Introduction
Lung cancer, a disease of notable global prevalence,
carries substantial morbidity and mortality rates, posing a severe
threat to human health (1-3).
Annually, ~11.6% of newly diagnosed cancer cases and 18.4% of all
cancer-related deaths are attributed to lung cancer (2,3).
This disease is primarily categorized into small cell lung cancer
and non-small cell lung cancer (NSCLC), with NSCLC comprising
80-85% of all lung cancer cases (4,5).
Among these cases, >55% of patients with NSCLC are diagnosed
with advanced cancer and have a poor prognosis (6,7).
The conventional treatment regimen for NSCLC includes surgical
resection of the primary tumor or metastatic lesions, radiotherapy
and chemotherapy. However, resistance to a number of chemotherapy
drugs, such as cisplatin (DDP), can develop, affecting patient
outcomes (8,9). Although immunotherapy based on
immune checkpoint inhibitors has been widely used clinically, only
<25% of patients can achieve a durable immune response, possibly
due to the immunosuppressive state of the tumor microenvironment
(TME) (10-12). Therefore, improving the
immunosuppressive state of the TME in patients with NSCLC and
conceiving new treatment strategies have become urgent issues that
need to be addressed.
Tumor invasion and metastasis are the results of the
interaction and co-development between tumor cells and the TME
(13). Macrophages in the TME are
termed tumor-associated macrophages (TAMs) and are the main cells
in the NSCLC microenvironment, not only enhancing immune evasion of
NSCLC as immunosuppressive cells but also directly participating in
cancer progression (14-16). Studies have shown that in the
initial stages of lung cancer formation, TAMs tend to be the M1
type, while during cancer invasion and migration, macrophages
gradually polarize from M1 to M2 (14-16). Yuan et al (17) discovered that M2 macrophages
promote A549 cell invasion and the growth of xenograft tumors,
while M1 macrophages significantly reduce the expression of
fibronectin and transforming growth factor-β (TGF-β), supporting
tumor progression. M2 TAMs stimulate tumor cell invasion and
migration, correlating with unfavorable outcomes in patients with
NSCLC (18). Furthermore, TAMs
foster an anti-inflammatory milieu and fuel tumor growth by
releasing cytokines, chemokines, matrix metalloproteinases (MMPs),
growth factors and other inflammatory agents (19). Notably, interleukin (IL)-10,
platelet-derived growth factor and vascular endothelial growth
factor (VEGF) have pivotal roles in NSCLC advancement (20). VEGF not only influences tumor
migration and angiogenesis but fosters tumor progression through
autocrine or paracrine signaling pathways (20). Upregulation of IL-10 in M2 TAMs is
positively correlated with advanced NSCLC, possibly by affecting
regulatory T lymphocytes to provide an immunosuppressive
environment for tumor cells (21,22). Therefore, inhibiting macrophage
polarization towards M2 and improving the balance of M1/M2
macrophages have become important strategies for NSCLC
treatment.
Research has found that the activation of peroxisome
proliferator-activated receptor γ (PPARγ) is a key factor in the
polarization of M2 TAMs (23).
PPARγ belongs to the nuclear receptor family and exerts
anti-inflammatory effects (23),
and is the main regulatory factor for adipocyte differentiation and
function, favoring M2 phenotype activation (24). Research has found that arginase 1
(Arg-1) and macrophage galactose type C-type lectin-1 are direct
target genes of PPARγ (25). In
addition, the expression of PPARγ is induced by IL-13 and IL-4,
both of which have been shown to induce M2 polarization (26). When macrophages are treated with
PPARγ-specific agonists such as rosiglitazone (RSG), the secretion
levels of pro-inflammatory factors are reduced (22). Therefore, PPARγ activation is
crucial for M2 polarization.
HuaChanSu injection is a commonly used antitumor
drug with significant clinical efficacy and is characterized by
broad-spectrum anticancer activity, diverse clinical application
modes and favorable tolerance in patients. In recent years, it has
been widely used to treat various malignant tumors such as liver
cancer, lung cancer, digestive tract malignant tumors, breast
cancer and cervical cancer (27-29). A study has demonstrated that
HuaChanSu injection combined with DDP significantly enhances
efficacy in the treatment of advanced NSCLC, resulting in an
improved patient quality of life and fewer adverse reactions
(30). As the main active
ingredient of HuaChanSu injection, cinobufagin (CB) is a
bufadienolide steroid similar to digoxin, which also exerts
antitumor activity (31).
Research has found that CB can inhibit STAT3 phosphorylation and
block the IL-6-induced nuclear translocation of STAT3, inhibiting
epithelial-mesenchymal transition (EMT) of colorectal cancer (CRC)
cells, thereby inhibiting the invasion and migration of CRC
(32). In osteosarcoma, CB can
enhance the transcription of the downstream genes of Forkhead Box
Protein O1 (FOXO1), such as Fc fragment of IgG binding protein,
regulating the expression of E-cadherin, transcription factor
twist1, vimentin and MMP9 in ectopic implants, thereby inhibiting
osteosarcoma progression (33).
In NSCLC, CB inhibits A549 cell progression by upregulating FOXO1
and downregulating histone methyltransferase G9a (34). However, there is limited research
on the inhibition of lung cancer progression by CB, and its
specific mechanism of action requires further validation.
Therefore, based on the notable clinical antitumor efficacy of
HuaChanSu injection (29), the
present study aimed to reveal the influence and mechanism of action
of its effective active ingredient, CB, on the polarization of TAMs
and on NSCLC progression, to provide a theoretical basis for
further exploring the pharmacological effects and clinical
applications of CB.
Materials and methods
Cell lines
The THP-1 human monocytic leukemia cell line (cat.
no. FH0112) was procured from Shanghai Fuheng Biotechnology Co.,
Ltd., while the A549 human lung adenocarcinoma cell line (cat. no.
CCL-185), LLC (cat. no. CRL-1642) and BEAS-2B human normal lung
epithelial cells (cat. no. CRL-3588), human umbilical vein
endothelial cells (HUVECs; cat. no. CRL-1730) were obtained from
the American Type Culture Collection. Upon revival, the THP-1,
BEAS-2B, A549 HUVEC and LLC cells were cultured in RPMI-1640 medium
(Beijing Solarbio Science & Technology Co., Ltd.) supplemented
with 10% fetal bovine serum (FBS; cat. no. 164210; Wuhan PuNuoSai
Biotech Co., Ltd.) and maintained in a 37°C and 5% CO2
incubator. Upon reaching 80-90% confluency, adherent cells were
detached using 0.25% EDTA-trypsin or directly pipetted, while
suspended cells were collected by centrifugation at 4°C and 1,200 x
g for 5 min. Cells in the logarithmic growth phase were utilized
for subsequent experiments.
Establishment of M1/M2 macrophage
models
The establishment of M1/M2 macrophage models was
performed according to previous studies (35,36). THP-1 cells into M0 macrophages
were induced using the widely employed phorbol 12-myristate
13-acetate (PMA; cat. no. P1585; Sigma-Aldrich; Merck KGaA), which
was followed by polarization into the M1 or M2 macrophage
phenotypes using interferon-γ (IFN-γ) or IL-4 (PeproTech China),
respectively. For the generation of M0 macrophages, THP-1 cells in
the logarithmic growth phase were centrifuged at 4°C and 1,200 x g
for 5 min, the supernatant was aspirated and the cells were
resuspended in RPMI-1640 medium supplemented with PMA (200 ng/ml)
for 24 h. Subsequently, the cell concentration was adjusted to
1×106 cells/ml before seeding into a 6-well plate. For
the generation of M1 macrophages, the M0 macrophages were incubated
in RPMI-1640 complete medium supplemented with a IFN-γ (20 ng/ml)
for 24 h. Similarly, to induce the generation of M2 macrophages, M0
macrophages were cultured in RPMI-1640 complete medium supplemented
with IL-4 (20 ng/ml) for 24 h.
Preparation of conditioned medium
Culture medium from the 6-well plate containing
macrophages in various polarization states was aspirated, and the
plate was washed twice with phosphate-buffered saline (PBS).
Subsequently, the macrophages were incubated in serum-free
RPMI-1640 medium at 37°C with 5% CO2 for 24 h. Following
this incubation period, the culture medium was harvested in a 5-ml
sterile Eppendorf tube and centrifuged at 4°C and 13,800 x g for 15
min. The resulting supernatant, designated as the conditioned
medium, was carefully transferred to another 5-ml sterile Eppendorf
tube and stored at −80°C for future use.
Treatments
For the CB treatment, macrophages were exposed to
various concentrations of CB (Sigma-Aldrich; Merck KGaA; 10, 15,
20, 25, 30, 35, 40 and 50 ng/ml) for 24 h before being prepared for
subsequent experiments. Similarly, for RSG treatment, macrophages
were exposed to various concentrations of RSG (Sigma-Aldrich; Merck
KGaA; 0.1, 0.5, 1, 5 and 10 μM) for 24 h before being
prepared for further experiments. In certain investigations,
macrophages were exposed to conditioned medium from M0, M1 or M2
macrophages for 24 h.
MTT assay
M0 macrophages and BEAS-2B, A549 and LLC cells were
prepared as single-cell suspensions. After counting, the cells were
seeded into a 96-well culture plate at a density of
1×104 cells in 200 μl of medium per well, with 6
replicate wells per group. The next day, after cell adherence,
various concentrations of CB or RSG were added to the wells for
intervention, in which the control group was treated with an
equivalent dose of DMSO (not exceeding 0.1% of the maximum dose).
Following a 24-h incubation, 20 μl of 5 mg/ml MTT solution
(Beijing Solarbio Science & Technology Co., Ltd.) was added to
each well, and the plate was further incubated for ~4 h.
Subsequently, 150 μl DMSO solution was added to each well
and was used to dissolve the purple formazan, and the plate was
shaken at room temperature for 15-20 min. The absorbance (A) values
of each well were measured at 490 nm, with blank wells set to 0.
The cell viability was then calculated using the following formula:
Cell viability (%)=(experimental group A value/control group A
value) ×100%.
Flow cytometry
The culture medium from the 6-well plate containing
macrophages in different polarization states was aspirated. The
plate was then washed with PBS, which was repeated twice. The cells
were scraped off using a cell scraper, repeating this step twice,
and collected in a centrifuge tube. The collected cells were gently
pipetted repeatedly and then centrifuged at 1,200 x g for 5 min at
room temperature. After centrifugation, the cells were resuspended
in PBS at a final concentration of 1×105 cells/ml, then
non-specific antigens were blocked using 5% BSA buffer
(MilliporeSigma) for 20 min at room temperature. For intracellular
CD86 staining, fixation in 4% paraformaldehyde for 20 min at room
temperature and permeabilization with 0.1% Triton X-100 were
performed prior to staining. The cells were then stained with
monoclonal mouse anti-human CD86 (1:50; cat. no. PE-65155) and
CD206 (1:100; cat. no. FITC-65165) antibodies (both from
Proteintech Group, Inc.) for 30 min at 4oC in the dark.
Subsequently, the cells were analyzed using a BD FACSCanto II flow
cytometer (BD Biosciences) and the FlowJo 10 software (BD
Biosciences). A non-specific mouse Ig was used as an isotype
control.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA in cells was extracted using the TRIzol
method (Thermo Fisher Scientific, Inc.). The total RNA
concentration of each sample was measured using a NanoDrop-2000 UV
spectrophotometer. RT was performed using the RevertAid First
Strand cDNA Synthesis Kit (cat. no. K1621; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol and qPCR
was conducted using the PowerUp™ SYBR™ Green Master Mix (cat. no.
A25743; Thermo Fisher Scientific, Inc.). PCR thermocycling
conditions were as follows: Denaturation at 94°C for 120 sec,
followed by 40 cycles consisting of melting (95°C; 15 sec) and
annealing/extension (60°C; 60 sec) phases. GAPDH was used as an
internal reference to calculate the relative transcription level of
the target gene by the 2−ΔΔCq method (37). The primer sequences are shown in
Table I.
 | Table ISequences of primers used for reverse
transcription-quantitative PCR. |
Table I
Sequences of primers used for reverse
transcription-quantitative PCR.
| Gene name | Primer sequence
(5′-3′) |
|---|
| CD68 | F:
GGAAATGCCACGGTTCATCCA |
| R:
TGGGGTTCAGTACAGAGATGC |
| CD206 | F:
CTACAAGGGATCGGGTTTATGGA |
| R:
TTGGCATTGCCTAGTAGCGTA |
| IL-10 | F:
GGTTGCCAAGCCTTGTCTGA |
| R:
AGGGAGTTCACATGCGCCT |
| MARCO | F:
CAGCGGGTAGACAACTTCACT |
| R:
TTGCTCCATCTCGTCCCATAG |
| Arg-1 | F:
CTGTGGGAAAAGCAAGCGAG |
| R:
CATGGCCAGAGATGCTTCCA |
| TNF-α | F:
CCTCTCTCTAATCAGCCCTCTG |
| R:
GAGGACCTGGGAGTAGATGAG |
| IL-1β | F:
ATGATGGCTTATTACAGTGGCAA |
| R:
GTCGGAGATTCGTAGCTGGA |
| IL-6 | F:
ACTCACCTCTTCAGAACGAATTG |
| R:
CCATCTTTGGAAGGTTCAGGTTG |
| PPARγ | F:
TTCAGAAATGCCTTGCAGTG |
| R:
GGGGGTGATGTGTTTGAACT |
| GAPDH | F:
TCCAAAATCAAGTGGGGCGA |
| R:
AGTAGAGGCAGGGATGATGT |
Immunofluorescence (IF)
Cells were seeded at a density of 5×105
cells/ml in confocal culture dishes to prepare macrophages in
different states. The culture medium was removed and the wells were
washed twice with PBS. Next, 500 μl of 4% paraformaldehyde
was added to each well and incubated at room temperature for 30
min, then 500 μl of 0.5% Triton X-100 was added to each well
and further incubated at room temperature for 10 min. Blocking
solution was next added to the wells and incubated at room
temperature for 15 min. Then, primary antibodies against PPARγ
(1:350; cat. no. 66936-1-Ig; Proteintech Group, Inc.) and
macrophage receptor with collagenous structure (MARCO; 1:400; cat.
no. bs-2659R; BIOSS) were added to each well and incubated
overnight at 4°C. The next day, the corresponding fluorescent
secondary antibody (1:200; cat. no. A23210, Abbkine Scientific Co.,
Ltd.) was added to the wells and incubated at room temperature for
1 h. DAPI (10 μg/ml) staining was performed for 5 min at
room temperature in the dark, followed by imaging using a laser
confocal microscope.
ELISA
Protein levels of tumor necrosis factor (TNF)-α
(cat. no. E-EL-H0109c; Elabscience Biotechnology Co., Ltd.), IL-6
(cat. no. E-EL-H0102c; Elabscience Biotechnology Co., Ltd.), IL-1β
(cat. no. E-EL-H0149c; Elabscience Biotechnology Co., Ltd.), TGF-β
(cat. no. MM-1774H1; Jiangsu Meimian Industrial Co., Ltd.) and
IL-10 (cat. no. MM-0066H1; Jiangsu Meimian Industrial Co., Ltd.)
were determined by respective ELISA assay kits.
Wound healing
Cells were seeded into a 6-well plate at a density
of 8×105 cells/ml. The macrophage-conditioned medium was
thawed and mixed with RPMI-1640 medium with 1% FBS. After the cells
had adhered to the plate and the cell confluency was >90%, a
vertical scratch was made in the center of each well using a 200
μl pipette tip. The culture medium was then removed, the
scratched cells were washed with sterile PBS to remove non-adherent
cells and fresh mixed culture medium was added to the plate. The
6-well plate was placed horizontally in a 5% CO2 and
37°C incubator. After 24 h, images were collected under a light
microscope, and the results were measured and analyzed using ImageJ
software version 1.48 (National Institutes of Health). Wound
healing rate=(0 h scratch width-24 h scratch width)/0 h scratch
width ×100%.
Cell invasion
The cell invasion assay was performed using a 6-well
8-μm Transwell chamber. For this, Matrigel was diluted with
ice-cooled serum-free medium, and 150 μl of the matrix gel
was added to each well. The 6-well plate was then placed in a cell
culture incubator at 37°C with 5% CO2 for 30 min. The
6-well plate was then placed in a cell culture incubator with 5%
CO2 at 37°C for 30 min. Next, 2 ml A549 or LLC cell
suspension (5×105 cells/ml) was added to the upper
chamber, and 2 ml macrophage-conditioned medium was added to the
lower chamber. After incubation for 48 h, the cells in the upper
chamber were wiped off with a cotton swab, and the invasive cells
were fixed with 4% paraformaldehyde at room temperature for 30 min,
washed with PBS, stained with 0.1% crystal violet solution for 30
min at room temperature, and then observed and imaged under a light
microscope. Cell counting was subsequently performed using
ImageJ.
Western blotting
The culture medium was aspirated and the wells were
washed twice with PBS. The 6-well plate was then transferred onto
ice and 250 μl pre-prepared RIPA lysis buffer (cat. no.
R0010; Beijing Solarbio Science & Technology Co., Ltd.)
containing protease and phosphatase inhibitors (cat. no. P0100;
Beijing Solarbio Science & Technology Co., Ltd.) was added to
each well. The plate was further cooled on ice and the cells lysed
for 30 min. The lysate was then transferred to a 1.5-ml sterile
Eppendorf tube and centrifuged at 4°C and 13,800 g for 15 min. The
protein concentration was determined using the BCA method and the
proteins were then denatured. Subsequently, protein samples (50
μg for each lane) were separated on 10% SDS-polyacrylamide
gels, followed by transferring onto PVDF membranes
(MilliporeSigma). The PVDF membranes were blocked with 5% BSA
(MilliporeSigma) for 2 h at room temperature. After that, primary
antibodies against Arg-1 (1:1,000; cat. no. bs-23837R; BIOSS),
inducible nitric oxide synthase (iNOS; 1:1,000; cat. no. bs-0162R;
BIOSS); phosphorylated (p)-PPARγ (1:1,000; cat. no. bs-3737R;
BIOSS), vimentin (1:1,000; cat. no. bsm-33170M; BIOSS), E-cadherin
(1:1,000; cat. no. bs-1519R; BIOSS), N-cadherin (1:1,000; cat. no.
bs-20623R; BIOSS) and GAPDH (1:3,000; cat. no. 60004-1-Ig;
Proteintech Group, Inc.) were incubated with the membrane overnight
at 4°C, followed by washing with Tris-buffered saline containing
0.05% Tween 20 and incubation with horseradish
peroxidase-conjugated secondary antibodies goat anti-mouse IgG
(1:10,000; cat. no. SA00001-1) and goat anti-rabbit IgG (1:10,000;
SA00001-2; both from Proteintech Group, Inc.) at room temperature
for 1 h. The western blot bands were visualized using an ECL kit
(cat. no. PE0010; Beijing Solarbio Science & Technology Co.,
Ltd.). Densitometric analysis was performed using ImageJ software
version 1.48 (National Institutes of Health).
Tube formation assay
For the preparation of conditioned medium,
macrophages were treated with the aforementioned drugs, the
drug-containing supernatant was removed and the cells were cultured
for 24 h. The supernatant was then collected as conditioned medium
for culturing cancer cells (A549 and LLC) for 24 h. After removing
the supernatant, fresh culture medium was added to the cancer cells
and the culturing was continued for another 24 h. The supernatant
was then collected as conditioned medium for culturing HUVECs for
24 h. Matrigel Basement Membrane Matrix (BD Biosciences) was
diluted with EBM-2 medium (Thermo Fisher Scientific, Inc.) and used
to coat 24-well plates at 37°C for 1 h. Subsequently,
5×104 HUVECs were treated with different conditioned
media from the A549 or LLC cells for 24 h. The tube formation
ability of HUVECs was then assessed by quantifying the number of
meshes, tube length and number of branches.
In vivo tumor growth
All animal studies were conducted following
protocols approved by the Animal Ethics Committee of Henan
University of Chinese Medicine (Zhengzhou, China; approval no.
IACUC-202302006). In this experiment, 18 6-8-week-old male BALB/c
nude mice (18-21 g) were randomly assigned to 3 groups (n=6 per
group) and housed in standard cages at 20-24°C with a 12/12-h
light-dark cycle and ad libitum access to food and water. A
100-μl PBS suspension containing 5×106 LLC cells
was subcutaneously injected into the right flank of each mouse. To
assess the effect of the LLC cells, data were collected every 5
days starting 4-5 days after tumor cell inoculation, which included
animal weight and tumor dimension measurements. The mice were
continuously fed for 25 days and received intraperitoneal
injections of CB (10 mg/kg/day) (38,39) or DDP (2 mg/kg/day). For
subcutaneous tumors, the maximum allowable diameter was 20 mm for
each mouse. The tumor size was checked every other 1-2 days. The
biggest tumor volume in the present study was 2,664.12
mm3. At the end of the experiments, the mice were
euthanized using CO2 (30-70% volume displacement of the
chamber air per min) followed by cervical dislocation as a
secondary method of euthanasia, in accordance with the approved
protocol of the Experimental Animal Ethics Committee. The tumors
from all animals were collected for subsequent analysis.
Statistical analysis
Experimental data were analyzed using GraphPad Prism
8.0 (Dotmatics) statistical software and are presented as the mean
± standard deviation. Normal distribution of the data was assessed
by the Kolmogorov-Smirnov test. The unpaired Student's t-test was
used for the comparison of differences between two groups, and
one-way ANOVA followed by Bonferroni's multiple comparison tests
was used for the comparison of differences among >2 groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
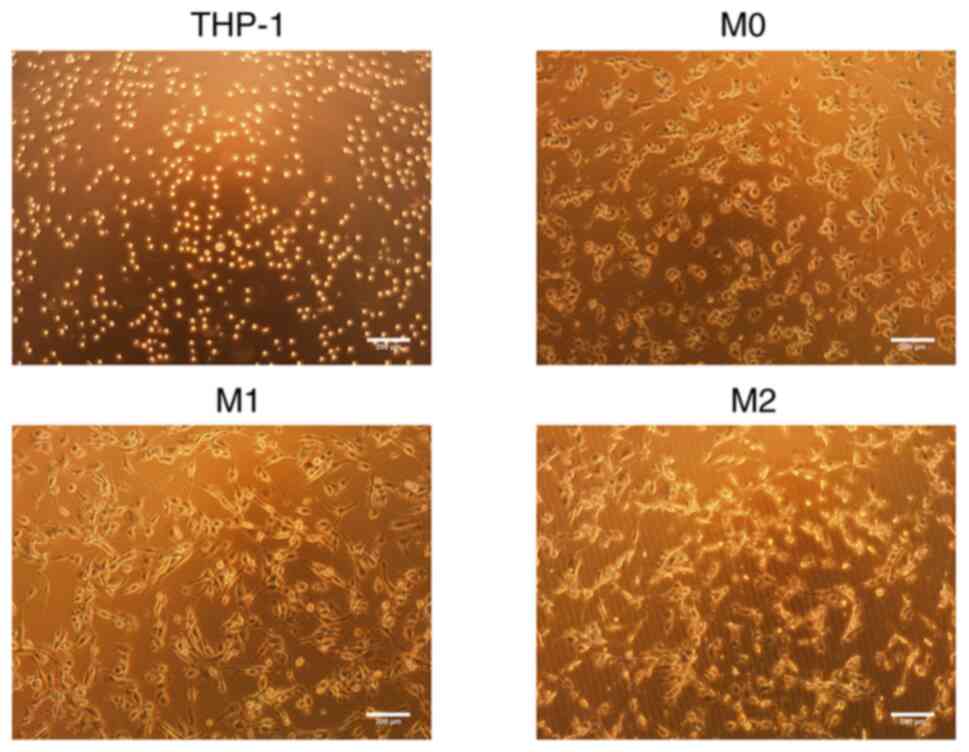
Morphology of the THP-1 cells and M0, M1
and M2 macrophages
The THP-1 cells appeared round and proliferated in
suspension. Following induction with PMA for 24 h, the THP-1 cells
underwent differentiation into M0 macrophages, which was
accompanied by observable morphological changes. These changes
included an increase in cell volume, resulting in an oval-shaped
appearance, with a few fibroblast-like pseudopodia extending from
the periphery. The cells also began to adhere to a surface. Upon
the subsequent induction with IFN-γ, the M1 macrophages exhibited
further changes, including an increased volume, enhanced surface
adherence and an elongated morphology. Conversely, upon induction
with IL-4, the M2 macrophages displayed an increase in volume,
maintained an oval-shaped morphology, exhibited extending
pseudopodia and tended to aggregate for growth (Fig. 1).
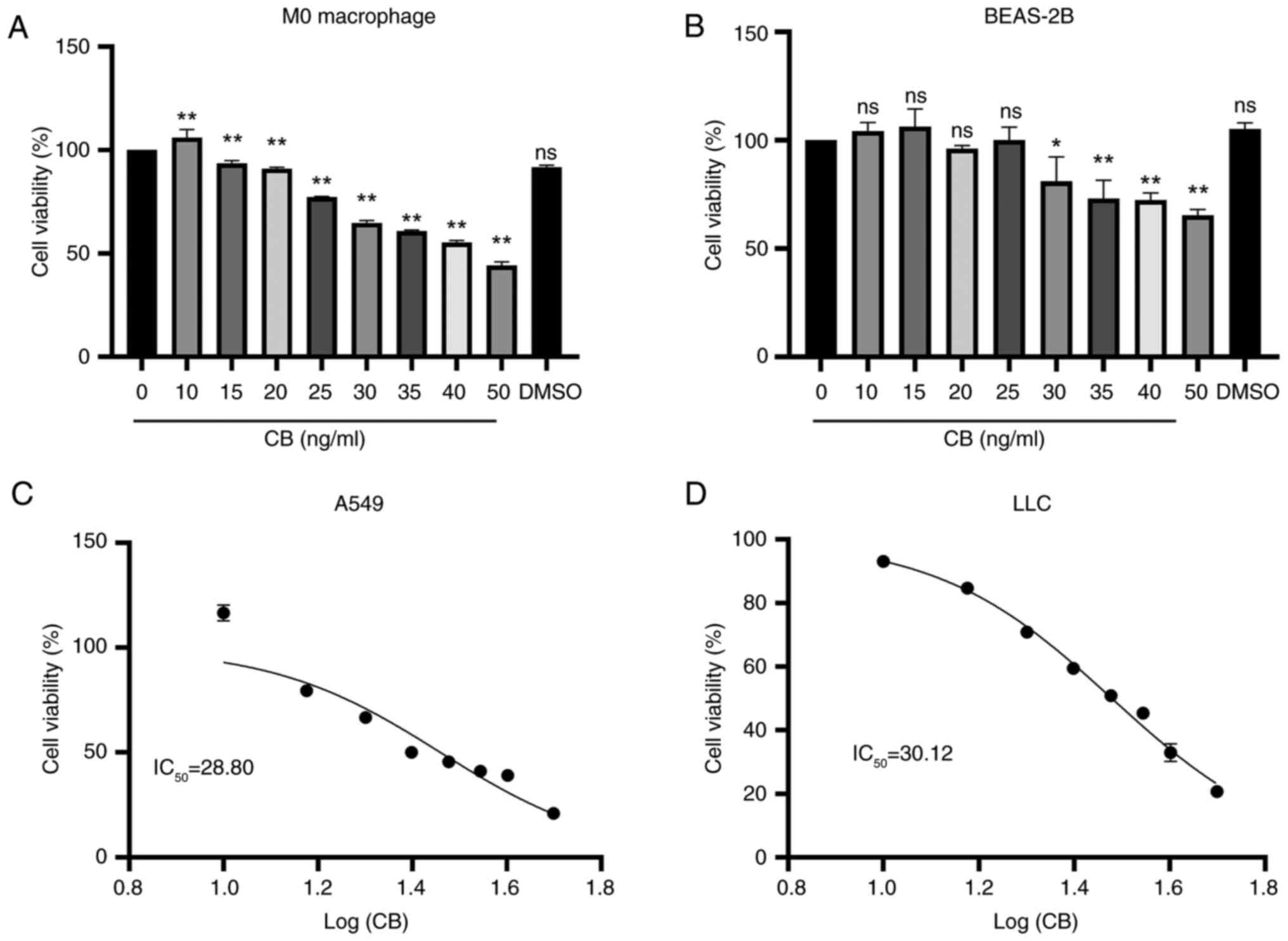
Effects of CB on the viability of M0
macrophages, BEAS-2B cells and lung cancer cells
The M0 macrophages were exposed to varying
concentrations of CB, resulting in a concentration-dependent
decrease in cell viability (Fig.
2A). Similarly, treatment with CB for 24 h resulted in a
decrease in normal lung epithelial cell (BEAS-2B) viability
starting at a concentration of 30 ng/ml (Fig. 2B). Additionally, CB exhibited a
concentration-dependent reduction in the viability of A549 and LLC
cells, with a calculated IC50 of 28.80 and 30.12 ng/ml,
respectively (Fig. 2C and D). CB
treatment inhibited the viability of A549 and LLC cells in a
concentration-dependent manner, while concentrations below 30 ng/ml
had no impact on the viability of M0 macrophages and BEAS-2B lung
epithelial cells.
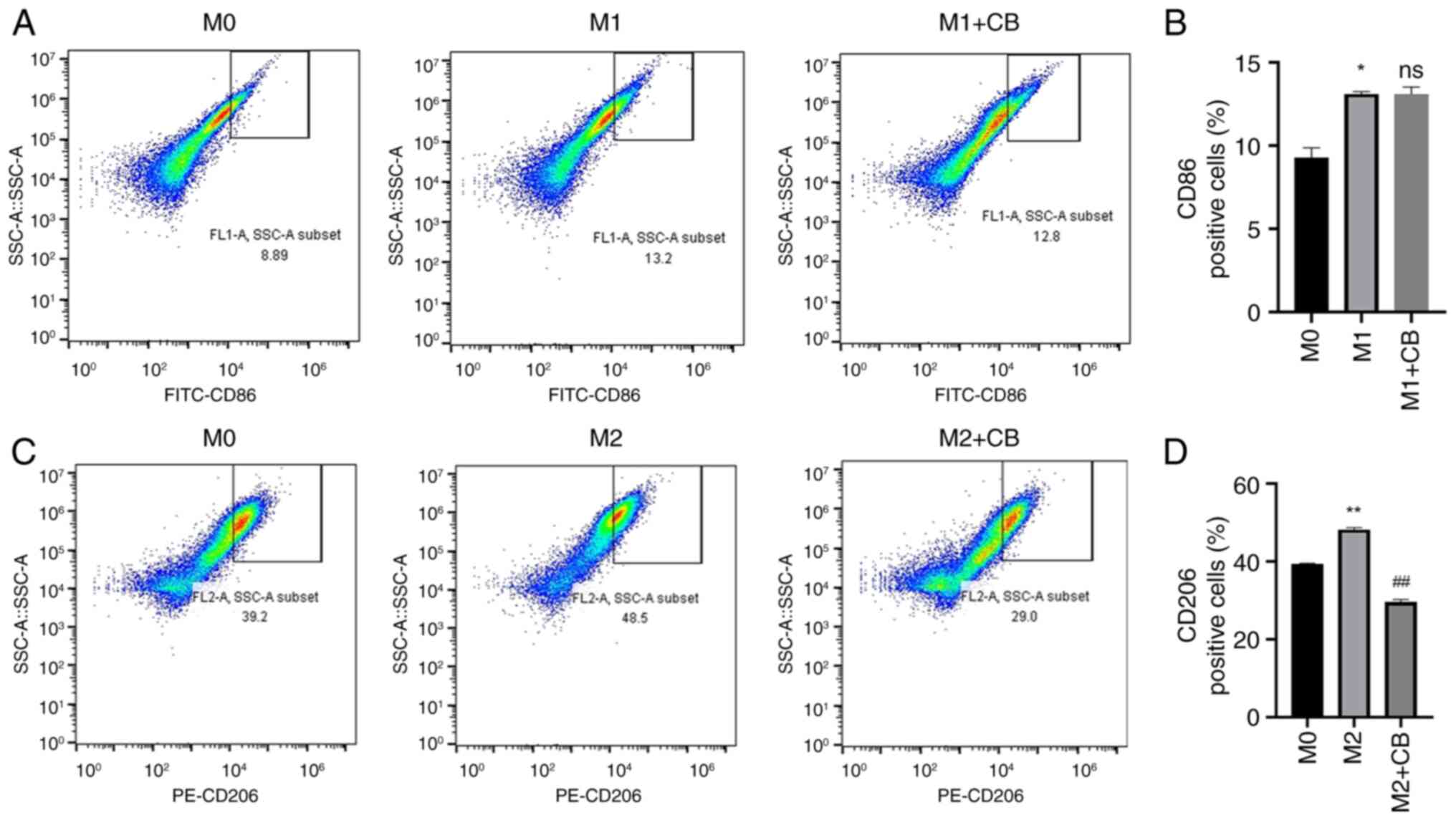
Effect of CB on macrophage surface
markers under different polarization states
THP-1 monocytes differentiate into macrophages when
induced by PMA, leading to changes in cell morphology and surface
markers (40). Therefore,
macrophage surface markers were assessed using flow cytometry in
the present study. The findings revealed a higher percentage of
CD86+ cells in M1 macrophages compared with M0
macrophages, and CB treatment did not influence the percentage of
CD86+ cells in the M1 macrophages (Fig. 3A and B). Furthermore, the
percentage of CD206+ cells was higher in M2 macrophages
compared with M0 macrophages. Nevertheless, CB treatment
significantly reduced the percentage of CD206+ cells in
the M2 macrophages (Fig. 3C and
D). The findings indicated that THP-1 monocytes, upon
differentiation into macrophages via PMA induction, exhibit
specific changes in surface markers.
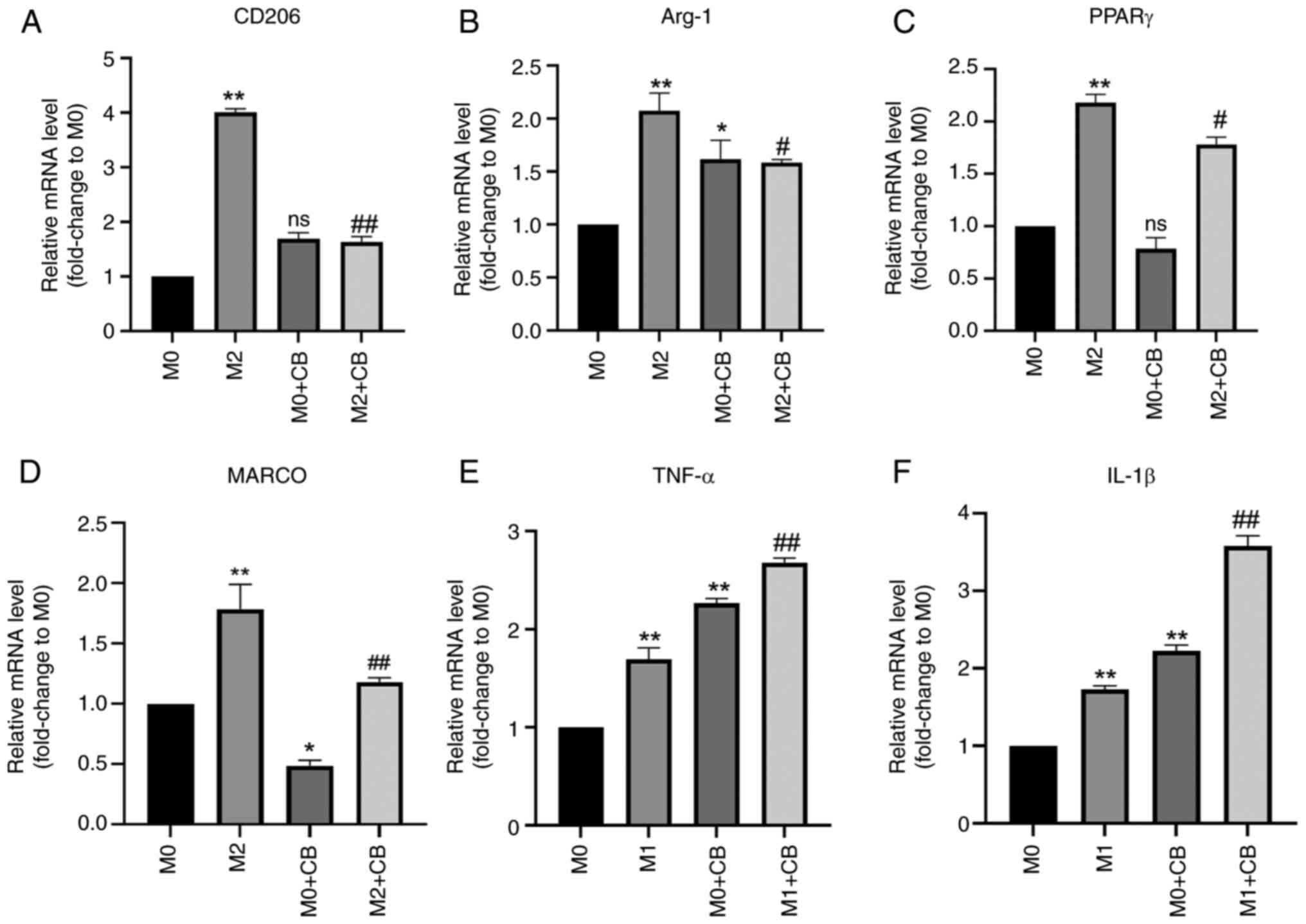
Effect of CB on the mRNA expression of
macrophage-related factors in different polarization states
The RT-qPCR results revealed significantly higher
levels of CD206, Arg-1 and PPARγ mRNA expression in M2 macrophages
compared with M0 macrophages (Fig.
4A-C). CB treatment failed to affect the CD206 and PPARγ levels
in M0 macrophages, while Arg-1 mRNA expression was upregulated in
the M0 + CB group compared with the M0 group (Fig. 4A-C). Notably, CB treatment led to
a significant downregulation of MARCO mRNA expression in M2
macrophages (Fig. 4D). Consistent
findings regarding the protein expression levels of Arg-1, PPARγ
and MARCO were detected by western blotting (Fig. S1). Additionally, the TNF-α and
IL-1β mRNA expression levels were upregulated in M1 macrophages
compared with M0 macrophages. Furthermore, CB treatment
significantly increased TNF-α and IL-1β mRNA expression in M0/M1
macrophages (Fig. 4E and F).
These results indicated that M2 macrophages had elevated CD206,
Arg-1 and PPARγ mRNA levels, which were unaffected by CB in M0
macrophages (except Arg-1 upregulation), while CB downregulated
MARCO in M2 macrophages and increased TNF-α and IL-1β mRNA in both
M0 and M1 macrophages.
Effect of CB on the protein levels of
macrophage-related factors in different polarization states
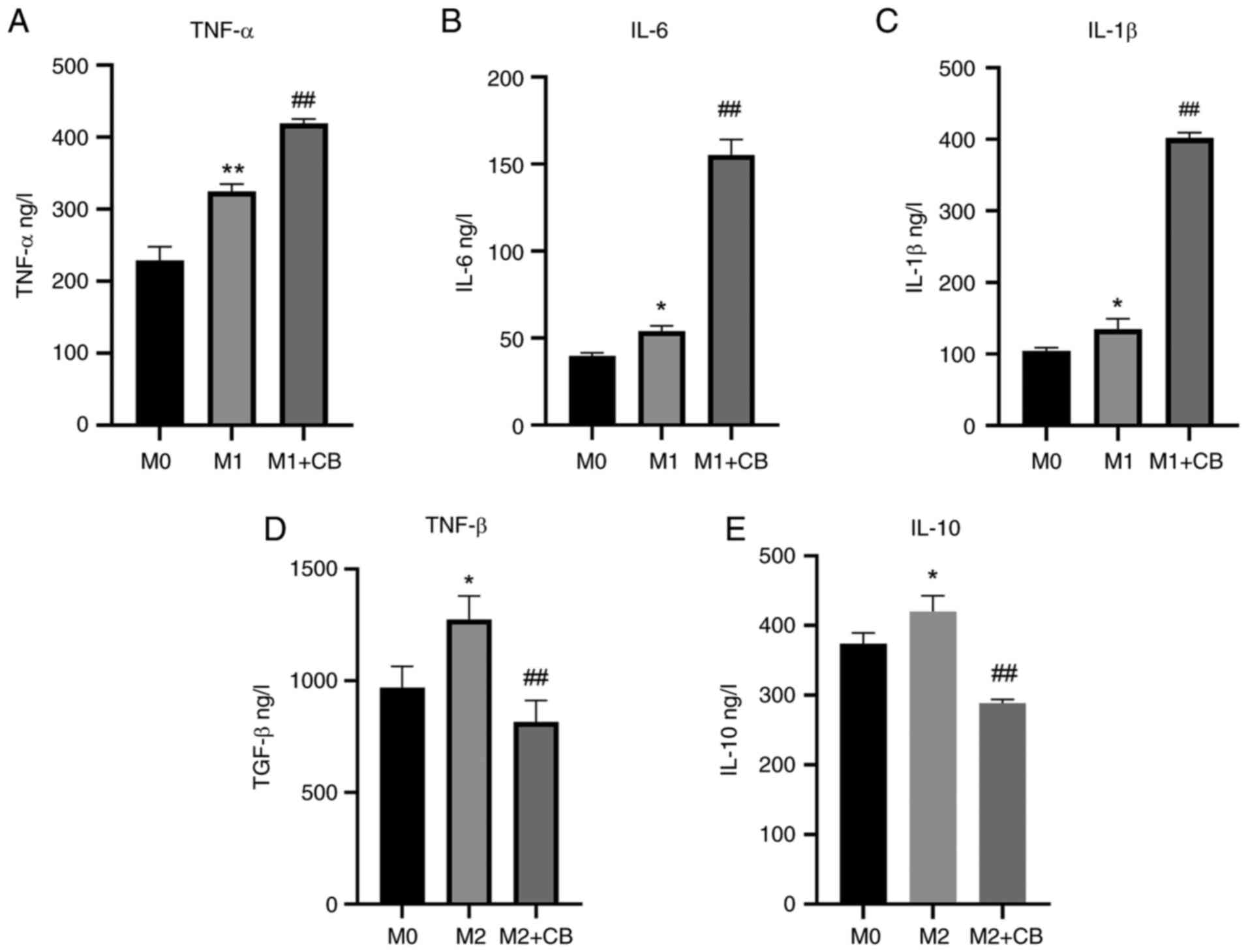
Supernatant proteins were assessed using ELISA.
There was a significant increase in the IL-6, IL-1β and TNF-α
protein levels in the supernatant of M1 macrophages compared with
M0 macrophages (Fig. 5A-C). Upon
CB treatment, there was a further augmentation in IL-6, IL-1β and
TNF-α protein levels in the supernatant of M1 macrophages (Fig. 5A-C). Additionally, the IL-10 and
TGF-β levels were higher in the supernatant of M2 macrophages
compared with M0 macrophages. However, CB treatment resulted in a
reduction in IL-10 and TGF-β protein levels in the supernatant of
M2 macrophages (Fig. 5D and E).
The results indicated that IL-6, IL-1β and TNF-α levels were
elevated in M1 macrophages and further increased with CB treatment,
while IL-10 and TGF-β levels were higher in M2 macrophages but
decreased with CB treatment.
Effect of CB-treated
macrophage-conditioned medium on the migration of A549 and LLC
cells
In the complex TME, TAMs interact with the TME,
undergo metabolic changes and alter the anticancer phenotype of
early M1-TAMs (38). Typically,
in the early stages of tumor development, M1-like TAMs predominate
the TME and inhibit tumor growth by secreting certain inflammatory
factors (39). As cancer
progresses, late-stage M2-like TAMs begin to dominate the TME,
promoting angiogenesis and aiding tumor cell dissemination and
metastasis, thus exhibiting pro-tumor effects (40). In the present study, M0
macrophage-conditioned medium treatment significantly improved the
wound healing of A549 and LLC cells compared with the control group
(Fig. 6A-D). Additionally, M2
macrophage-conditioned medium further enhanced the migratory
abilities of A549 and LLC cells compared with the M0 group
(Fig. 6A-D). However, CB-treated
M0 macrophage-conditioned medium showed no significant effect on
the migration of A549, but attenuated the migration of LLC cells
compared with the M0 group (Fig.
6A-D). Furthermore, CB-treated M2 macrophage-conditioned medium
reduced A549 and LLC cell migration compared with the M2 group
(Fig. 6A-D). The results
indicated that M0 macrophage-conditioned medium significantly
enhanced wound healing in A549 and LLC cells, with M2
macrophage-conditioned medium further boosting their migratory
abilities, while CB-treated M0 medium had no significant effect on
A549 migration but reduced LLC migration, and CB-treated M2 medium
decreased migration in both cell types.
Effect of CB-treated
macrophage-conditioned medium on A549 and LLC cell invasion
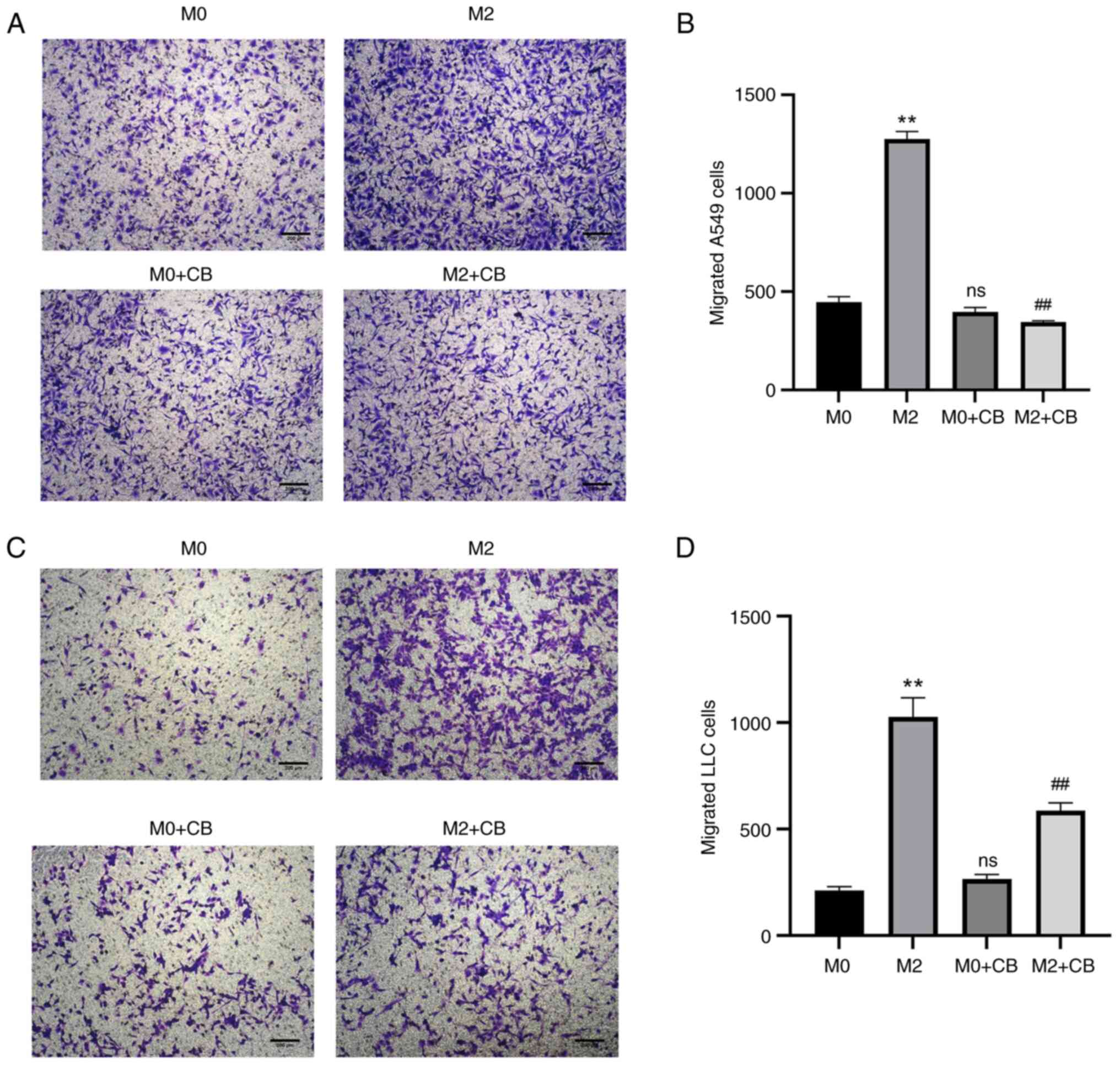
M2 macrophage-conditioned medium enhanced A549 and
LLC cell invasion compared with the M0 group (Fig. 7A-D). However, CB-treated M0
macrophage-conditioned medium showed no significant effect on A549
and LLC cell invasion compared with the M0 group (Fig. 6A-D). Furthermore, CB-treated M2
macrophage-conditioned medium reduced A549 and LLC cell invasion
compared with the M2 group (Fig.
7A-D). These results indicated that M2 macrophage-conditioned
medium enhanced A549 and LLC cell invasion, whereas CB treatment
had no significant effect on M0 macrophage-conditioned medium but
reduced invasion in CB-treated M2 macrophage-conditioned
medium.
Effects of conditioned medium from lung
cancer cells treated with macrophage-conditioned medium on
angiogenesis
First, lung cancer cells (A549 and LLC) were treated
with conditioned medium from M0, M2 or CB-treated M2 macrophages.
Then, the supernatants of the treated lung cancer cells were used
to treat HUVECs. As shown in Figs.
S2 and S3, the tube formation ability of HUVECs was
significantly enhanced in the M2 group compared with the M0 group,
and the tube formation ability was attenuated in the M2 + CB group
compared with the M2 group.
Effects of RSG on cell viability and
PPARγ and MARCO expression in macrophages with different
polarization states
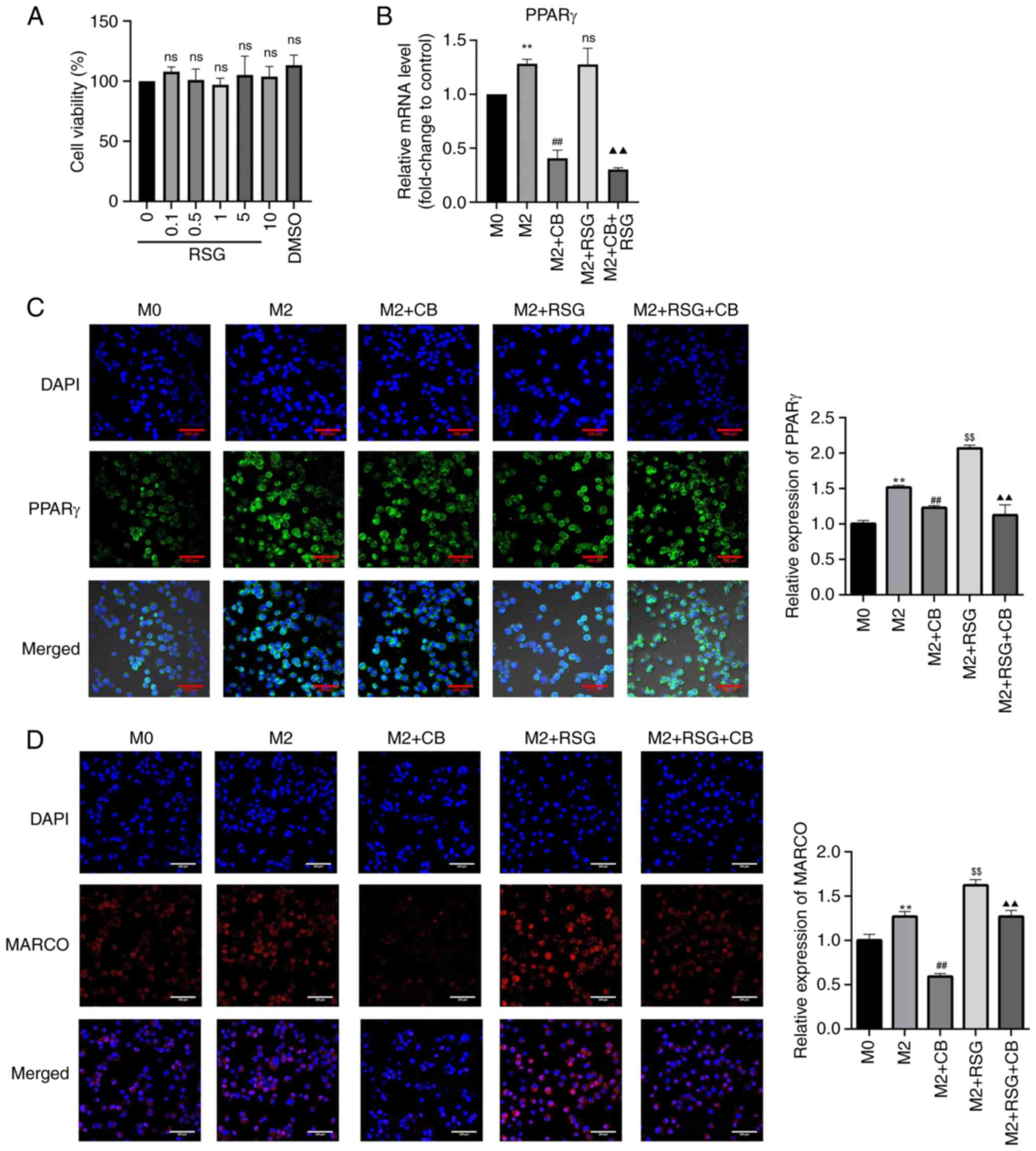
Research has found that PPARγ can regulate the
expression of the M2 macrophage-related factor, Arg-1 (25). Additionally, PPARγ can promote the
progression of colon cancer cells and the growth of mouse tumors by
affecting M2 polarization. RSG is an agonist of PPARγ. As
demonstrated in Fig. 8A, RSG
concentrations ranging from 0.1-10 μM had no effect on M0
macrophage viability. PPARγ mRNA expression in M2 macrophages was
higher than that in M0 macrophages, which was significantly
downregulated by CB treatment but not by RSG treatment (Fig. 8B). Additionally, CB treatment
downregulated PPARγ mRNA expression in RSG-treated M2 macrophages
(Fig. 8B). The nuclear
translocation of PPARγ was enhanced in M2 macrophages compared with
M0 macrophages. CB treatment decreased PPARγ expression in the
nucleus, whereas RSG treatment increased PPARγ expression in the
nucleus of M2 macrophages. Furthermore, CB treatment reversed the
RSG-induced increase in nuclear expression of PPARγ in M2
macrophages (Fig. 8C). MARCO
belongs to the class A scavenger receptor molecules. Research has
revealed that macrophages express MARCO extensively on their
surface, and MARCO+ TAMs in the TME tend to exhibit the
M2 phenotype (40). In the
present study, the expression of MARCO was elevated in M2
macrophages compared with M0 macrophages. CB treatment decreased
MARCO expression in the nucleus, while RSG treatment increased
MARCO expression in M2 macrophages. Furthermore, RSG treatment
partially restored the CB-induced decrease in MARCO expression in
M2 macrophages (Fig. 8D). These
results indicated that CB treatment significantly downregulated
PPARγ mRNA expression and nuclear translocation in M2 macrophages,
whereas RSG treatment had opposite effects, with CB reversing the
RSG-induced increase in PPARγ expression; additionally, CB reduced
MARCO expression in M2 macrophages, while RSG partially restored
CB-induced MARCO downregulation.
Effects of CB on the expression of
macrophage polarization-related proteins
As shown in Fig.
9A, the effects of CB on macrophage polarization-related
proteins were assessed. Arg-1 protein expression was upregulated in
M2 macrophages compared with M0 macrophages. CB treatment repressed
Arg-1 protein expression, whereas RSG elevated Arg-1 protein
expression in M2 macrophages (Fig. 9A
and B). Furthermore, CB treatment attenuated the RSG-induced
increase in Arg-1 protein expression in M2 macrophages (Fig. 9A and B). No difference in iNOS
protein expression between M0 and M2 macrophages was observed
(Fig. 9A-C). CB treatment
increased iNOS protein expression, while RSG had no effect on iNOS
expression in M2 macrophages (Fig. 9A
and C). Additionally, RSG treatment partially mitigated the
CB-induced increase in iNOS expression in M2 macrophages (Fig. 9A and C). p-PPARγ expression was
upregulated in M2 macrophages compared with M0 macrophages. CB
treatment repressed p-PPARγ protein expression, while RSG elevated
p-PPARγ protein expression in M2 macrophages (Fig. 9A and D). Furthermore, CB treatment
attenuated the RSG-induced increase in p-PPARγ protein expression
in M2 macrophages (Fig. 9A and
D). These results revealed that in M2 macrophages, CB treatment
decreased Arg-1 and p-PPARγ protein expression while increasing
iNOS protein expression; conversely, RSG treatment increased Arg-1
and p-PPARγ expression without affecting iNOS, with RSG partially
reversing the CB-induced effects on these proteins.
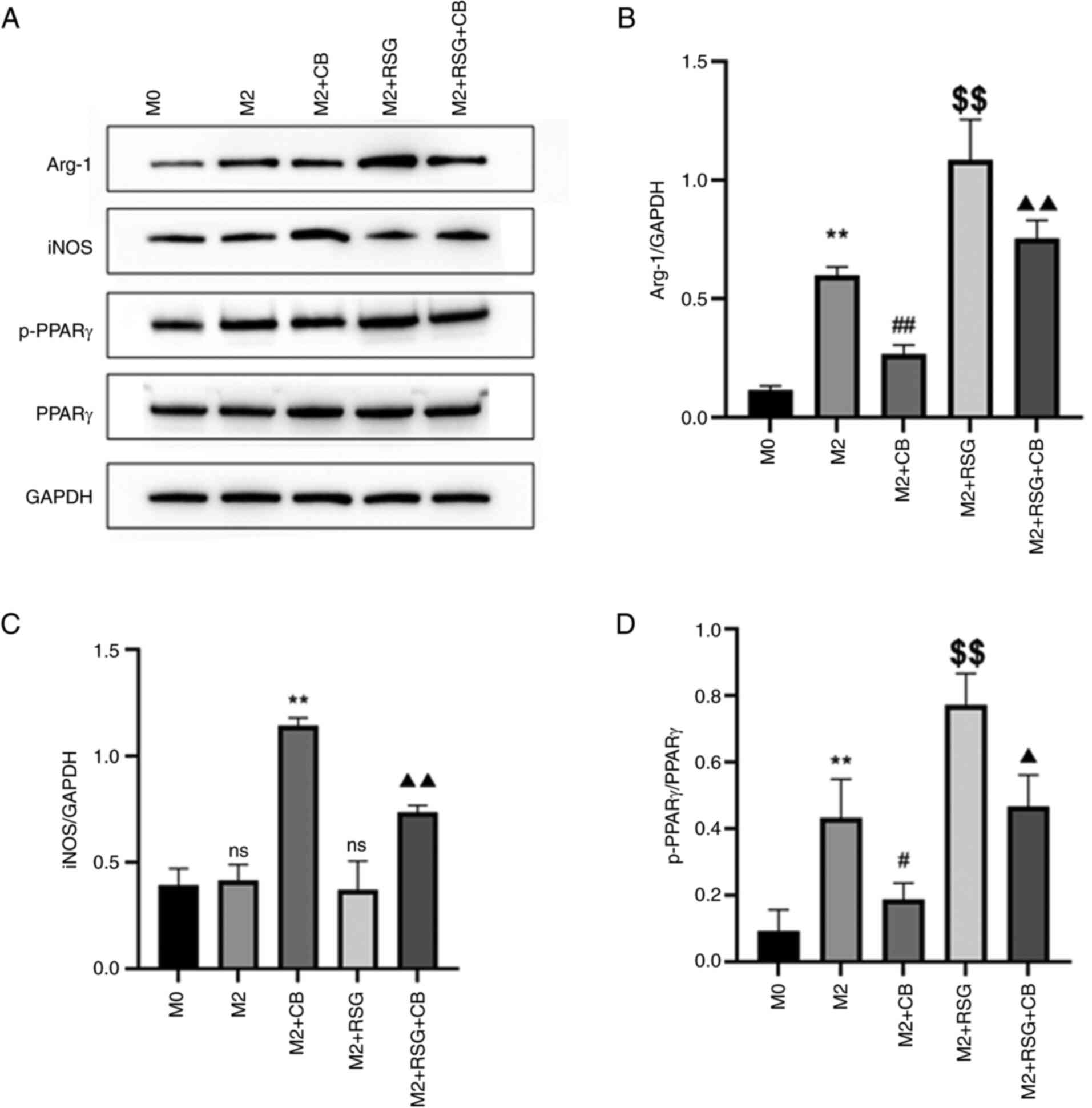 | Figure 9Effects of CB on the expression of
macrophage polarization-related proteins. (A) Gel blots showing
protein expression of Arg-1, iNOS, p-PPARγ and PPARγ in cells with
different treatment groups. (B-D) Quantification of (B) Arg-1, (C)
iNOS and (D) p-PPARγ based on the western blot assay (n=3).
**P<0.01 compared with M0 group;
#P<0.05, ##P<0.01 and
$$P<0.01 compared with M2 group;
▲P<0.05 and ▲▲P<0.01 compared with M2 +
RSG group. CB, cinobufagin; Arg-1, arginase 1; iNOS, inducible
nitric oxide synthase; p-, phosphorylated; PPARγ, peroxisome
proliferator-activated receptor γ; RSG, rosiglitazone; ns from M2
group, not statistically significant compared with M0 group; ns
from M2 + RSG group, not statistically significant compared with M2
group. |
Effects of CB-treated
macrophage-conditioned medium on EMT-related protein expression in
lung cancer cells
EMT is the process by which polarized epithelial
cells transform into mesenchymal-like cells. During this
transformation, cell surface epithelial markers such as E-cadherin
and desmoplakin are downregulated, while mesenchymal markers such
as N-cadherin and vimentin are upregulated, resulting in the loss
of cell adhesion and apical-basal polarity, which promotes tumor
progression and metastasis (41).
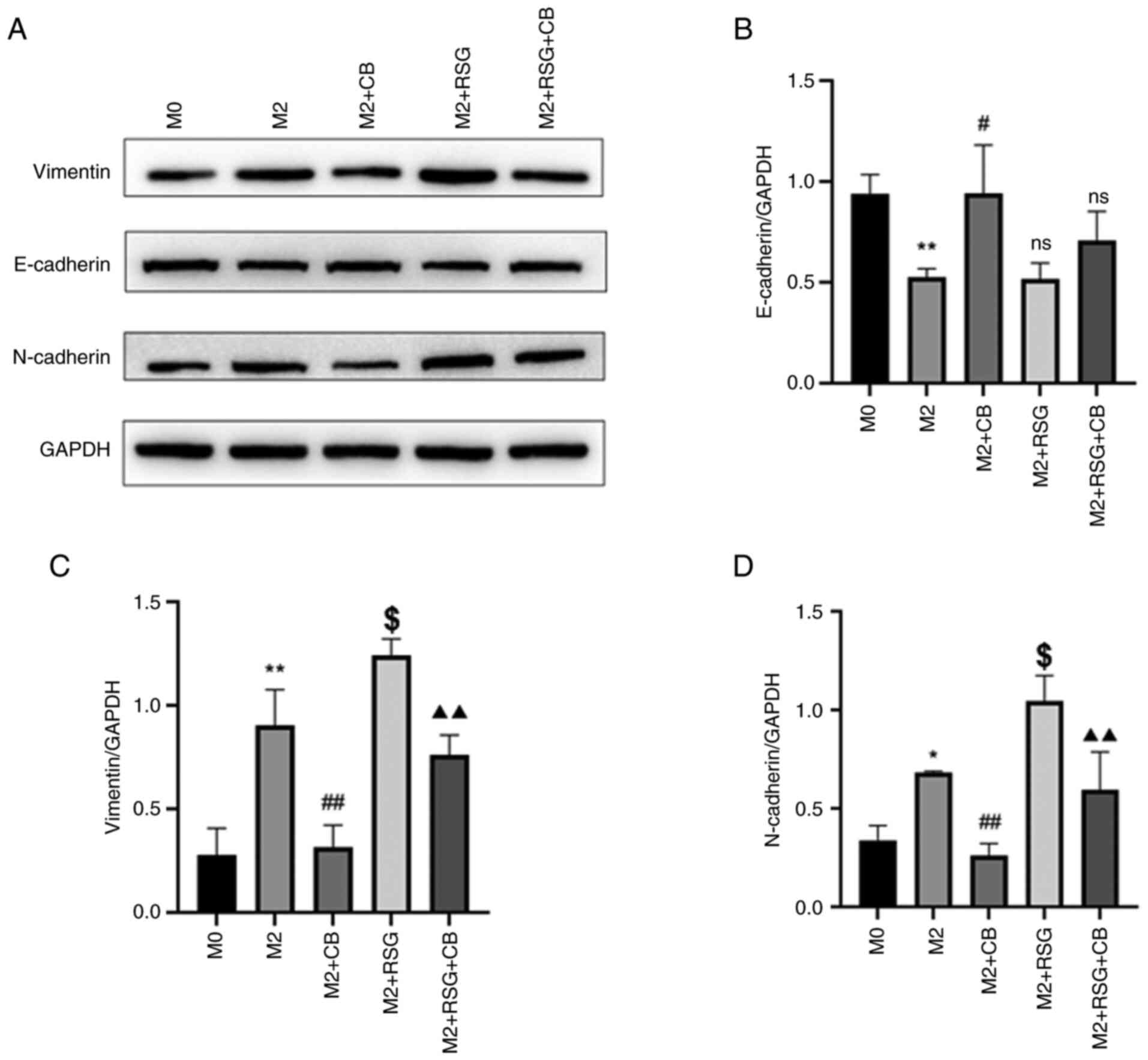
As demonstrated in Fig. 10A, the
effects of CB on EMT-related proteins in A549 lung cancer cells
were assessed using western blotting. E-cadherin was downregulated
in A549 cells treated with M2 macrophage-conditioned medium
compared with M0 macrophage-condition medium (Fig. 10A and B). The E-cadherin protein
level was increased in A549 cells cultured with CB-treated M2
macrophage-conditioned medium compared with M2
macrophage-conditioned medium, while RSG-treated M2
macrophage-conditioned medium had no effect on E-cadherin
expression in A549 cells compared with M2 macrophage-conditioned
medium (Fig. 10A and B).
Vimentin and N-cadherin were upregulated in A549
cells treated M2 macrophage-conditioned medium compared with M0
macrophage-condition medium (Fig.
10A, C and D). The vimentin and N-cadherin protein levels were
decreased in A549 cells cultured with CB-treated with M2
macrophage-conditioned medium compared with M2
macrophage-conditioned medium, while RSG-treated M2
macrophage-conditioned medium increased vimentin and N-cadherin
protein levels in A549 cells compared with M2
macrophage-conditioned medium (Fig.
10A, C and D). Furthermore, the RSG-mediated effects on
vimentin and N-cadherin expression in A549 cells were attenuated by
CB treatment (Fig. 10A, C and
D). The results indicated that in A549 lung cancer cells,
treatment with M2 macrophage-conditioned medium downregulated
E-cadherin and upregulated vimentin and N-cadherin levels, effects
attenuated by CB treatment but exacerbated by RSG treatment,
indicating modulation of EMT-related protein expression.
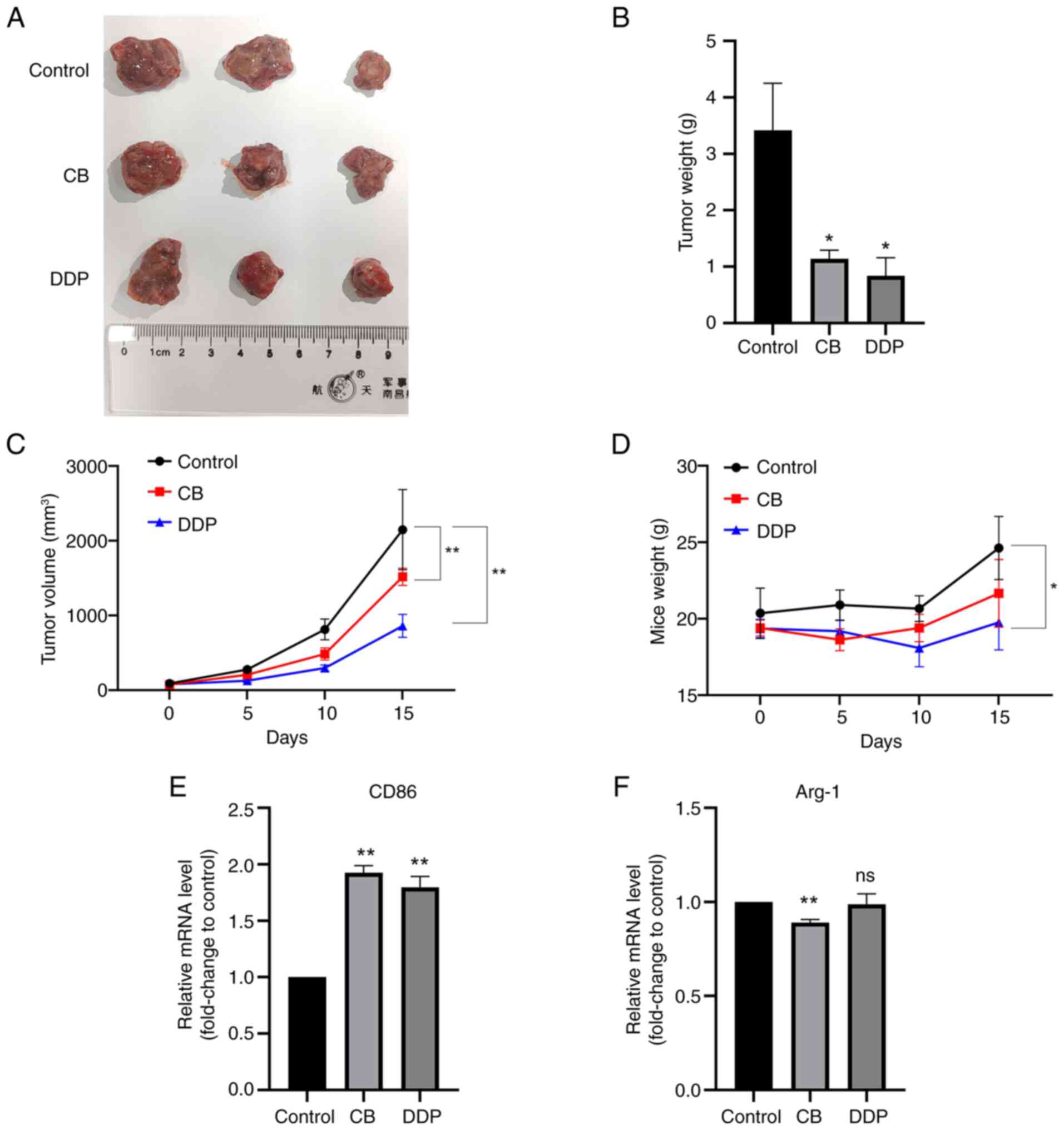
Effects of CB and DDP on in vivo tumor
growth
The effects of CB on in vivo tumor growth
were further examined using a nude mice xenograft model. As
revealed in Fig. 11A-C, both DDP
and CB treatments inhibited the in vivo tumor growth of LLC
cells. However, DDP, but not CB, reduced the body weight of the
nude mice. Additionally, both CB and DDP treatments significantly
upregulated CD86 mRNA expression in the tumor tissues (Fig. 11E). However, CB, but not DDP,
downregulated Arg-1 expression in the tumor tissues compared with
the control group (Fig. 11F).
These results indicated that both CB and DDP treatments effectively
suppressed LLC tumor growth and increased CD86 mRNA expression in
tumor tissues, with DDP additionally reducing body weight and CB
uniquely downregulating Arg-1 expression compared with
controls.
Discussion
Lung cancer is one of the most common malignant
tumors worldwide, with its incidence and mortality rates increasing
annually (1). In the TME,
interactions between tumor and immune cells directly influence
tumor progression (10). TAMs
have a dual regulatory role in lung cancer malignancy, among which
the quantity, activity and function of M2 macrophages are closely
related to tumor progression (10,14). HuaChanSu injection has been used
for treating lung cancer clinically (29). Furthermore, a HuaChanSu injection
and DDP combination improves efficacy in the treatment of advanced
NSCLC, with a higher quality of life for patients and fewer adverse
reactions (7). Bufadienolide
glycosides, including CB and bufalin, are the main antitumor active
ingredients in HuaChanSu injection, but their exact mechanisms of
action remain unclear (42). At
present, most research on the antitumor mechanism of CB focus on
inhibiting tumor cell proliferation and promoting apoptosis, with
fewer studies on the molecular mechanisms of tumor invasion and
migration. Therefore, based on the significant clinical antitumor
efficacy of HuaChanSu injection, the present study aimed to reveal
the influence and mechanism of action of its effective active
ingredient, CB, on the polarization of TAMs, to provide a basis for
further exploration of the pharmacological actions and clinical
applications of CB.
In the complex TME, TAMs interact with the TME,
undergo metabolic changes and alter the anticancer phenotype of
early M1-TAMs (43). Typically,
in the early stages of tumor development, M1-like TAMs predominate
the TME and inhibit tumor growth by secreting certain inflammatory
factors (44). As cancer
progresses, late-stage M2-like TAMs begin to dominate the TME,
promoting angiogenesis and aiding in tumor cell dissemination and
metastasis, thus exhibiting pro-tumor effects (40). THP-1 monocytes differentiate into
macrophages when induced by PMA, leading to changes in cell
morphology and surface markers (45). Exposure to the Th1 cytokine,
IFN-γ, induces differentiation into M1-type macrophages, while
exposure to the Th2 cytokine, IL-4, induces differentiation into
M2-type macrophages, which is typically accompanied by changes in
cell surface marker expression (46). Therefore, the widely used PMA was
adopted in the present study to induce the differentiation of THP-1
cells into M0 macrophages, which were then separately induced into
M1 or M2 macrophage models using human IFN-γ or IL-4, respectively.
The results demonstrated that after PMA stimulation, the cells
enlarged and adhered to the wall, and the mRNA levels of the M0
macrophage surface marker CD86 increased, indicating the successful
induction of M0 macrophages. Under IFN-γ stimulation, the surface
expression of the M1 polarization marker CD86 increased,
accompanied by secretion of high levels of pro-inflammatory
cytokines. After IL-4 stimulation, the levels of CD206 increased.
These results were consistent with previous studies (36,47) indicating the successful
replication of macrophage models with different polarization
phenotypes.
Pharmacological studies have demonstrated that
HuaChanSu injection has significant antitumor, analgesic and
immune-enhancing effects, and is commonly used clinically to treat
primary liver cancer, lung cancer, CRC and other diseases (48). CB possesses antitumor, analgesic,
cardiotonic and local anesthetic effects, but it also has certain
toxicities, particularly cardiac toxicity (49,50). In the present study, the MTT
method was used to assess the effects of different concentrations
(0, 10, 15, 20, 25, 30, 35, 40 and 50 ng/ml) of CB on the viability
of M0 macrophages. The results indicated that, as the drug
concentration increased the cell viability gradually decreased, and
when the concentration was <30 ng/ml, the viability of M0 cells
was >80%. In addition, to further evaluate the safety of CB, the
viability of BEAS-2B cells was assessed. The results showed that
when the concentration was <30 ng/ml, CB had no clear toxic
effect on BEAS-2B cells. Ultimately, 25 ng/ml was selected as the
CB concentration for subsequent experiments.
The balance between M1 and M2 macrophages will
affect the overall antitumor or pro-tumor effects of macrophages.
Therefore, maintaining the M1/M2 macrophage balance in the body has
become an important strategy for antitumor therapy. At present,
immunotherapy based on macrophages aims to reduce the proportion of
M2 macrophages or repolarize M2 into M1 macrophages to inhibit
tumor invasion and metastasis (51). Traditional Chinese Medicine and
its active ingredients can regulate the balance of M1/M2
macrophages. The active ingredient, β-elemene, in Curcuma
aromatica can inhibit the migration, invasion and EMT of LLC
cells induced by M2 macrophage-conditioned medium (52). β-elemene downregulates Arg-1 and
upregulates iNOS expression, suggesting that its effect on tumor
cell invasion and migration may be achieved through regulating the
balance of M1/M2 macrophages (52). Consistently, the results of the
present study suggested that CB is key in maintaining the M1/M2
macrophage balance, promoting M1 polarization and inhibiting M2
polarization.
Macrophages participate in various stages of tumor
metastasis, in which M2 macrophages can assist tumor cells in
penetrating blood vessels and entering the bloodstream in the form
of circulating tumor cells, thus facilitating the subsequent
settlement of tumor cells (53).
Additionally, the infiltration density of M2 macrophages is
correlated with the generation of microvessels within tumors and is
negatively correlated with patient survival (54). In the present study, M2
macrophage-conditioned medium was prepared and separately cultured
with the A549 and LLC lung cancer cell lines, to observe the
effects on the invasion, migration and angiogenic abilities of
these cells. The results showed that M2 macrophage-conditioned
medium promoted the invasion, migration and angiogenesis of A549
and LLC cells, while pre-treatment with CB significantly inhibited
the invasion, migration and angiogenesis of these cells in M2
macrophage-conditioned medium. A study has shown that M2-like TAMs
can induce EMT through the secretion of TGF-β1, activating the
TGF-β signaling pathway via Smad-dependent or Smad-independent
signaling pathways (55). In the
present study, the expression levels of the EMT-related proteins,
vimentin, E-cadherin and N-cadherin, in A549 lung cancer cells were
also examined. The A549 cells cultured in M2 macrophage-conditioned
medium exhibited a significant increase in vimentin and N-cadherin
levels and a decrease in E-cadherin levels, supporting the notion
that M2 macrophages promote lung cancer cell progression. When the
A549 cells were cultured in M2 macrophage-conditioned medium
pre-treated with CB, the vimentin and N-cadherin levels
significantly decreased, while E-cadherin expression increased.
These results suggested that the inhibition of lung cancer cell
invasion and migration by CB may be achieved through regulating
macrophage polarization to influence EMT-related protein expression
in lung cancer cells.
Research has demonstrated that MARCO is extensively
expressed on the surface of macrophages, and MARCO+TAMs
in the TME tend to exhibit the M2 phenotype (40). In a mouse glioblastoma model,
peritoneal macrophages overexpressing PPARγ increased the
transcription levels of MARCO, driving the formation of M2
macrophages (56). Whether the
CB-inhibited M2 polarization is related to MARCO expression has
not, to the best of our knowledge, been reported. In the present
study, IF techniques were used to detect MARCO expression in
macrophages. The results demonstrated that the average fluorescence
intensity of MARCO in M2 macrophages was higher than that in M0
macrophages; however, after pretreatment with CB, the average
fluorescence intensity of MARCO decreased, suggesting that CB
downregulated MARCO expression on the surface of M2
macrophages.
The present study demonstrated that there was no
significant change in the PPARγ mRNA level in M2 macrophages
following RSG stimulation. It was hypothesized that PPARγ might
exert its effects through self-phosphorylation and/or nuclear
translocation. The experimental results showed that the
phosphorylation level of PPARγ in M2 macrophages significantly
increased after RSG stimulation, and a large amount of PPARγ was
translocated to the nucleus. Collectively, these results indicated
that the transcription factor, PPARγ, can promote M2 polarization
through phosphorylation and nuclear translocation. In the present
study, to verify whether the observed CB-inhibited M2 polarization
was related to the regulation of PPARγ, macrophages were pretreated
with CB, and after RSG stimulation, CB partially inhibited the
phosphorylation and nuclear translocation of PPARγ in M2
macrophages. These results suggested that CB can prevent PPARγ
activation and thus affect macrophage polarization.
In the present study, to further clarify whether the
impact of CB on NSCLC progression was related to PPARγ, RSG was
used to treat and collect macrophage-conditioned medium for
culturing A549 cells. Upon examination, it was found that RSG
pre-treated macrophage-conditioned medium induced a high expression
of vimentin and N-cadherin in A549 cells, while E-cadherin
expression remained unchanged; meanwhile, CB reversed these
changes. These results suggested that CB may inhibit M2
polarization by targeting PPARγ in M2 macrophages, thereby
affecting lung cancer cell progression.
However, the present study still has certain
limitations and further research is needed on the mechanism by
which CB inhibits lung cancer invasion and migration. Further
research is planned in the following three areas. First, the
present study lacked verification at the in vivo animal
level regarding whether CB inhibits lung cancer invasion and
migration by affecting macrophage polarization. The present study
preliminarily demonstrated that CB inhibited LLC xenograft growth
in mice and modulated the gene expression levels of the M1
macrophage surface marker, CD86, and the M2 macrophage surface
marker, Arg-1, in tumor tissues, but detailed experimental research
is still ongoing. Second, TAM phenotype and function in the TME are
also influenced by metabolic reprogramming, including glutamine,
glucose and fatty acid metabolisms. Therefore, it remains to be
further clarified whether CB regulates macrophage polarization by
modulating metabolism. Third, communication between TAMs and lung
cancer cells can also be achieved through exosomes. Therefore,
exosomes could be used as carriers for anticancer drug delivery
systems. Compared with other types of macrophages, M1 macrophages
have stronger phagocytic capacity for loading anticancer drug
nanoparticles (57). Using M1
macrophage exosomes as carriers and utilizing Traditional Chinese
Medicine nanotechnology for the precise targeting of tumor sites
may improve anticancer effects. Fourth, the detailed interactions
between MARCO and PPARγ were not fully elucidated in the present
work, which should be further deciphered. Fifth, the present study
primarily focused on the effects of CB, with limited exploration of
alternative treatments or combination therapies. Considering the
complex nature of cancer and macrophage biology, comparative
studies with other treatments or synergistic combinations may
provide valuable insights into potential therapeutic strategies.
Furthermore, the expression of CD86 and Arg-1 in tumor tissues was
detected only the mRNA level, and further studies should determine
the protein levels of CD86 and Arg-1 in the tumor tissues to
consolidate the findings.
In summary, the inhibition of NSCLC invasion,
migration and angiogenesis by CB potentially operates through
several mechanisms: Suppressing PPARγ phosphorylation and nuclear
translocation, lowering MARCO expression, hindering M2 macrophage
polarization, enhancing secretion of inflammatory factors linked to
M1 macrophages, balancing the M1/M2 macrophage ratios, ameliorating
NSCLC TME immunosuppression and impacting EMT-related protein
expression levels.
Supplementary Data
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YS, YL and XM conceived and designed the
experiments. YS, YL, XM, JX, LF and JG performed the experiments.
HX XZ, HY, XH and YF analyzed the data. HY, XH and YF contributed
to the provision of reagents/materials/analysis tools. YS, YL and
XM confirm the authenticity of all the raw data. All authors
drafted and edited the manuscript. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
IACUC-202302006) by the animal Ethics Committee of Henan University
of Chinese Medicine (Zhengzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Nature Science
Foundation of China (grant no. 81803863), the Programs for Science
and Technology Development of Henan (grant no. 232102311111) and
the Henan Provincial College Youth Key Teacher Training Program
(grant no. 2020GGJS103).
References
|
1
|
Kratzer TB, Bandi P, Freedman ND, Smith
RA, Travis WD, Jemal A and Siegel RL: Lung cancer statistics, 2023.
Cancer. 130:1330–1348. 2023. View Article : Google Scholar
|
|
2
|
Lee E and Kazerooni EA: Lung cancer
screening. Semin Respir Crit Care Med. 43:839–850. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schabath MB and Cote ML: Cancer progress
and priorities: Lung cancer. Cancer Epidemiol Biomarkers Prev.
28:1563–1579. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng M: Classification and pathology of
lung cancer. Surg Oncol Clin N Am. 25:447–468. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rodriguez-Canales J, Parra-Cuentas E and
Wistuba II: Diagnosis and molecular classification of lung cancer.
Cancer Treat Res. 170:25–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Srivastava S, Mohanty A, Nam A, Singhal S
and Salgia R: Chemokines and NSCLC: Emerging role in prognosis,
heterogeneity, and therapeutics. Semin Cancer Biol. 86:233–246.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duma N, Santana-Davila R and Molina JR:
Non-small cell lung cancer: Epidemiology, screening, diagnosis, and
treatment. Mayo Clin Proc. 94:1623–1640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miller M and Hanna N: Advances in systemic
therapy for non-small cell lung cancer. BMJ. 375:n23632021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Osmani L, Askin F, Gabrielson E and Li QK:
Current WHO guidelines and the critical role of immunohistochemical
markers in the subclassification of non-small cell lung carcinoma
(NSCLC): Moving from targeted therapy to immunotherapy. Semin
Cancer Biol. 52:103–109. 2018. View Article : Google Scholar
|
|
10
|
Wang Y, Chen R, Wa Y, Ding S, Yang Y, Liao
J, Tong L and Xiao G: Tumor immune microenvironment and
immunotherapy in brain metastasis from non-small cell lung cancer.
Front Immunol. 13:8294512022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cascone T, Fradette J, Pradhan M and
Gibbons DL: Tumor immunology and immunotherapy of non-small-cell
lung cancer. Cold Spring Harb Perspect Med. 12:a0378952022.
View Article : Google Scholar :
|
|
12
|
Sivori S, Pende D, Quatrini L, Pietra G,
Della Chiesa M, Vacca P, Tumino N, Moretta F, Mingari MC, Locatelli
F and Moretta L: NK cells and ILCs in tumor immunotherapy. Mol
Aspects Med. 80:1008702021. View Article : Google Scholar
|
|
13
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng D, Ge K, Yao X, Wang B, Chen R, Zhao
W, Fang C and Ji M: Tumor-associated macrophages mediate resistance
of EGFR-TKIs in non-small cell lung cancer: Mechanisms and
prospects. Front Immunol. 14:12099472023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sedighzadeh SS, Khoshbin AP, Razi S,
Keshavarz-Fathi M and Rezaei N: A narrative review of
tumor-associated macrophages in lung cancer: Regulation of
macrophage polarization and therapeutic implications. Transl Lung
Cancer Res. 10:1889–1916. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Genova C, Dellepiane C, Carrega P,
Sommariva S, Ferlazzo G, Pronzato P, Gangemi R, Filaci G, Coco S
and Croce M: Therapeutic implications of tumor microenvironment in
lung cancer: Focus on immune checkpoint blockade. Front Immunol.
12:7994552022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan A, Hsiao YJ, Chen HY, Chen HW, Ho CC,
Chen YY, Liu YC, Hong TH, Yu SL, Chen JJ and Yang PC: Opposite
effects of M1 and M2 macrophage subtypes on lung cancer
progression. Sci Rep. 5:142732015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sumitomo R, Hirai T, Fujita M, Murakami H,
Otake Y and Huang CL: M2 tumor-associated macrophages promote tumor
progression in non-small-cell lung cancer. Exp Ther Med.
18:4490–4498. 2019.PubMed/NCBI
|
|
19
|
Hu JM, Liu K, Liu JH, Jiang XL, Wang XL,
Yang L, Chen YZ, Liu CX, Li SG, Cui XB, et al: The increased number
of tumor-associated macrophage is associated with overexpression of
VEGF-C, plays an important role in Kazakh ESCC invasion and
metastasis. Exp Mol Pathol. 102:15–21. 2017. View Article : Google Scholar
|
|
20
|
Frezzetti D, Gallo M, Maiello MR,
D'Alessio A, Esposito C, Chicchinelli N, Normanno N and De Luca A:
VEGF as a potential target in lung cancer. Expert Opin Ther
Targets. 21:959–966. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeni E, Mazzetti L, Miotto D, Lo Cascio N,
Maestrelli P, Querzoli P, Pedriali M, De Rosa E, Fabbri LM, Mapp CE
and Boschetto P: Macrophage expression of interleukin-10 is a
prognostic factor in nonsmall cell lung cancer. Eur Respir J.
30:627–632. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vahl JM, Friedrich J, Mittler S, Trump S,
Heim L, Kachler K, Balabko L, Fuhrich N, Geppert CI, Trufa DI, et
al: Interleukin-10-regulated tumour tolerance in non-small cell
lung cancer. Br J Cancer. 117:1644–1655. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maltarollo VG, Kronenberger T, Windshugel
B, Wrenger C, Trossini GHG and Honorio KM: Advances and challenges
in drug design of PPARδ ligands. Curr Drug Targets. 19:144–154.
2018. View Article : Google Scholar
|
|
24
|
Tontonoz P and Spiegelman BM: Fat and
beyond: The diverse biology of PPARgamma. Annu Rev Biochem.
77:289–312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gallardo-Soler A, Gómez-Nieto C, Campo ML,
Marathe C, Tontonoz P, Castrillo A and Corraliza I: Arginase I
induction by modified lipoproteins in macrophages: A peroxisome
proliferator-activated receptor-gamma/delta-mediated effect that
links lipid metabolism and immunity. Mol Endocrinol. 22:1394–1402.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang JT, Welch JS, Ricote M, Binder CJ,
Willson TM, Kelly C, Witztum JL, Funk CD, Conrad D and Glass CK:
Interleukin-4-dependent production of PPAR-gamma ligands in
macrophages by 12/15-lipoxygenase. Nature. 400:378–382. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bu ZJ, Wan SR, Steinmann P, Yin ZT, Tan
JP, Li WX, Tang ZY, Jiang S, Ye MM and Xu JY: Effectiveness and
safety of Chinese herbal injections combined with SOX chemotherapy
regimens for advanced gastric cancer: A Bayesian network
meta-analysis. J Cancer. 15:889–907. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tarasiuk A, Mirocha G and Fichna J:
Current status of complementary and alternative medicine
interventions in the management of pancreatic cancer-an overview.
Curr Treat Options Oncol. 24:1852–1869. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu YF, Chen YR, Bu FL, Huang YB, Sun YX,
Li CY, Sellick J, Liu JP, Qin DM and Liu ZL: Chinese herbal
injections versus intrapleural cisplatin for lung cancer patients
with malignant pleural effusion: A Bayesian network meta-analysis
of randomized controlled trials. Front Oncol. 12:9429412022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tan X, Liang X, Xi J, Guo S, Meng M, Chen
X and Li Y: Clinical efficacy and safety of Huachansu injection
combination with platinum-based chemotherapy for advanced non-small
cell lung cancer: A systematic review and meta-analysis of
randomized controlled trials. Medicine (Baltimore). 100:e271612021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He K, Wang GX, Zhao LN, Cui XF, Su XB, Shi
Y, Xie TP, Hou SW and Han ZG: Cinobufagin is a selective
anti-cancer agent against tumors with EGFR amplification and PTEN
deletion. Front Pharmacol. 12:7756022021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bai Y, Wang X, Cai M, Ma C, Xiang Y, Hu W,
Zhou B, Zhao C, Dai X, Li X and Zhao H: Cinobufagin suppresses
colorectal cancer growth via STAT3 pathway inhibition. Am J Cancer
Res. 11:200–214. 2021.PubMed/NCBI
|
|
33
|
Ma X, Suo Z, Ma X, Zhan C, Luo G and Song
J: Cinobufagin inhibits tumor progression and reduces doxorubicin
resistance by enhancing FOXO1-mediated transcription of FCGBP in
osteosarcoma. J Ethnopharmacol. 296:1154332022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang L, Liang B, Xu H, Gong Y, Hu W, Jin
Z, Wu X, Chen X, Li M, Shi L, et al: Cinobufagin induces
FOXO1-regulated apoptosis, proliferation, migration, and invasion
by inhibiting G9a in non-small-cell lung cancer A549 cells. J
Ethnopharmacol. 291:1150952022. View Article : Google Scholar
|
|
35
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu F, Cui WQ, Wei Y, Cui J, Qiu J, Hu LL,
Gong WY, Dong JC and Liu BJ: Astragaloside IV inhibits lung cancer
progression and metastasis by modulating macrophage polarization
through AMPK signaling. J Exp Clin Cancer Res. 37:2072018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
38
|
Niu J, Wang J, Zhang Q, Zou Z and Ding Y:
Cinobufagin-induced DNA damage response activates G2/M checkpoint
and apoptosis to cause selective cytotoxicity in cancer cells.
Cancer Cell Int. 21:4462021. View Article : Google Scholar :
|
|
39
|
Lu XS, Qiao YB, Li Y, Yang B, Chen MB and
Xing CG: Preclinical study of cinobufagin as a promising
anti-colorectal cancer agent. Oncotarget. 8:988–998. 2017.
View Article : Google Scholar :
|
|
40
|
Murray PJ, Allen JE, Biswas SK, Fisher EA,
Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence
T, et al: Macrophage activation and polarization: Nomenclature and
experimental guidelines. Immunity. 41:14–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mittal V: Epithelial mesenchymal
transition in tumor metastasis. Annu Rev Pathol. 13:395–412. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qi F, Inagaki Y, Gao B, Cui X, Xu H,
Kokudo N, Li A and Tang W: Bufalin and cinobufagin induce apoptosis
of human hepatocellular carcinoma cells via Fas- and
mitochondria-mediated pathways. Cancer Sci. 102:951–958. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Netea-Maier RT, Smit JWA and Netea MG:
Metabolic changes in tumor cells and tumor-associated macrophages:
A mutual relationship. Cancer Lett. 413:102–109. 2018. View Article : Google Scholar
|
|
44
|
Redente EF, Dwyer-Nield LD, Merrick DT,
Raina K, Agarwal R, Pao W, Rice PL, Shroyer KR and Malkinson AM:
Tumor progression stage and anatomical site regulate
tumor-associated macrophage and bone marrow-derived monocyte
polarization. Am J Pathol. 176:2972–2985. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xue J, Fu C, Cong Z, Peng L, Peng Z, Chen
T, Wang W, Jiang H, Wei Q and Qin C: Galectin-3 promotes
caspase-independent cell death of HIV-1-infected macrophages. FEBS
J. 284:97–113. 2017. View Article : Google Scholar
|
|
46
|
Genin M, Clement F, Fattaccioli A, Raes M
and Michiels C: M1 and M2 macrophages derived from THP-1 cells
differentially modulate the response of cancer cells to etoposide.
BMC Cancer. 15:5772015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen R, Lu X, Li Z, Sun Y, He Z and Li X:
Dihydroartemisinin prevents progression and metastasis of head and
neck squamous cell carcinoma by inhibiting polarization of
macrophages in tumor microenvironment. Onco Targets Ther.
13:3375–3387. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang J, Chen F, Zhong Z, Tan HY, Wang N,
Liu Y, Fang X, Yang T and Feng Y: Interpreting the pharmacological
mechanisms of huachansu capsules on hepatocellular carcinoma
through combining network pharmacology and experimental evaluation.
Front Pharmacol. 11:4142020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Dai CL, Zhang RJ, An P, Deng YQ, Rahman K
and Zhang H: Cinobufagin: A promising therapeutic agent for cancer.
J Pharm Pharmacol. 75:1141–1153. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Asrorov AM, Kayumov M, Mukhamedov N,
Yashinov A, Mirakhmetova Z, Huang Y, Yili A, Aisa HA,
Tashmukhamedov M, Salikhov S and Mirzaakhmedov S: Toad venom
bufadienolides and bufotoxins: An updated review. Drug Dev Res.
84:815–838. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li X, Bu W, Meng L, Liu X, Wang S, Jiang
L, Ren M, Fan Y and Sun H: CXCL12/CXCR4 pathway orchestrates
CSC-like properties by CAF recruited tumor associated macrophage in
OSCC. Exp Cell Res. 378:131–138. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Schmall A, Al-Tamari HM, Herold S,
Kampschulte M, Weigert A, Wietelmann A, Vipotnik N, Grimminger F,
Seeger W, Pullamsetti SS and Savai R: Macrophage and cancer cell
cross-talk via CCR2 and CX3CR1 is a fundamental mechanism driving
lung cancer. Am J Respir Crit Care Med. 191:437–447. 2015.
View Article : Google Scholar
|
|
53
|
Nakatsumi H, Matsumoto M and Nakayama KI:
Noncanonical pathway for regulation of CCL2 expression by an
mTORC1-FOXK1 axis promotes recruitment of tumor-associated
macrophages. Cell Rep. 21:2471–2486. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu CY, Cherng JY, Yang YH, Lin CL, Kuan
FC, Lin YY, Lin YS, Shu LH, Cheng YC, Liu HT, et al: Danshen
improves survival of patients with advanced lung cancer and
targeting the relationship between macrophages and lung cancer
cells. Oncotarget. 8:90925–90947. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fu XH, Li JP, Li XY, Tan Y, Zhao M, Zhang
SF, Wu XD and Xu JG: M2-macrophage-derived exosomes promote
meningioma progression through TGF-β signaling pathway. J Immunol
Res. 2022:83265912022. View Article : Google Scholar
|
|
56
|
Sa JK, Chang N, Lee HW, Cho HJ, Ceccarelli
M, Cerulo L, Yin J, Kim SS, Caruso FP, Lee M, et al:
Transcriptional regulatory networks of tumor-associated macrophages
that drive malignancy in mesenchymal glioblastoma. Genome Biol.
21:2162020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Yu J, Deng H and Xu Z: Targeting
macrophage priming by polyphyllin VII triggers anti-tumor immunity
via STING-governed cytotoxic T-cell infiltration in lung cancer.
Sci Rep. 10:213602020. View Article : Google Scholar : PubMed/NCBI
|