiguous with the nuclear membrane and/or fragmented
nuclei. Overall, these results are consistent with cell apoptosis
by Hoechst 33258 staining and flow cytometry analysis, suggesting
that the inhibition of cell growth by caudatin may be associated
with induction of apoptosis.
Caudatin-induced apoptosis is mediated
via caspase activation in uterine cancer cell lines
To confirm the effect of caudatin on apoptosis, we
next detected the expression of some pro-apoptotic and
antiapoptotic proteins, cytochrome c (Cyt c), BAX,
Bcl-2 caspase-3/9 and PARP in HeLa and HEC-1A cells by western blot
analysis. Caudatin markedly increased the expression levels of Bax
protein, but decreased the Bcl-2 protein levels, as compared with
the control group. The release of Cyt-c from the
mitochondrial inter-membrane space into the cytosol is the
precondition of caspase-dependent apoptosis pathway. The expression
level of full-length caspase-9 and −3 was decreased, suggesting
cleavage and activation of the caspase pathway. Furthermore, PARP,
which is the prime marker for caspase-dependent apoptosis, showed
cleavage in a dose-dependent manner (Fig. 2C). These data demonstrate that
caudatin is a potent inducer of apoptosis in cervical carcinoma and
endometrial carcinoma cells, and the apoptosis involves the
caspase-related pathway.
Caudatin inhibited cell migration
We also tested the effect of caudatin on cell
migration by employing a wound-healing assay. As shown in Fig. 3A, the part of the wounding space
between cell layers after making a scratch was occupied completely
by the migrating cells after 72 h in the control group and the
group treated with 25 μmol/l caudatin in the HeLa cell line.
However, the empty space of the cells was not occupied by the
migrating cells treated with 50 or 100 μmol/l caudatin. Similar
result of HEC-1A cell line is shown in Fig. 3B. These results demonstrate the
potential of caudatin in inhibiting cell migration in uterine tumor
cells.
Caudatin inhibits spheroid formation of
HeLa cells
The 3-D cellular model should be used to better
mimic the in vivo environments (e.g., diffusion and
transport conditions of drugs, nutrients, and oxygen). Formation of
cell spheroids is one of the essential tools for studying the
behaviors of a 3-D cellular model. Cell spheroids are also more
reliable material than two-dimensional (2-D) cellular models for
drug screening in clinical research (30,31).
We next determined the effects of caudatin on cervical carcinoma
cell spheroid formation. As shown in Fig. 4A, caudatin treatment significantly
inhibited cell sphere formation in a dose-dependent manner in HeLa
cells. The spheroids size was counted (Fig. 4B). These results demonstrated
caudatin treatment reduced spheroid formation.
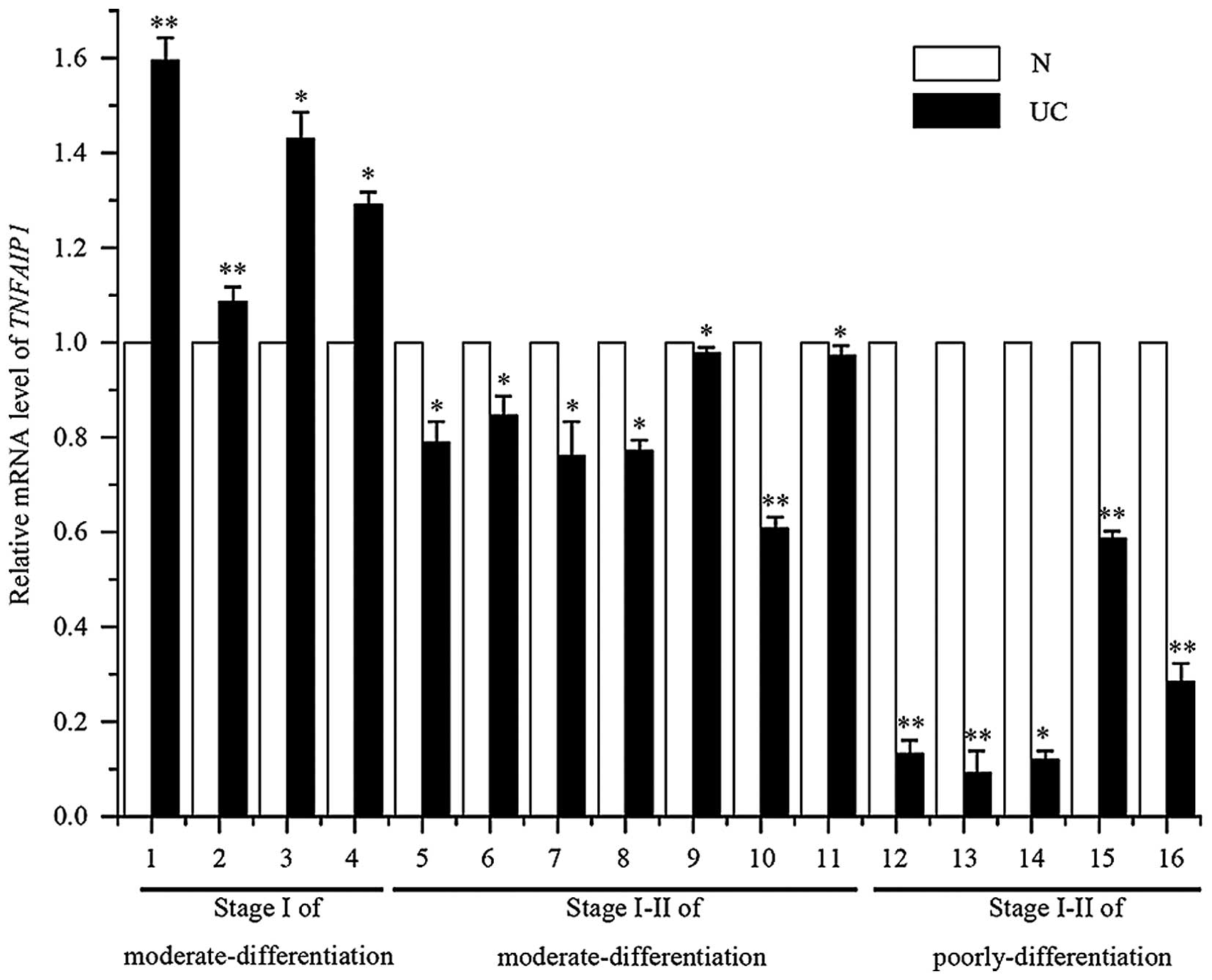
TNFAIP1 is downregulated in human uterine
carcinoma tissues
The correlation of TNFAIP1 expression with various
clinicopathologic factors was analyzed. To confirm the expression
level of TNFAIP1 in uterine cancer tissues and normal uterine
tissues, total RNA was extracted from 16 uterine cancer samples,
consisting of four stage I of moderate differentiation, seven stage
I–II of moderate differentiation and six stage I–II of poorly
differentiation uterine cancer tissues, 16 matched normal tissues,
and then quantitative real-time PCR was performed to analyze the
expression profile. As shown in Fig.
5, we found that TNFAIP1 had lower expression levels in the 12
uterine cancer tissues (UC; 5–11, stage I–II of moderate
differentiation; and 12–16, stage I–II of poor differentiation)
when compared with that in the paired adjacent non-tumorous tissues
(N), and 4 tissues had higher expression (UC; 1–4, stage I of
moderate differentiation). This study has shown the expression of
TNFAIP1 in uterine cancer tissues to be consistent with a previous
study in cancer cell lines, indicating a possible role as an
antioncogene.
Caudatin regulates the expression of NFκB
(p65) through TNFAIP1
NF-κB has been regarded as the hallmark of
carcinogenesis and the expression of NF-κB is tightly controlled by
TNFAIP1. In this study, we found that TNFAIP1 is expressed at low
level in HeLa and HEC-1A cell lines. After pCMV-Myc-TNFAIP1 was
transfected into the uterine cancer cells for 24 h, the expression
levels of TNFAIP1 protein levels of TNFAIP1 and p65NF-κB were
detected by western blotting, indicating increased expression of
TNFAIP1 and decreased expression of p65NF-κB in the overexpression
TNFAIP1 group compared with the NC and MOCK groups (Fig. 6A). The expression level of p65NF-κB
protein was found to be significantly downregulated in the
pCMV-Myc-TNFAIP1 group when compared with the level in the NC and
NC groups. To determine whether caudatin targets the TNFAIP1/NF-κB
signaling, we treated HeLa and HEC-1A cells with caudatin (25, 50
or 100 μmol/l) and examined the expression of TNFAIP1 and NF-κB
proteins by western blotting, respectively. These results
demonstrate that caudatin upregulated expression of TNFAIP1 in a
dose-dependent manner. Along with TNFAIP1 expression improved by
induction of caudatin in the HeLa and HEC-1A cells, NF-κB showed
downregulation (Fig. 6B). TNFAIP1
regulated by caudatin may finally have an effect on NF-κB
signaling. Consequently, caudatin that can regulate the expression
of TNFAIP1 could potentially have therapeutic value.
The effect of TNFAIP1 overexpression is
consistent with caudatin treated in HEC-1A cells
To confirm the effect of TNFAIP1 on uterine cancer
growth, we examined cell proliferative activities by MTT assay and
colony formation. The results showed that overexpression of TNFAIP1
suppressed the proliferative activities of the uterine cancer cells
in a time- and dose-dependent manner compared to the NC group
(Fig. 7A) and inhibited colony
formation (Fig. 7B). Moreover,
Hoechst 33258 staining indicated that typical apoptotic cell
morphology, such as the formation of apoptotic bodies, appeared
after cells were transfected with TNFAIP1, while the control cells
did not show evident apoptotic morphological changes (Fig. 7C). In brief, these results
demonstrated that TNFAIP1 produces the same effect as caudatin
treatment in uterine cancer cells and indicated that caudatin
regulate cell growth, apoptosis of uterine cancer cells by
targeting TNFAIP1.
Caudatin inhibits tumorigenicity in
vivo
We then tested the caudatin inhibition of
tumorigenicity in vivo. HeLa cells (1×106) were
resuspended in 0.1 ml Dulbecco’s modified Eagle’s medium and
inoculated subcutaneously. Seven days after inoculation, when
tumors became palpable, mice were subdivided into two groups of 5
animals each in which the tumor volumes were equally distributed
between the groups. One group of mice was treated daily with 100
mg/kg caudatin. In the experiment presented, the animal was treated
with the drugs for a period of 14 days. Compared to the vehicle
(60% DMSO and 40% alcohol), caudatin significantly decreased tumor
size, overall tumor weight, and mean tumor volume in 2 weeks after
injection. In order to verify the results, we repeated the
experiment. The general conditions and the body weight of the
animals treated with caudatin showed nearly no change for the
period of caudatin application, which implies that the compound has
no obvious toxicity to experiment animals (Fig. 8A and B). These results indicate
caudatin has potential as a novel tumor suppressor that can
suppress tumor growth. We also observed a significant inverse
correlation between TNFAIP1 and NF-κB by treatment with caudatin.
In the two tests, the tumours of two mice were small in the treated
group and therefore total RNA extraction could not be performed,
our qRT-PCR assay indicates that caudatin increased the mRNA level
of TNFAIP1 (Fig. 8C) and
downregulated the mRNA level of NF-κB (Fig. 8D) in the other tumours of eight
mice.
Discussion
Uterine cancer is a common type of cancer among
women, and its incidence is increasing worldwide. Tumor metastasis
poses a predominant threat to cancer-related mortality (34). Despite uterine tumor being
frequently diagnosed cancer and the recent advances in the
treatment of uterine tumor, there are patients for whom no targeted
therapies are available. The significant morbidity, toxicity and
poor response rates of current chemotherapy regimens have led to
search for less toxic alternative therapies (35). It was previously reported that
caudatin suppresses proliferation and induces apoptosis in a
variety of tumor cells; however, the molecular mechanisms
underlying these effects are still unclear.
MTT assay was performed to quantify the effects of
caudatin on HeLa and HEC-1A cell growth inhibition. The data
presented here showed that caudatin treatment resulted in a dose-
and time-dependent inhibition of proliferation in HeLa and HEC-1A
cells. The IC50 values for caudatin in HeLa cells at 12,
24, and 36 h were 86.73, 65.85 and 61.60 μmol/l, respectively. The
IC50 values for caudatin in HEC-1A cells at 12, 24, and
36 h were 121.72, 97.49 and 70.70 μmol/l, respectively. Moreover,
caudatin treatment suppressed colony formation in HeLa and HEC-1A
cells tested, suggesting that the caudatin effect on the tumor
cells is irreversible. In addition to inappropriate growth signals,
many cancer cells lose their ability to undergo apoptosis.
Disturbances in apoptosis are important for the development of
cancer (36). Therefore, the
killing of tumors through induction of apoptosis has been
recognized as a novel strategy for the identification of
anti-cancer drugs (37). In order
to distinguish that the cell death caused by caudatin is due to
apoptosis or necrosis, Annexin V/PI double-labeling analysis was
performed which enables further distinction of necrotic/late
apoptotic (Annexin V+/PI+) and early
apoptotic (Annexin V+/PI−) cells. Our results
showed that treatment of HeLa cells with caudatin resulted in a
dose-dependent increase in the numbers of both early apoptotic and
late apoptotic/necrotic cells. The treatment of HeLa cells with
various concentrations of caudatin (25, 50 and 100 μmol/l) resulted
in apoptosis rates of 4.19±0.344, 5.16±1.15 and 8.73±0.78%,
respectively, which were significantly higher than that of the
control group (3.14±0.50%). Indeed, we observed a significant
increase in dead cells in the HeLa cells even at 12 h following
incubation with caudatin, which subsequently led to cell death.
Hoechst staining showed that the typical morphological changes
attributed to apoptosis, such as formation of apoptotic bodies,
appeared after the cells were treated for 24 h with 25–100 μmol/l
caudatin, whereas the control cells did not show evident apoptotic
morphological changes. Caspases, a family of cysteine proteases,
are synthesized as inactive pro-enzymes which are processed to an
active form in cells undergoing apoptosis (38,39).
In this study, we demonstrated that caudatin treatment triggered
cytochrome c release from mitochondrial inter-membrane space
into cytosol, affected expression of BAX and Bcl-2, and promoted
cleavage of caspase-3, −9 and PARP, thereby activating apoptotic
pathway. Thus, the induction of HeLa and HEC-1A cell apoptosis in
response to caudatin is an important mechanism for its preventative
and antitumor activity in uterine cancer.
Wound-healing assay was a mature approach to test
cell migration ability. Our experimental results suggested that
caudatin markedly reduced the migration ability of cells compared
to scramble group cells. As shown in Fig. 3, the part of the wounding space
between cell layers after making a scratch was occupied completely
by the migrating cells after 72 h in the control group. However,
the empty space of the cells was not occupied by the migrating
cells treated with 50 and 100 μmol/l caudatin. These results
demonstrated the potential of caudatin in changing cell morphology
and inhibiting cell migration in uterine cancer cells. Cancer cells
possess varying capacities for spheroid formation and although a
positive correlation with tumorigenicity has been suggested
(40,41), another study reported an inverse
association between brain tumor cell spheroid cohesiveness and
invasive potential (42). Although
spheroid formation affords protection of cancer cells against some
chemotherapeutic agents, it has not been established whether a
relationship exists between invasive behavior and predisposition to
spheroid formation. The cell behavior necessary for compact
spheroid formation may also promote uterine cancer progression
(43). In this study, HeLa cells
were grown in low adherent plates and treated with increasing
concentrations of caudatin (0–100 μmol/l) and performed in the
spheroid assay. Caudatin treatment significantly inhibited uterine
cancer cells spheroids. The results revealed caudatin play a
potential suppressive role and antitumor activity in uterine
cancer.
Advances in the understanding of the molecular
mechanisms of apoptosis have identified the apoptotic pathway as a
promising target to increase the effectiveness of cancer treatment.
It has been previously reported that TNFAIP1 was shown to be
expressed at a high level in normal cells and was downregulated in
several cancer derived cell lines. In tumor cells, TNFAIP1 can act
through pleiotropic mechanisms, including stimulation of cell
proliferation, survival, migration, and invasion (24,27).
Initially found to be an anti-oncogene in metastatic breast cancer
(44). NF-κB is one of the most
important intracellular nuclear transcription factors, and it plays
a central role in the transcriptional regulation of many genes that
are influenced by various stimuli. It has also been shown that
NF-κB activity inhibits apoptosis in cancer cells. In a previous
study, we found that TNFAIP1 and KCTD10 suppressed the
transcriptional activities of NF-κB, leading to discreased cell
survival by transactivating anti-apoptotic genes downstream of
NF-κB (29,45). A recent study indicated that high
expression of TNFAIP1 was associated with distant metastasis of
osteosarcoma, and knockdown of TNFAIP1 inhibited the growth and
invasion, and induced apoptosis in osteosarcoma cells through
inhibition of the NF-κB pathway, suggesting that TNFAIP1 may act as
a potential therapeutic target for the treatment of cancer
(25,26). Whereas TNFAIP1 upregulation
inhibited tumor growth, suggesting that TNFAIP1 might play a key
role in maintaining cancer cell survival. In this study, we found
that TNFAIP1 is frequently downregulated in uterine cancer tissues
(Fig. 5). We found that while
pCMV-Myc-TNFAIP1 was transfected to HeLa or HEC1A cells, NF-κB
presented downregulation accompanied by increased TNFAIP1.
Moreover, the overexpression of TNFAIP1 could inhibit cell
proliferation, colony formation, and induces apoptosis in HEC-1A
cells similarly to those induced by caudatin. Therefore, we
hypothesized that caudatin exerts its effects through the
modulation of the expression of TNFAIP1. As shown in Fig. 6B, experimental confirmation
demonstrated that TNFAIP1 gene is a potential target of caudatin
and can be regulated by caudatin in a dose-dependent manner.
Moreover, we found that caudatin treatment led to an ~50%
increasion in TNFAIP1 and an ~40% reduction in NF-κB protein
levels. Collectively, our data indicate that tumor suppression
function of caudatin may be through control of cell growth,
migration and cell apoptosis by upregulating the TNFAIP1 and
negatively regulating NF-κB expression.
To further explore the role of caudatin in tumor
growth in vivo, HeLa cells (1×106) were injected
subcutaneously into nude mice. Seven days after inoculation, when
tumors became palpable, mice were subdivided into two groups of 5
animals each with the tumor volumes equally distributed between the
two groups. One group of mice was injected daily with 100 mg/kg
caudatin. Compared to the vehicle (DMSO), caudatin significantly
decreased tumor size, overall tumor weight, and mean tumor volume
in 2 weeks after injection. Treatment with caudatin induced smaller
tumors than the control in vivo and their average volume was
36.9% of the control group at day 14 after injection (P<0.05)
(Fig. 8B). As expected, the
expression of TNFAIP1 was increased in xenograft animal tumors
treated with caudatin (Fig. 8C).
However, the expression of NF-κB was significantly suppressed in
caudatin-treated tumors (Fig. 8D).
These results indicated that caudatin might function as a tumor
suppressor drug partly mediated by repressing TNFAIP1 expression in
uterine cancer. However, TNFAIP1 is not the only pathway inhibited
in uterine cancer, as many other signal transduction pathways are
inhibited, and these pathways will be the focus of our future
research.
In conclusion, we demonstrated that caudatin
exhibited anti-proliferative and pro-apoptotic activities in
uterine cancer cells, at least partially, via the caspase-dependent
apoptotic pathway and the TNFAIP1/NF-κB signaling pathway. Thus,
targeting TNFAIP1 may be an effective strategy to control tissue
destruction in uterine cancer patients. Our findings provide new
insights into exploring the potential therapeutic strategies and
novel targets for human uterine cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81071696 and 81372157),
Project of Inquiry Learning and Innovative Experiment of Hunan
Province (2014-075), Project of Innovative Experiment of Hunan
Normal University (043-0094), The Cooperative Innovation Center of
Engineering and New Products for Developmental Biology of Hunan
Province (20134486), and the Construct Program of the Key
Discipline of Basic Medicine in Hunan Province in China.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar
|
|
2
|
Barbera L and Thomas G: Management of
early and locally advanced cervical cancer. Semin Oncol.
36:155–169. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Y, Li Y, Li J, Wang J and Yan Z:
Tendency and strategy of younger patients with cervical carcinoma.
Med Natl Defending Forces Southwest China. 1:53–55. 2008.
|
|
4
|
Huang CY, Chen CA, Chen YL, Chiang CJ, Hsu
TH, Lin MC, Lai MS, Chen CJ, You SL and Cheng WF: Nationwide
surveillance in uterine cancer: survival analysis and the
importance of birth cohort: 30-year population-based registry in
Taiwan. PLoS One. 7:e513722012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Z, Li B, Meng X, Yao S, Jin L, Yang
J, Wang J, Zhang H, Zhang Z, Cai D, et al: Berberine prevents
progression from hepatic steatosis to steatohepatitis and fibrosis
by reducing endoplasmic reticulum stress. Sci Rep. Feb 9–2016.(Epub
ahead of print). View Article : Google Scholar
|
|
6
|
Yu D, An F, He X and Cao X: Curcumin
inhibits the proliferation and invasion of human osteosarcoma cell
line MG-63 by regulating miR-138. Int J Clin Exp Pathol.
8:14946–14952. 2015.
|
|
7
|
Tian L, Shen D, Li X, Shan X, Wang X, Yan
Q and Liu J: Ginsenoside Rg3 inhibits epithelial-mesenchymal
transition (EMT) and invasion of lung cancer by down-regulating
FUT4. Oncotarget. 7:1619–1632. 2016.
|
|
8
|
Vistad I, Fosså SD and Dahl AA: A critical
review of patient-rated quality of life studies of long-term
survivors of cervical cancer. Gynecol Oncol. 102:563–572. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ma XX, Wang D, Zhang YJ and Yang CR:
Identification of new qingyangshengenin and caudatin glycosides
from the roots of Cynanchum otophyllum. Steroids. 76:1003–1009.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin ZQ, Yu SL, Wei YJ, Ma L, Wu ZF, Wang
L, Zhang QW, Zhao M, Ye WC, Che CT, et al: C21 steroidal glycosides
from Cynanchum Stauntonii induce apoptosis in HepG2 cells.
Steroids. 106:55–61. 2016. View Article : Google Scholar
|
|
11
|
Wang YQ, Zhang SJ, Lu H, Yang B, Ye LF and
Zhang RS: A C21-steroidal glycoside isolated from the
roots of cynanchum auriculatum induces cell cycle arrest and
apoptosis in human gastric cancer SGC-7901 cells. Evid Based
Complement Alternat Med. 2013:1808392013.
|
|
12
|
Fu XY, Zhang S, Wang K, Yang MF, Fan CD
and Sun BL: Caudatin inhibits human glioma cells growth through
triggering DNA damage-mediated cell cycle arrest. Cell Mol
Neurobiol. 35:953–959. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fei HR, Cui LY, Zhang ZR, Zhao Y and Wang
FZ: Caudatin inhibits carcinomic human alveolar basal epithelial
cell growth and angiogenesis through modulating GSK3β/β-catenin
pathway. J Cell Biochem. 113:3403–3410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li X, Zhang X, Liu X, Tan Z, Yang C, Ding
X, Hu X, Zhou J, Xiang S, Zhou C, et al: Caudatin induces cell
apoptosis in gastric cancer cells through modulation of
Wnt/β-catenin signaling. Oncol Rep. 30:677–684. 2013.PubMed/NCBI
|
|
15
|
Peng Y and Ding Y: Pharmacokinetics and
tissue distribution study of caudatin in normal and
diethylnitrosamine-induced hepatocellular carcinoma model rats.
Molecules. 20:4225–4237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo Y, Sun Z, Li Y, Cai X and Li Z:
Caudatin inhibits human hepatoma cell growth and metastasis through
modulation of the Wnt/β-catenin pathway. Oncol Rep. 30:2923–2928.
2013.PubMed/NCBI
|
|
17
|
Wang LJ, Geng CA, Ma YB, Luo J, Huang XY,
Chen H, Zhou NJ, Zhang XM and Chen JJ: Design, synthesis, and
molecular hybrids of caudatin and cinnamic acids as novel
anti-hepatitis B virus agents. Eur J Med Chem. 54:352–365. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peng YR, Ding YF, Wei YJ, Shu B, Li YB and
Liu XD: Caudatin-2,6-dideoxy-3-O-methy-β-D-cymaropyranoside 1
induced apoptosis through caspase 3-dependent pathway in human
hepatoma cell line SMMC7721. Phytother Res. 25:631–637. 2011.
View Article : Google Scholar
|
|
19
|
Wolf FW, Marks RM, Sarma V, Byers MG, Katz
RW, Shows TB and Dixit VM: Characterization of a novel tumor
necrosis factor-alpha-induced endothelial primary response gene. J
Biol Chem. 267:1317–1326. 1992.PubMed/NCBI
|
|
20
|
Link CD, Taft A, Kapulkin V, Duke K, Kim
S, Fei Q, Wood DE and Sahagan BG: Gene expression analysis in a
transgenic Caenorhabditis elegans Alzheimer’s disease model.
Neurobiol Aging. 24:397–413. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang L, Liu N, Hu X, Zhang W, Wang T, Li
H, Zhang B, Xiang S, Zhou J and Zhang J: CK2 phosphorylates TNFAIP1
to affect its subcellular localization and interaction with PCNA.
Mol Biol Rep. 37:2967–2973. 2010. View Article : Google Scholar
|
|
22
|
Zhou C, Li X, Zhang X, Liu X, Tan Z, Yang
C and Zhang J: microRNA-372 maintains oncogene characteristics by
targeting TNFAIP1 and affects NFκB signaling in human gastric
carcinoma cells. Int J Oncol. 42:635–642. 2013.
|
|
23
|
Zhang X, Li X, Tan Z, Liu X, Yang C, Ding
X, Hu X, Zhou J, Xiang S, Zhou C, et al: MicroRNA-373 is
upregulated and targets TNFAIP1 in human gastric cancer,
contributing to tumorigenesis. Oncol Lett. 6:1427–1434.
2013.PubMed/NCBI
|
|
24
|
Zhu Y, Yao Z, Wu Z, Mei Y and Wu M: Role
of tumor necrosis factor alpha-induced protein 1 in paclitaxel
resistance. Oncogene. 33:3246–3255. 2014. View Article : Google Scholar
|
|
25
|
Tian X, Zhang J, Yan L, Dong JM and Guo Q:
miRNA-15a inhibits proliferation, migration and invasion by
targeting TNFAIP1 in human osteosarcoma cells. Int J Clin Exp
Pathol. 8:6442–6449. 2015.PubMed/NCBI
|
|
26
|
Zhang CL, Wang C, Yan WJ, Gao R, Li YH and
Zhou XH: Knockdown of TNFAIP1 inhibits growth and induces apoptosis
in osteosarcoma cells through inhibition of the nuclear factor-κB
pathway. Oncol Rep. 32:1149–1155. 2014.PubMed/NCBI
|
|
27
|
Yang LP, Zhou AD, Li H, Zhang WF, Wu YY,
Zhang J and Han M: Expression profile in the cell lines of human
TNFAIP1 gene. Yi Chuan. 28:918–922. 2006.(In Chinese). PubMed/NCBI
|
|
28
|
Kim DM1, Chung KS, Choi SJ, Jung YJ, Park
SK, Han GH, Ha JS, Song KB, Choi NS, Kim HM, et al: RhoB induces
apoptosis via direct interaction with TNFAIP1 in HeLa cells. Int J
Cancer. 125:2520–2527. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hu X, Yan F, Wang F, Yang Z, Xiao L, Li L,
Xiang S, Zhou J, Ding X and Zhang J: TNFAIP1 interacts with KCTD10
to promote the degradation of KCTD10 proteins and inhibit the
transcriptional activities of NF-κB and AP-1. Mol Biol Rep.
39:9911–9919. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin RZ and Chang HY: Recent advances in
three-dimensional multicellular spheroid culture for biomedical
research. Biotechnol J. 3:1172–1184. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Patra B, Peng CC, Liao WH, Lee CH and Tung
YC: Drug testing and flow cytometry analysis on a large number of
uniform sized tumor spheroids using a microfluidic device. Sci Rep.
Feb 15–2016.(Epub ahead of print). View Article : Google Scholar
|
|
32
|
Daniyal M, Akhtar N, Ahmad S, Fatima U,
Akram M and Asif HM: Update knowledge on cervical cancer incidence
and prevalence in Asia. Asian Pac J Cancer Prev. 16:3617–3620.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh R: Review literature on uterine
carcinosarcoma. J Cancer Res Ther. 10:461–468. 2014.PubMed/NCBI
|
|
34
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J,
Murray T and Thun MJ: Cancer statistics, 2008. CA Cancer J Clin.
58:71–96. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
May BH, Lu C, Bennett L, Hügel HM and Xue
CC: Evaluating the traditional Chinese literature for herbal
formulae and individual herbs used for age-related dementia and
memory impairment. Biogerontology. 13:299–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular mechanisms of apoptosis and roles in cancer
development and treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen CT, Chen YC, Yamaguchi H and Hung MC:
Carglumic acid promotes apoptosis and suppresses cancer cell
proliferation in vitro and in vivo. Am J Cancer Res. 5:3560–3569.
2015.
|
|
38
|
Heath-Engel HM, Chang NC and Shore GC: The
endoplasmic reticulum in apoptosis and autophagy: Role of the BCL-2
protein family. Oncogene. 27:6419–6433. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yun SI, Yoon HY and Chung YS: Glycogen
synthase kinase-3beta regulates etoposide-induced apoptosis via
Bcl-2 mediated caspase-3 activation in C3H10T1/2 cells. Apoptosis.
14:771–777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kelm JM, Timmins NE, Brown CJ, Fussenegger
M and Nielsen LK: Method for generation of homogeneous
multicellular tumor spheroids applicable to a wide variety of cell
types. Biotechnol Bioeng. 83:173–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Weiswald LB, Bellet D and Dangles-Marie V:
Spherical cancer models in tumor biology. Neoplasia. 17:1–15. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Winters BS, Shepard SR and Foty RA:
Biophysical measurement of brain tumor cohesion. Int J Cancer.
114:371–379. 2005. View Article : Google Scholar
|
|
43
|
López J, Poitevin A, Mendoza-Martínez V,
Pérez-Plasencia C and García-Carrancá A: Cancer-initiating cells
derived from established cervical cell lines exhibit stem-cell
markers and increased radioresistance. BMC Cancer. Jan
28–2012.(Epub ahead of print). View Article : Google Scholar
|
|
44
|
Grinchuk OV, Motakis E and Kuznetsov VA:
Complex sense-antisense architecture of TNFAIP1/POLDIP2 on 17q11.2
represents a novel transcriptional structural-functional gene
module involved in breast cancer progression. BMC Genomics.
11(Suppl 1): S92010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Skoblov M, Marakhonov A, Marakasova E,
Guskova A, Chandhoke V, Birerdinc A and Baranova A: Protein
partners of KCTD proteins provide insights about their functional
roles in cell differentiation and vertebrate development.
Bioessays. 35:586–596. 2013. View Article : Google Scholar : PubMed/NCBI
|