Introduction
Higher recurrence rate and poorer survival prognosis
have made esophageal cancer one of the most lethal malignancies,
ranked the fourth in cancer-related mortality (1–3). The
overall 5-year survival rate varies from 15 to 25%, and still no
efficient treatments are available (2). Non-coding Let-7 could target and
degrade its downstream CCND1, HMGA2, RAS and other oncogenic
factors to function as one of the strongest suppressors in both
cancer cells and cancer stem cells (4,5). The
novel regulative mechanisms of miRNAs were defined as 'Sponge
action' (6,7). The miRNA sponge was accepted as an
innovative concept to regulate miRNAs (8), which could produce segments of RNA,
containing repeats of tandem-binding sites, which were
complementary to seed regions of certain miRNAs (9). Through base-pair-dependent
interaction to the seed region, the sponge leads to a reduction of
active miRNAs. Adenoviral and lentiviral constructs of miRNA
sponges utilize RNA polymerase III for transcription (10), and in detail, H19, CCAT1 of
Lnc-RNAs and other circular-RNAs were the molecular sponge
corresponding to Let-7 expression (11,12).
Tumorigenic transformation occurs in the immortal or
repeatedly dividing cells more commonly, and cancer stem-like cells
(CSCs) were blamed for tumor recurrence and resistance (5,13),
whereas, how these CSCs emerge is still unclear. Cancer stem cells
could be identified and isolated by FACS sorting in cell lines, and
be identified in cancer tissues by immunofluorescence or
immunohistochemical staining (14–16).
Esophageal cancer stem cells could be identified with surface
markers of CD133 (17,18) and ALDH1 (19–23).
The treatments aiming to eliminate the stem cells will help in
cancer treatment, yielding diagnostic and therapeutic approaches
(4,5). The way stem cells divide affects
greatly the stem cell numbers, but how the division influence the
cell renewal capacity is still in debate. Carcinogenesis may arise
as a consequence of adult stem-cell dysfunction, which fails to
undergo asymmetric cell division (ACD) (24,25).
The fine regulations of stem cells allow themselves to self-renew
and generate the differentiated cells, forming and maintaining
mature tissues and organs. The uncontrolled symmetric cell division
(SCD) will expand the stem cell pool, resulting in numerous
stem-like cells in carcinoma (26,27).
The aim of ACD is to create two different daughter
cells; one is to sustain the stem cell group, and another is to
differentiate into certain type. The way to achieve this is the
asymmetric segregation of cell fate determinants, such as Numb,
PKC, and p53 (28–31), which could instruct the cell that
inherits it to adopt a certain identity (27). The asymmetric distribution of cell
fate determinants makes the cells segregate in a polarized way,
with the mitotic spindle enriched asymmetrically. The influences on
ACD decrease the stem cell number, determining the stem cells fate.
The division manner in esophageal cancer cells, and the
relationship between the stem-like cells and cancer biology are
barely known in esophageal cancer. In the present study, we
explored the mechanistic phenotypes of division of stem-like cells
in esophageal cancer, and the application of tentative usage of
nanoliposomal non-coding RNA in cancer treatment.
Materials and methods
Enrollment of patients
From July 2008 to November 2014, 317 Chinese
patients consecutively underwent radical esophagectomy and
reconstruction for esophageal tract at the First Affiliated
Hospital of Xi'an Jiaotong University, were evaluated and enrolled.
The pathological examinations were confirmed and filed in order.
The clinicopathological characteristics, and results of
pathological sections were collected and shown in detail in
Table I. Patients diagnosed with
phase IV carcinoma preoperatively were not enrolled, and grouped
patients with phase IV were all diagnosed postoperatively.
Histopathologic evaluation was confirmed by two separate pathologic
professors, and patients were diagnosed with no other system
malignancies. Written informed consent was obtained from each
patient, in accordance with the Declaration of Helsinki before
sample collection. The study protocol and patients' informed
consent statements were approved and supervised by the Ethics
committee of the First Affiliated Hospital and the Second
Affiliated Hospital of Xi'an Jiaotong University.
 | Table IThe clinicopathological
characteristics of patients enrolled (N=317). |
Table I
The clinicopathological
characteristics of patients enrolled (N=317).
| Category | RE |
|---|
| Sex | |
| Male/Female | 255/62 |
| Age (median) | 53.2 |
| <65/≥65 | 204/113 |
| Location of
tumor | |
|
Upper/middle/lower | 44/151/122 |
| Histological
type | |
| SC/AC/AS | 260/39/18 |
| Neoadjuvant or
adjuvant therapy | |
| Yes/No | 270/47 |
| Neoadjuvant
therapy/adjuvant therapy | |
| Yes/No | 29/241 |
| Recurrence within 2
years | |
| Yes/No | 74/243 |
| Pathological tumor
stage | |
| I/II/III/IV | 14/156/135/12 |
| Operation time | |
| <4/≥4
hours | 90/227 |
Isolation and culturing of stem
cells
Esophageal cancer cells were maintained at 37°C, 5%
CO2 in RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA)
supplemented with 10% fetal bovine serum (Hyclone, Salt Lake City,
UT, USA) and 1% penicillin/streptomycin (Cellgro, Lowell, MA, USA).
fluorouracil and docetaxel were used at a concentration of 0.2 mM.
The stem-like cells were cultured as we previously described.
Briefly, cells were grown in ultra-low attachment dishes (Corning
Inc., Lowell, MA, USA), supplemented with serum-free medium
(DMEM/F12), 1:50 B27 (Invitrogen), 20 ng/ml recombinant human basic
FGF (Invitrogen), 5 μg/ml insulin, 0.5 mg/ml hydrocortisone
and 20 ng/ml epidermal growth factor (Invitrogen). The sphere
forming efficiency was calculated as the ratio of obtained spheres
verses number of plated cells (spheres/1,000 cells). All sphere
formation experiments were performed in triplicate
independently.
RNA and protein detection
Total RNA was extracted from cells with
TRIzol® reagent (Invitrogen) according to the
manufacturer's instructions. The reverse-transcription was
conducted by using Prime Script™ RT reagent kit (Takara, Dalian,
China). The qRT-PCR was performed on a CFX96™ Real-Time PCR
Detection system (Bio-Rad, USA) with SYBR® Premix
Ex-Taq™ II (Takara). The primers for qRT-PCR detection were
synthesized by Invitrogen (Shanghai, China). RNU6B (for mature
miRNA) or 18S was taken as internal control. Fold change was
determined as 2−ΔΔCt. All experiments were performed in
triplicate, independently. Cell lysates were prepared with RIPA
buffer containing Protease Inhibitor Cocktail Tablets (Roche) for
15 min on ice. The total protein concentrations were determined
using Protein BCA assay kit (Bio-Rad). Protein samples were
denatured with 5X loading buffer at 100°C for 5 min. Equal amounts
of protein were separated by 10% SDS-PAGE and transferred onto NC
membranes (Bio-Rad). The membrane was blocked with 5% non-fat milk
for 2 h at room temperature, and subsequently incubated with
primary antibody overnight at 4°C. The primary antibodies used were
as follows: HMGA2 (1:1,000, ab52039; Abcam, Cambridge, MA, USA),
CCND1 (1:1,000; #2922; Cell Signaling Technology, Inc., USA), TCF-4
(1:1,000, ab60727; Abcam), β-catenin (1:2,000, ab78483; Abcam).
Then the membranes were incubated with HRP-conjugated secondary
antibody (1:5,000; Santa Cruz Biotechnology, Inc.) for 2 h. An
anti-vinculin antibody (1:5,000, #4650; Cell Signaling Technology,
Inc.) was used as internal control.
Construction of CCAT-1 and Let-7b
deregulated cells
For constructing cells with enforced CCAT1, a
genomic region encoding CCAT1 was PCR-amplified using
PrimeSTAR® HS DNA Polymerase (Takara) and subcloned into
the pcDNA3.1 vector (Invitrogen), named pcDNA3.1-CCAT1. The
pcDNA3.1 vector was used as a negative control. The primers were as
follows: 5′-CTAGCTAGCACAACATCGACTTTGAAGTT-3′ (sense) and
5′-CCCAAGCTTAAGACTTAATATACTTATATTTA-3′ (antisense). To obtain cell
lines stably expressing CCAT1, ECA-109 and ECA-9706 cells were
transfected with the plasmid pcDNA3.1-CCAT1 or pcDNA3.1 vector by
using Lipofectamine 2000 according to the manufacturer's
instructions. Cells were selected with neomycin (800 μg/ml)
for four weeks. Nanoliposomes containing Let-7b mimics were
prepared as previously described (32–34).
Briefly, let-7b mimics were mixed with 1,
2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC) (Avanti Polar
Lipids, Alabaster, AL, USA) in the presence of excess tertiary
butanol at a ratio of 1:10 (w/w) let-7b mimics/DOPC. The mixture
was vortexed and lyophilized. Before experiments, this mixture was
hydrated with normal 0.9% saline and purified by separating free
mimics from liposomes with 30,000 nominal molecular weight limit
filter units (Millipore, Billerica, MA, USA).
Immunochemistry and immunofluorescence
staining
The cells were fixed in 4% formaldehyde, washed with
PBS for 15 min, and permeabilized with 0.2% Triton X-100 for 20
min. After permeabilization, the cells were blocked with bovine
serum albumin (BSA) at 37°C for 30 min. Fixed cells were incubated
with the antibodies against H3K9me2 (1:200, ab1220; Abcam) at 4°C
overnight, followed by Alexa Fluor 594 goat anti-rabbit IgG (H+L)
secondary antibody (1:1,000, A-11012; Life Technologies,
Gaithersburg, MD, USA) for 1 h at room temperature. The nuclei were
counterstained with 4, 6-diamidino-2-phenylindole (DAPI, 1:10,000;
4084; Cell Signaling Technology, Inc.). The fluorescence images
were obtained using an Olympus microscope.
For immunochemistry test of clinical samples,
briefly, formalin-fixed paraffin-embedded samples were prepared as
4-μm-thick sections as previously described (35). The sections were incubated with
primary antibodies against CD133 (ab19898; Abcam) and ALDH1
(sc-166362, Santa Cruz Biotechnology, Inc.) at 4°C overnight, and
subjected to be incubated with the appropriate secondary antibody
for 30 min at room temperature. IHC staining results in each sample
was scored using semiquantitative scoring system, taking into
consideration the staining intensity obtained and the proportion of
positive cells observed.
Statistical analysis
The association between the postoperative
complications, recurrence ratio, the ratio of stem-like cell
markers and the clinicopathological factors was assessed using the
Chi-square two-tailed test, ANOVA analysis or Fisher's exact test.
The independent factor associated with clinicopathological factors
and the ratio of stem-like cell markers were evaluated using a
logistic regression analysis and Cox regression analysis. All
statistical analyses were performed using the GraphPad Prism 5.01
or Microsoft Excel 2011, and a P-value <0.05 was considered to
indicate a statistically significant difference.
Results
The ratio of stem-like cells was
responsible for prognosis of postoperative survivors
Specimens were tested for the ratio of cancer
stem-like cells from each patient, and for the correlation between
certain stem cell marker and the ratio of patients who were
diagnosed with recurrent esophageal carcinoma after receiving
radical esophagectomy. Stem-like cells were more likely to be
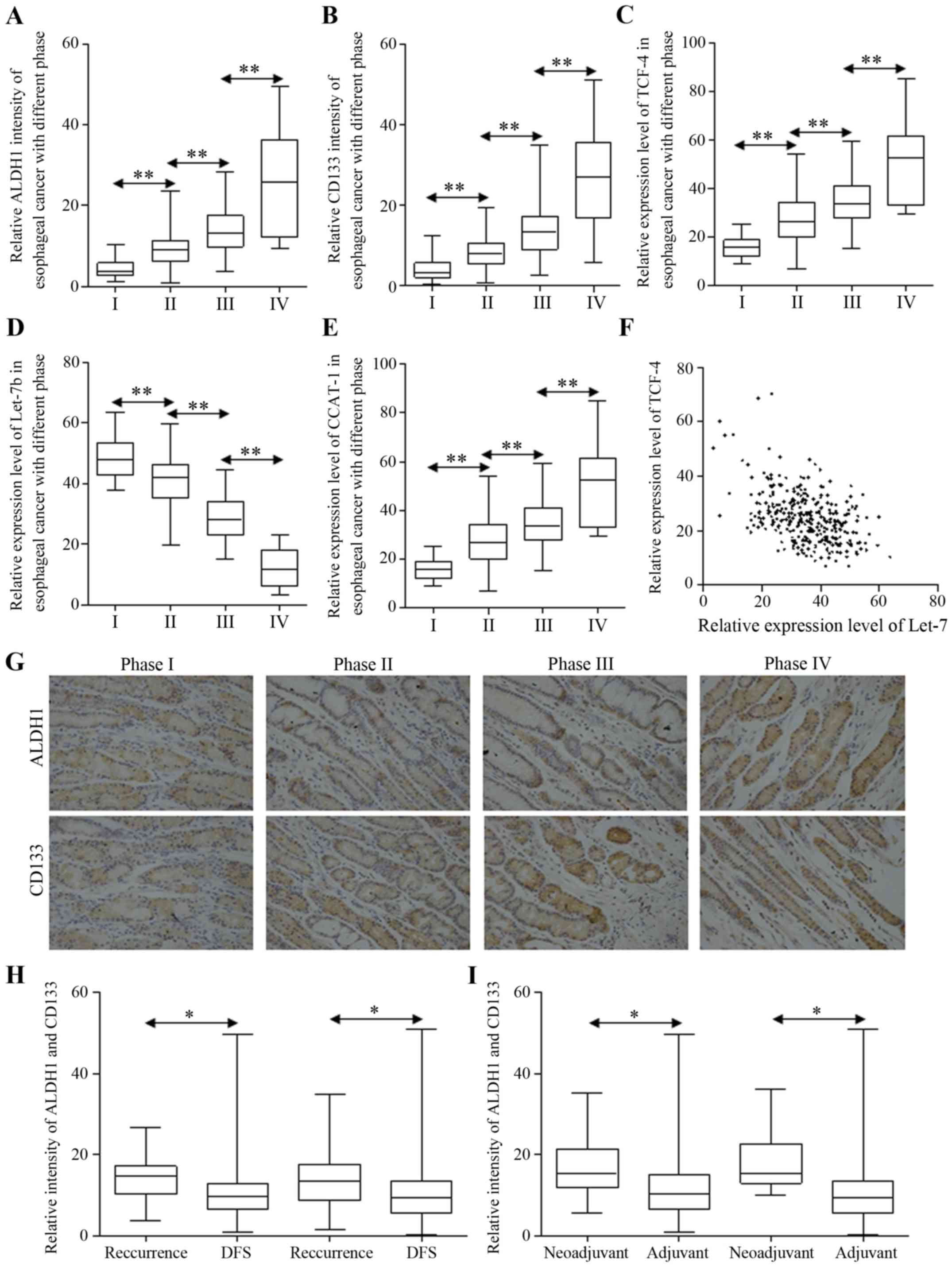
enriched in carcinoma of later stages (Fig. 1A and B). Furthermore, esophageal
carcinoma with more stem-like cancer cells tends to relapse more
often in two years postoperatively (Table II). Wnt signaling activation and
Let-7b repression were both involved in carcinoma progression,
strengthened in esophageal cancer of later stages (Fig. 1C and D). The potential miRNA sponge
of CCAT1 was increased as Wnt signaling did (Fig. 1E). The correlation was also
observed between Let-7b and TCF-4, indicating the indirect
regulation (Fig. 1F). The staining
of ALDH1 and CD133 are shown in Fig.
1G. Last but not least, patients diagnosed with recurrent
esophageal carcinoma presented different patterns of stem cell
numbers, and carcinoma harbored less stem-like cells indicated
longer disease-free survival time, while more cancer stem-like
cells were correlated to poorer survival (Fig. 1H). Additionally, we identified the
enriched stem-like cells in patients who received neo-adjuvant
chemotherapy, compared to patients undergoing radical esophagectomy
with none preoperative treatment (Fig.
1I).
 | Table IIThe phenotypes and signatures of stem
cell potency involving in recurrence after patients receiving
radical esophagectomy. |
Table II
The phenotypes and signatures of stem
cell potency involving in recurrence after patients receiving
radical esophagectomy.
| Category | Recurrence
| P-value | Chi-square |
|---|
| Yes | No |
|---|
| Relative ALDH1
intensity | | | | |
| +/++/+++/++++ | 6/16/26/26 | 20/97/108/18 | <0.0001 | 37.91 |
| Relative CD133
intensity | | | | |
| +/++/+++/++++ | 4/10/38/22 | 25/85/124/22 | 0.0005 | 17.63 |
| Relative β-catenin
intensity | | | | |
| +/++/+++/++++ | 6/16/30/22 | 47/156/39/1 | <0.0001 | 106.1 |
| Relative Wnt1
intensity | | | | |
| +/++/+++/++++ | 8/12/26/28 | 62/70/60/51 | 0.0005 | 17.77 |
| Relative TCF-4
intensity | | | | |
| +/++/+++/++++ | 2/10/28/34 | 49/86/70/38 | <0.0001 | 44.15 |
| Relative Let-7
expression | | | | |
| +/++/+++/++++ | 16/46/8/4 | 23/31/104/85 | <0.0001 | 97.91 |
| Relative CCAT1
expression | | | | |
| +/++/+++/++++ | 4/12/34/24 | 12/58/81/92 | 0.2147 | 4.473 |
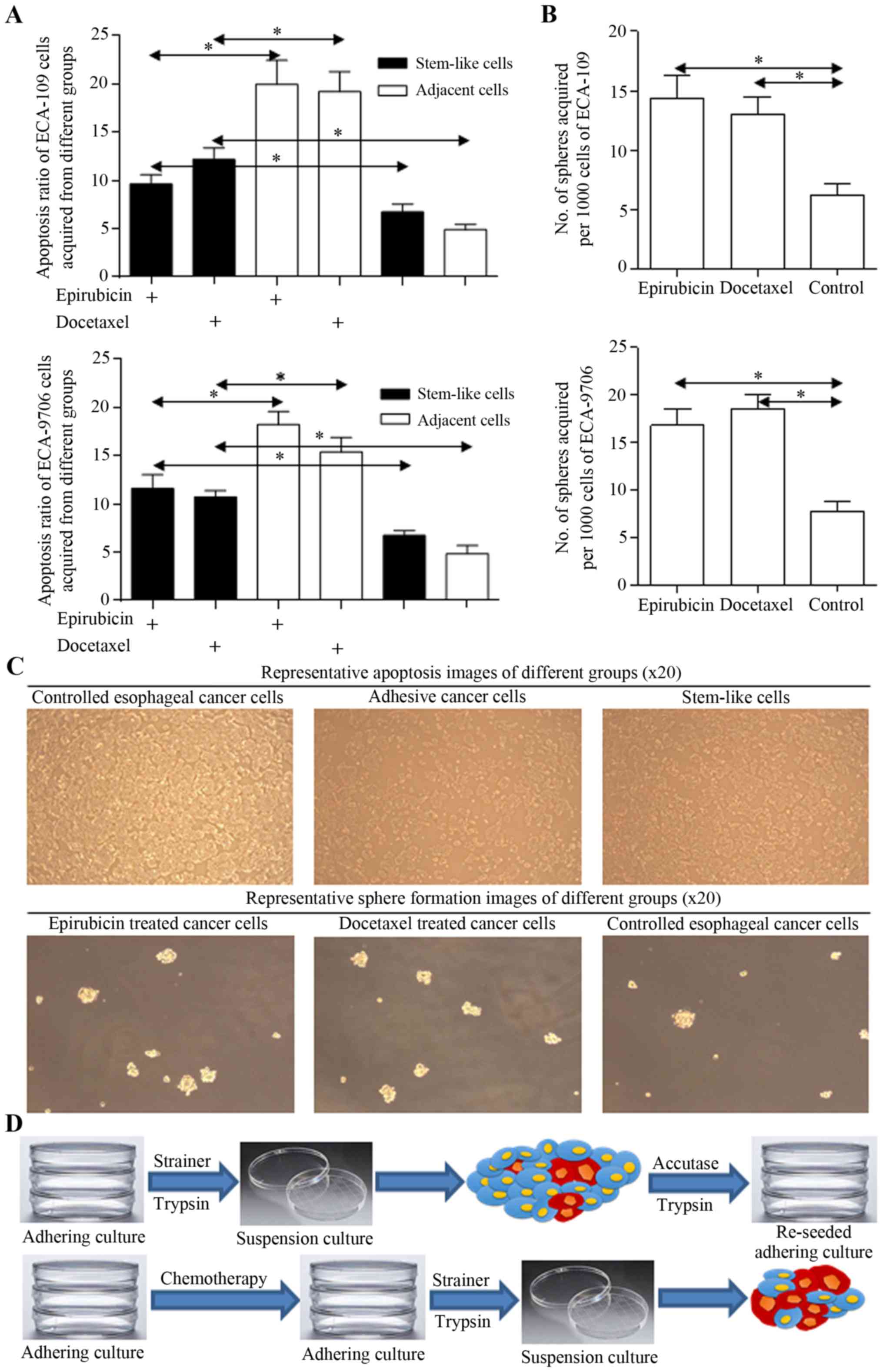
Harbored stem-like cells could revive
from therapeutic procedure
Stem cells harboring in cancer cells group could
survive through multidisciplinary treatment, for their less
proliferative signatures and native drug resistant nature. Stem
cells acquired from spheres were found to be naturally resistant to
chemotherapy (Fig. 2A), and more
spheres could be enriched from cancer cells treated with
fluorouracil or docetaxel (Fig.
2B), supporting the hypothesis we concluded. Representative
images and the scheme are illustrated in Fig. 2C and D.
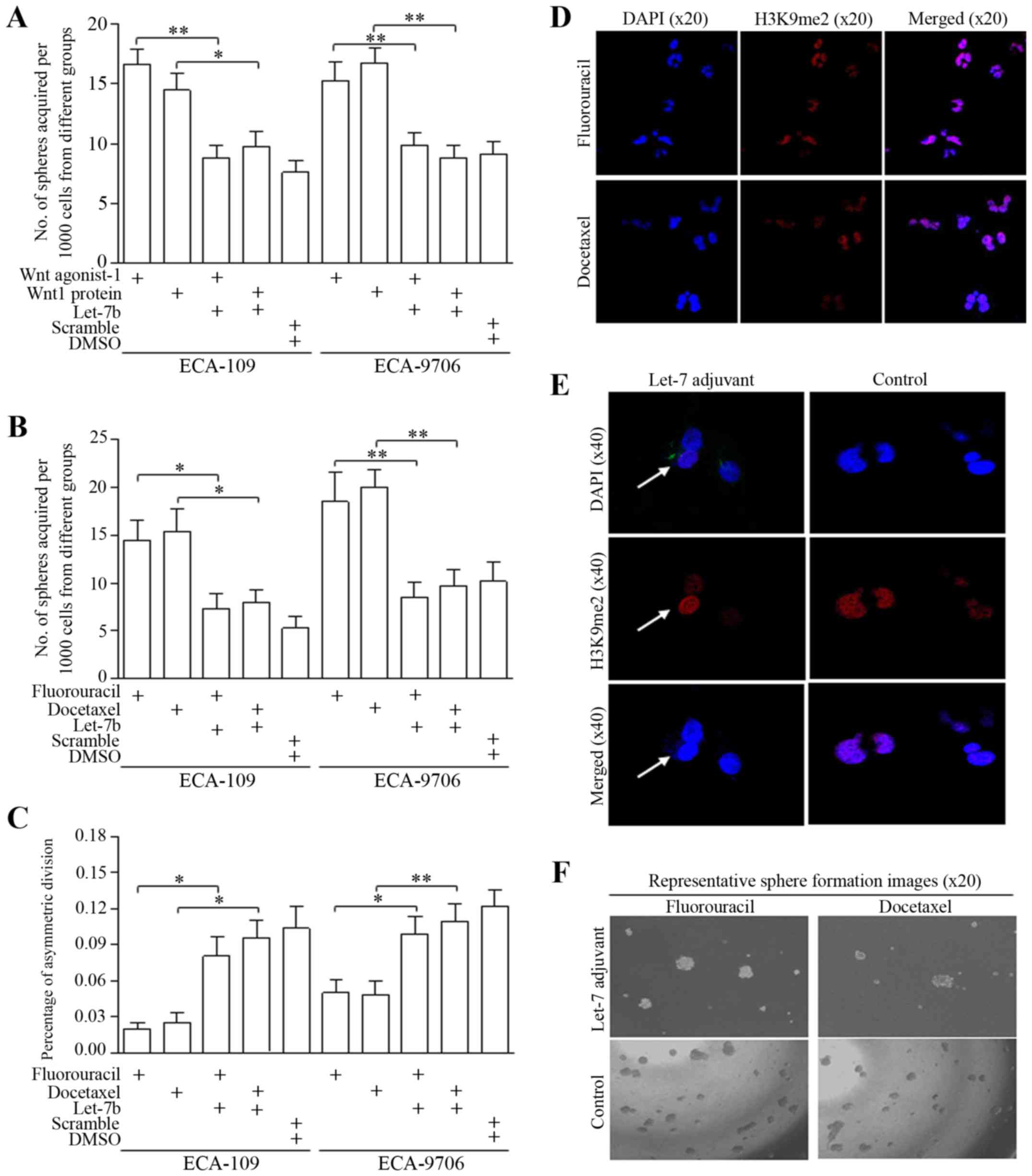
Wnt signaling activation drives stem-like
cells to divide symmetrically to form more spheres
Stem cells and cancer stem-like cells divide
asymmetrically to generate two daughter cells with diverse
phenotype, as illustrated in Fig.
3A. Cancer stem-like cells tend to divide symmetrically
generating two unique stem cells to expand the stem cell pool
(36,37). In stem cells enriched from spheres
of esophageal cancer cells, we identified the frequent occurrence
of symmetric division through H3K9me2 staining (24,25,38–40),
as presented in Fig. 3B and C.
Both Wnt pathway agonist-1 (50 nM, S8178, Selleckchem, USA) and
recombinant Wnt1 protein (50 ng/ml; Gibco, Life Technologies, USA)
decreased ratio of asymmetric division than that of controlled
group significantly (Fig. 3D,
upper), and to the contrary, increased the ratio of symmetric
division (Fig. 3D, lower). The
manner of deregulated division contributed to sphere number
increasing (Fig. 3E).
Delivering nanoliposome of Let-7b
promotes asymmetric division of cancer stem-like cells
Let-7 family of miRNAs includes of
Let-7a/b/c/d/e/f/g/I and miRNA-98, and Let-7b was selected as the
candidate for its stably inhibitive function after we deeply
explored their roles in multiple malignancies. For the first time,
we tentatively used the nanoliposome based Let-7 which is closer to
the clinical application and has not been explored. The delivery of
nanoliposomal Let-7b attenuated the Wnt activator induction of
self-renewal (Fig. 4A). In groups
treated with either fluorouracil or docetaxel, Let-7b counteracted
the stemness enrichment of chemotherapeutic agents, as was shown in
Fig. 4B (ECA-109) and Fig. 4C (ECA-9706). Representative images
are shown in Fig. 4D–F.
Regulatory feedback loop of Let-7 and Wnt
signaling was connected through CCAT1
Either Wnt activation or therapeutic agents
stimulated the TCF-4/Wnt activation greatly, detected by luciferase
assay (Fig. 5A). Let-7 decreased
self-renewal of esophageal cancer stem-cells via direct inhibition
on TCF-4/β-catenin complex activity, and only the TCF-4 promoter
activity of wild-type (compared with mutant group) was inhibited
(Fig. 5B). Enforced Let-7b via
Nano-delivery also blocked the Wnt signaling activators (Fig. 5B). Wnt stimulators accounted for
CCAT1 overexpression, formed the Let-7/Wnt/CCAT1 signaling
(Fig. 5C). We further identified
that the TCF-4 inhibition stimulated cells to divide
asymmetrically, and Let-7b functioned through TCF-4 repression
(Fig. 5D). Wnt activators of
inhibited asymmetrically division could be reversed by Let-7b
delivery (Fig. 5D).
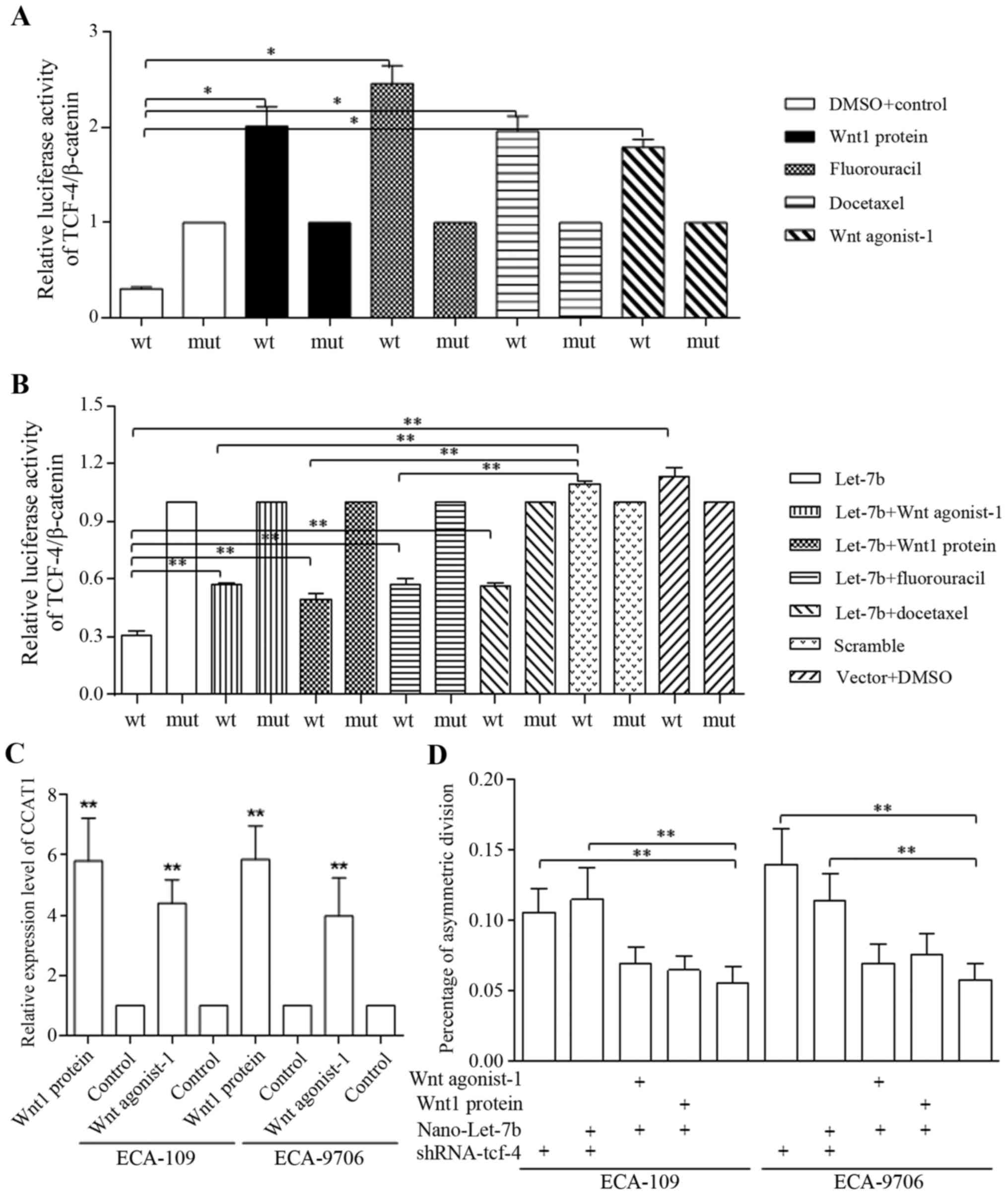 | Figure 5Nano-Let-7b promotes asymmetric
division via direct inhibition on TCF-4. (A) The addition of Wnt
agonist-1, Wnt1 protein, fluorouracil and docetaxel alone
stimulated the activation of TCF-4 promoter significantly in H293-T
cells. (B) We identified the direct repression of TCF-4 promoter
caused by nano-Let-7. Both Wnt sinaling activators of agonist and
recombinant protein, and therapeutic agents of fluorouracil and
docetaxel stimulated TCF-4 promoter functions of wild-type (wt)
could be blocked by Nano-Let-7b, compared with negative control
group of mutant-type (mut). In detail, Let-7b alone affected the
TCF-4 promoter activity effectively, and wnt signaling activators
of either Wnt agonist-1 or Wnt protein attenuated the Let-7b
functions significantly. Combined usage of therapeutic agent of
either fluorouracil or docetaxel with Let-7b increased the TCF-4
activity when compared to groups treated with Let-7b alone,
however, the TCF-4 activity when applying combination was still
significantly lower than that of controlled group (C) Wnt signaling
activation increased CCAT1 level effectively, forming the
Let-7/Wnt/CCAT1 cascade. (D) Let-7b sustained the ratio of
asymmetric division as shRNA of TCF-4 did, and counteracted the
functions of Wnt signaling activator through a TCF-4
inhibition-dependent maner. *P<0.05,
**P<0.01. |
Let-7b decreased HMGA2/Wnt signaling factors through
repressing TCF-4 activity in ECA-9706 (Fig. 6A) and ECA-109 (Fig. 6B) cells. CCAT1 overexpression
released the downstream oncogenes of CCND1 and HMGA2, which were
directly targeted and degraded by Let-7b in ECA-9706 (Fig. 6A) and ECA-109 (Fig. 6B) cells. In spheres of ECA-109
cells, Wnt signaling activators sustained HMGA2 and CCND1 level
could be equaled by the overexpression of CCAT1 (Fig. 6C). Enforced CCAT1 expression
released the downstream oncogenes of Let-7b effectively, with
Let-7b level staying stable (Fig.
6D), and further, Let-7b downstream genes were identified to be
increased with CCAT1 enforcement (Fig.
6E), which further proving the hypothesis of CCAT1 interaction
with Let-7/Wnt regulatory feedback loop. We found higher level of
miRNA sponge of CCAT1 and TCF-4 in esophageal cancer stem-like
cells (Fig. 6F), indicating the
crucial oncogenic roles in tumor formation and progression.
 | Figure 6Regulatory feedback loop of Let-7 and
Wnt signaling was connected through CCAT1. Wnt signaling factors
including β-catenin, HMGA2, CCND1 and TCF-4, and Let-7b decreased
HMGA2/Wnt signaling factors through repressing TCF-4 activity in
ECA-9706 (A) and ECA-109 (B) cells. CCAT1 overexpression released
the downstream oncogenes inhibited by Let-7b, counteracted Let-7b
functions effectively in ECA-9706 (A) and ECA-109 (B) cells. (C) In
spheres of ECA-109 cells, Wnt signaling activators sustained HMGA2
and CCND1 level equally by the overexpression of CCAT1. The
expression of CCAT1, Let-7b, CCND1 and TCF-4 was detected by
qRT-PCR. CCAT1 overexpression (D, left) did not alternate Let-7b
level (D, right). Enforced CCAT1 increased CCND1 (E, left) and
TCF-4 (E, right) expression significantly. (F) The cancer stem-like
cells derived from spheres exhibited higher expression levels of
CCAT1 and TCF-4, compared to that of adjacent cells.
*P<0.05, **P<0.01. |
Discussion
Esophageal carcinoma is one of the lethal
malignancies, especially in East Asia and China. Due to its
specific biological location and inevitable surgery wound, patients
diagnosed suffered greatly from preclinical malaise, dysphagia,
malnutrition and slowing postoperative recovery. Apart from the
above, poorer prognosis and recurrence are the main obstacles in
treatment of esophageal cancer. Cancer possesses mutations that
impair the capacity of normal cells responding to the signals that
regulate proliferation. However, the theory of CSCs reversed this
opinion, meaning that cancer could arise from a few cells that have
the capacity to generate the numerous different cells types in a
tumor. We previously studied the mechanisms through which the CSCs
may emerge, and paid close attention to the formation of different
cell types through ACD and SCD, which are crucial to understand
carcinogenesis from the viewpoint of stem cells (4). ACD will decrease the stem cell
population through inhibiting the self-renewal and then blocking
the proliferating rates of cancer cells. We were determined to find
new strategies and reagents to induce more ACD of cancer stem
cells, and thought that the decreased stem cell population will
inhibit malignancy and prevent tumor recurrence.
Stem cell signatures could be influenced by multiple
non-coding RNAs, which are also known as fates' determinations.
Let-7 and other suppressive miRNAs could decrease the stem cell
numbers via inhibition on self-renewal, which were confirmed in
multiple systemic malignancies. In the present study, we found that
the higher ratio of ALDH1 or CD133-positive cancer stem cells,
which was identified with higher intensity of protein level by IHC
or IF, is associated with later clinical stages and 2-year
recurrence, as was expected. Besides, Wnt signaling activation was
more frequent in later esophageal cancer. Cancer stem cells derived
from spheres naturally divided symmetrically and are therapy
resistant with lower apoptosis ratio consequently. Furthermore, we
found that Let-7b directly inhibited TCF-4/β-catenin complex
activity in a promoter alternation manner, functioning as negative
regulator of self-renewal. Wnt activation released CCAT1
overexpression and rescued the downstream oncogenes of Let-7b
effectively, with Let-7b level staying stable. We also demonstrated
that a regulatory feedback loop of Let-7 and Wnt signaling was
connected through CCAT1, indicated as Let-7/Wnt/CCAT1.
The ratio of stem cells harbored in esophageal
carcinoma indicates the prognosis of patients undergoing
esophagectomy, and neoadjuvant chemotherapy could enlarge the stem
cells pool. Let-7 promotes asymmetric division of esophageal cancer
stem cells, which resulted in stem cell renewal repression. Wnt
activation of self-renewal could be blocked by Let-7
overexpression, and Let-7 sensitization of adjuvant therapy of
fluorouracil and docetaxel was achieved through Wnt signaling
inhibition. Moreover, the interaction between non-coding genes
greatly expanded the non-coding RNAs controlling the stem cell
fate. Traditionally, invisible miRNA and lncRNA functioned through
alternating downstream effectors, however, importance of their
mutual effect was noted. The basic findings of Let-7 inhibited Wnt
signaling, the feedback loop of Let-7b/Wnt was linked via lncRNA of
CCAT-1, proving the cascade of Let-7b/Wnt/CCAT-1 signaling. The
clear focus of mechanistic regulation of Let-7 and its downstream
oncogenic signaling will help to define the prospect application of
Nano-Let-7b. Based on the novel roles of stem cell ratio and
crucial suppressive functions of Let-7, the detection and targeted
therapy of cancer stem cells will pave the way for improving
prognosis and the response of comprehensive treatment.
Acknowledgments
The authors acknowledge assistants in the Center for
Translational Medicine of The First Affiliated Hospital of Xi'an
Jiaotong University, for their technical assistance. The team
appreciate Prof. Peijun Liu for help in experiments and technique
guidance. This study was mainly supported by National Science
Foundation for Young Scientists of China, grant no. 81602597 (to
Xin Sun). This study was also supported in part by National Natural
Science Foundation of China, grant no. 81272418 (to Hong Ren),
Natural Science Foundation of Shaanxi Province, grant no.
2016JM8007 (to Jing Zhang), and National Science Foundation for
Young Scientists of China, grant no. 81402506 (to Sida Qin).
References
|
1
|
Rustgi AK and El-Serag HB: Esophageal
carcinoma. N Engl J Med. 371:2499–2509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song Y, Li L, Ou Y, Gao Z, Li E, Li X,
Zhang W, Wang J, Xu L, Zhou Y, et al: Identification of genomic
alterations in oesophageal squamous cell cancer. Nature. 509:91–95.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sun X, Liu J, Xu C, Tang SC and Ren H: The
insights of Let-7 miRNAs in oncogenesis and stem cell potency. J
Cell Mol Med. 20:1779–1788. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun X, Jiao X, Pestell TG, Fan C, Qin S,
Mirabelli E, Ren H and Pestell RG: MicroRNAs and cancer stem cells:
The sword and the shield. Oncogene. 33:4967–4977. 2014. View Article : Google Scholar
|
|
6
|
Wan L, Zhang L, Fan K, Cheng ZX, Sun QC
and Wang JJ: Circular RNA-ITCH suppresses lung cancer proliferation
via inhibiting the Wnt/β-catenin pathway. BioMed Res Int.
2016:15794902016. View Article : Google Scholar
|
|
7
|
Blagodatski A, Poteryaev D and Katanaev
VL: Targeting the Wnt pathways for therapies. Mol Cell Ther.
2:282014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dhar SK, Tangpong J, Chaiswing L, Oberley
TD and St Clair DK: Manganese superoxide dismutase is a
p53-regulated gene that switches cancers between early and advanced
stages. Cancer Res. 71:6684–6695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirsch HA, Iliopoulos D and Struhl K:
Metformin inhibits the inflammatory response associated with
cellular transformation and cancer stem cell growth. Proc Natl Acad
Sci USA. 110:972–977. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhou D, Shao L and Spitz DR: Reactive
oxygen species in normal and tumor stem cells. Adv Cancer Res.
122:1–67. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Printz C: Radiation treatment generates
therapy-resistant cancer stem cells from less aggressive breast
cancer cells. Cancer. 118:3225. 2012.PubMed/NCBI
|
|
12
|
Deng L, Yang S-B, Xu F-F and Zhang J-H:
Long noncoding RNA CCAT1 promotes hepatocellular carcinoma
progression by functioning as let-7 sponge. J Exp Clin Cancer Res.
34:182015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun X, Xu C, Tang SC, Wang J, Wang H, Wang
P, Du N, Qin S, Li G, Xu S, et al: Let-7c blocks estrogen-activated
Wnt signaling in induction of self-renewal of breast cancer stem
cells. Cancer Gene Ther. 23:83–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beck B and Blanpain C: Unravelling cancer
stem cell potential. Nat Rev Cancer. 13:727–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Taylor MD, Poppleton H, Fuller C, Su X,
Liu Y, Jensen P, Magdaleno S, Dalton J, Calabrese C, Board J, et
al: Radial glia cells are candidate stem cells of ependymoma.
Cancer Cell. 8:323–335. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Atkinson RL, Yang WT, Rosen DG, Landis MD,
Wong H, Lewis MT, Creighton CJ, Sexton KR, Hilsenbeck SG, Sahin AA,
et al: Cancer stem cell markers are enriched in normal tissue
adjacent to triple negative breast cancer and inversely correlated
with DNA repair deficiency. Breast Cancer Res. 15:R772013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hang D, Dong HC, Ning T, Dong B, Hou DL
and Xu WG: Prognostic value of the stem cell markers CD133 and
ABCG2 expression in esophageal squamous cell carcinoma. Dis
Esophagus. 25:638–644. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu Y, Luo M, Brooks M, Clouthier SG and
Wicha MS: Biological and clinical significance of cancer stem cell
plasticity. Clin Transl Med. 3:322014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Molavi O, Su M and Lai R: The
clinical and biological significance of STAT1 in esophageal
squamous cell carcinoma. BMC Cancer. 14:7912014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang G, Ma L, Xie YK, Miao XB and Jin C:
Esophageal cancer tumorspheres involve cancer stem-like populations
with elevated aldehyde dehydrogenase enzymatic activity. Mol Med
Rep. 6:519–524. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang L, Ren Y, Yu X, Qian F, Bian BS, Xiao
HL, Wang WG, Xu SL, Yang J, Cui W, et al: ALDH1A1 defines invasive
cancer stem-like cells and predicts poor prognosis in patients with
esophageal squamous cell carcinoma. Mod Pathol. 27:775–783. 2014.
View Article : Google Scholar
|
|
22
|
Rodriguez-Torres M and Allan AL: Aldehyde
dehydrogenase as a marker and functional mediator of metastasis in
solid tumors. Clin Exp Metastasis. 33:97–113. 2016. View Article : Google Scholar :
|
|
23
|
Hwang C-C, Nieh S, Lai C-H, Tsai CS, Chang
LC, Hua CC, Chi WY, Chien HP, Wang CW, Chan SC, et al: A
retrospective review of the prognostic value of ALDH-1, Bmi-1 and
Nanog stem cell markers in esophageal squamous cell carcinoma. PLoS
One. 9:e1056762014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dey-Guha I, Wolfer A, Yeh AC, G Albeck J,
Darp R, Leon E, Wulfkuhle J, Petricoin EF III, Wittner BS and
Ramaswamy S: Asymmetric cancer cell division regulated by AKT. Proc
Natl Acad Sci USA. 108:12845–12850. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cicalese A, Bonizzi G, Pasi CE, Faretta M,
Ronzoni S, Giulini B, Brisken C, Minucci S, Di Fiore PP and Pelicci
PG: The tumor suppressor p53 regulates polarity of self-renewing
divisions in mammary stem cells. Cell. 138:1083–1095. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gómez-López S, Lerner RG and Petritsch C:
Asymmetric cell division of stem and progenitor cells during
homeostasis and cancer. Cell Mol Life Sci. 71:575–597. 2014.
View Article : Google Scholar :
|
|
27
|
Fürthauer M and González-Gaitán M:
Endocytosis, asymmetric cell division, stem cells and cancer: Unus
pro omnibus, omnes pro uno. Mol Oncol. 3:339–353. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
El-Hashash AHK and Warburton D: Numb
expression and asymmetric versus symmetric cell division in distal
embryonic lung epithelium. J Histochem Cytochem. 60:675–682. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kechad A, Jolicoeur C, Tufford A, Mattar
P, Chow RWY, Harris WA and Cayouette M: Numb is required for the
production of terminal asymmetric cell divisions in the developing
mouse retina. J Neurosci. 32:17197–17210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martin-Blanco NM, Checquolo S, Del Gaudio
F, Palermo R, Franciosa G, Di Marcotullio L, Gulino A, Canelles M
and Screpanti I: Numb-dependent integration of pre-TCR and p53
function in T-cell precursor development. Cell Death Dis.
5:e14722014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Brand AH: A new dawn for Aurora? Nat Cell
Biol. 10:1253–1254. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Landen CN Jr, Chavez-Reyes A, Bucana C,
Schmandt R, Deavers MT, Lopez-Berestein G and Sood AK: Therapeutic
EphA2 gene targeting in vivo using neutral liposomal small
interfering RNA delivery. Cancer Res. 65:6910–6918. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Seviour EG, Sehgal V, Mishra D, Rupaimoole
R, Rodriguez-Aguayo C, Lopez-Berestein G, Lee J-S, Sood AK, Kim MP,
Mills GB, et al: Targeting KRas-dependent tumour growth,
circulating tumour cells and metastasis in vivo by clinically
significant miR-193a-3p. Oncogene. 36:1339–1350. 2017. View Article : Google Scholar
|
|
34
|
Rupaimoole R, Ivan C, Yang D, Gharpure KM,
Wu SY, Pecot CV, Previs RA, Nagaraja AS, Armaiz-Pena GN, McGuire M,
et al: Hypoxia-upregulated microRNA-630 targets Dicer, leading to
increased tumor progression. Oncogene. 35:4312–4320. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun X, Jiang S, Liu J, Wang H, Zhang Y,
Tang SC, Wang J, Du N, Xu C, Wang C, et al: MiR-208a stimulates the
cocktail of SOX2 and β-catenin to inhibit the let-7 induction of
self-renewal repression of breast cancer stem cells and formed
miR208a/let-7 feedback loop via LIN28 and DICER1. Oncotarget.
6:32944–32954. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hwang W-L, Jiang J-K, Yang S-H, Huang TS,
Lan HY, Teng HW, Yang CY, Tsai YP, Lin CH, Wang HW, et al:
MicroRNA-146a directs the symmetric division of Snail-dominant
colorectal cancer stem cells. Nat Cell Biol. 16:268–280. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lathia JD, Hitomi M, Gallagher J, Gadani
SP, Adkins J, Vasanji A, Liu L, Eyler CE, Heddleston JM, Wu Q, et
al: Distribution of CD133 reveals glioma stem cells self-renew
through symmetric and asymmetric cell divisions. Cell Death Dis.
2:e2002011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Todaro M, Francipane MG, Medema JP and
Stassi G: Colon cancer stem cells: Promise of targeted therapy.
Gastroenterology. 138:2151–2162. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Morrison SJ and Kimble J: Asymmetric and
symmetric stem-cell divisions in development and cancer. Nature.
441:1068–1074. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Berika M, Elgayyar ME and El-Hashash AHK:
Asymmetric cell division of stem cells in the lung and other
systems. Front Cell Dev Biol. 2:332014. View Article : Google Scholar : PubMed/NCBI
|