Prostate cancer (PCa) is the second leading cause of
cancer-related death in men and ranks as the fifth most prevalent
cancer globally (1,2). Over the past few years, the 5-year
survival rate for patients with non-metastatic PCa has continued to
increase and is almost 100%. However, some individuals who undergo
castration therapy will ultimately develop incurable
castration-resistant PCa (CRPC) (3-5).
Research has indicated that 80-90% of individuals diagnosed with
advanced PCa will ultimately experience bone metastasis (6,7).
Metastasis of PCa to the bones frequently leads to skeletal related
events (SREs) and a range of complications, primarily affecting the
pelvis and spine (8,9), which lead to a lower quality life
and death (10,11). Patients with PCa but without bone
metastasis have a survival rate of 87% at 1 year and 56% at 5
years. However, patients with bone metastasis have a survival rate
of 47% at 1 year and 3% at 5 years (12). Hence, it is crucial to investigate
therapeutic approaches for PCa bone metastasis to enhance patient
prognosis.
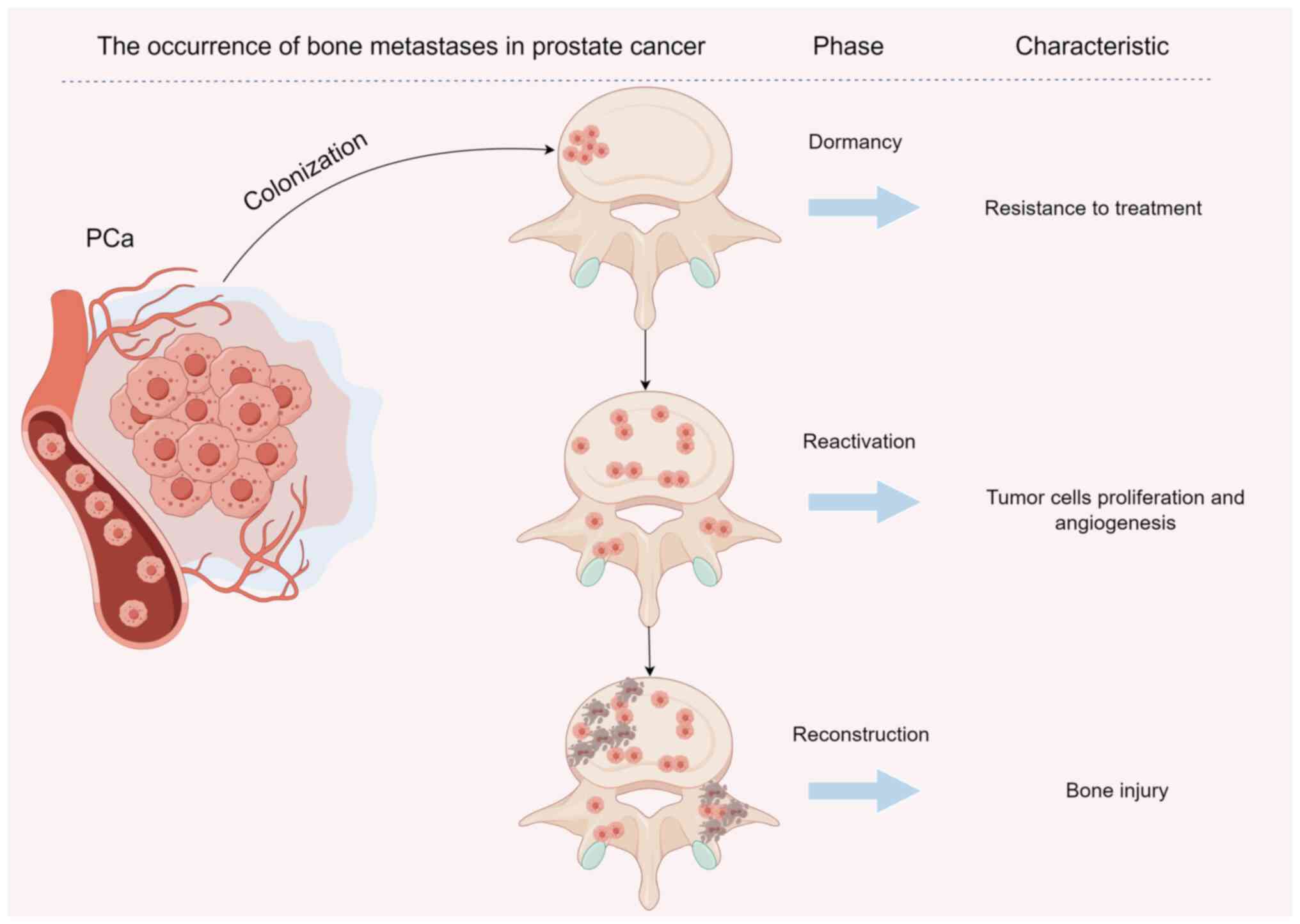
PCa bone metastasis involves four stages:
Colonization, dormancy, reactivation and reconstruction (13). Numerous investigations have
concentrated on the interplay between tumor cells and the tumor
microenvironment (TME) when examining the mechanism of bone
metastasis (14-16). Maintaining the integrity of bone
structure is achieved by the relative equilibrium between
osteoblasts and osteoclasts in the bone microenvironment (17). Various degrees of participation in
bone homeostasis regulation are observed from bone cells, bone
marrow endothelial cells (BMECs) and the immune environment
(15). Research has indicated
that a range of cytokines play a role in the progression of
metastasis of PCa to the bones (18). For a number of decades, the
development of therapeutic approaches has focused on directly
addressing the tumor. Nevertheless, the emergence of drug
resistance poses great difficulties. Despite the approval of
bisphosphonate, dinomumab, radium-223 and other medications for
preventing and treating PCa bone metastasis, there is still a need
to investigate the underlying mechanisms and develop more targeted
therapeutic drugs for the bone metastasis (19,20).
Hence, comprehending the molecular mechanism behind
PCa bone metastasis would aid in the exploration of novel
therapeutic approaches. The present review provides an overview of
the bone metastasis process in PCa, including the associated
signaling pathways and molecular interaction mechanisms.
Additionally, it examines the findings from clinical research on
targeted drugs. Finally, the possibilities and challenges in
treating bone metastasis of PCa is explored, with the goal of
offering fresh perspectives for its treatment.
The process by which PCa cells enter bone tissue
through the blood circulation is defined as colonization (Fig. 1). Research has indicated that bone
stroma-released cytokines facilitate the establishment of PCa cells
in the bone (21). Chemokine and
receptor interactions have been shown to have a notable role in the
bone metastasis of PCa. An increase in C-X-C motif chemokine ligand
12 (CXCL12) in bone tissues is associated with tumor metastasis.
CXCL12 binds to C-X-C motif chemokine receptor (CXCR) 4 to induce
the adhesion, invasion and migration of PCa cells, thereby
promoting the colonization of cancer cells in bone tissue (22,23). Research has shown that, after
knocking out androgen receptor (AR) signals in tumor-associated
fibroblasts, the expression of chemokine ligand (CCL) 2 is
significantly increased, and the migration ability of PCa cells is
improved (24). Additional
research has indicated that CCL2 and receptor activator of nuclear
factor-κB (NF-κB) ligand (RANKL) stimulate the formation of
osteoclasts, enhance the activity of osteoblasts and facilitate the
spread of PCa in the bones (25).
CXCR2 induces the release of vascular endothelial growth factor
(VEGF), facilitates the creation of the pre-metastasis environment
in bone tissue and amplifies the ability of PCa cells to migrate
towards the bone (26). Research
has additionally discovered that integrin is controlled by various
cytokines and contributes to altering the cytoskeleton, thereby
enhancing the metastatic potential of PCa. By binding to its
designated receptor, CXCR6, CXCL16 induces dynamic alterations in
tumor cells and enhances the migratory, invasive and adhesive
properties of endothelial cells, primarily through the activation
of integrin αvβ3 (27).
Furthermore, there is a notable abundance of integrin αv in the
bone metastasis of PCa, while integrin α5 is exclusively present in
the tumor stroma and endothelial cells of the bone metastasis,
excluding the primary tumor (28).
Secondary tumors of the bone are often derived from
diffuse tumor cells that first enter a dormant state (29) (Fig.
1). Due to the dormant state of cells, bone metastasis often
has resistance to conventional chemotherapy drugs, which hinders
drug clearance of tumor cells (30). Following the spread of PCa to the
bones, dormant cancer cells gather close to osteoblasts and express
a significant amount of receptor tyrosine kinases (RTKs), which
play a role in controlling the expression of transforming growth
factor β (TGF-β) and its receptor (31). TGF-β2 secreted by bone marrow
stromal cells can upregulate the expression of growth arrest
specific protein 6 (GAS6). GAS6 is involved in the regulation of
PCa cell dormancy by specifically binding to Axl protein.
Therefore, specific blockade of TGF-β signaling may limit the
osteoblast-induced dormancy of PCa cells (31). Activation of p38 mitogen-activated
protein kinase and upregulation of the cell cycle inhibitor, p21,
and metastasis suppressor, N-myc downstream-regulated gene 1, by
bone morphogenetic protein (BMP) 7 leads to the induction of
senescence in PCa stem cell-like cells. PCa dormancy and recurrence
are significantly influenced by the involvement of BMP7 (32). Additionally, it has been
discovered that osteoblasts secrete RANKL, which can bind with
receptor activator of NF-κB, a protein that is abundantly present
in PCa. The expression of the Wnt signaling pathway is increased by
RANKL, which specifically stimulates the epithelial-mesenchymal
transformation (EMT) of PCa cells (33). The Wnt/β catenin signaling pathway
is related to the dormancy of PCa. Wnt5α, an important member of
this pathway, induces and maintains the dormancy of PCa cells in
the bone through the Wnt5α/receptor tyrosine kinase-like orphan
receptor 2/Siah E3 ubiquitin protein ligase 2 signaling axis
(34).
Dormant PCa cells are activated by specific factors
to become active and proliferating (Fig. 1). The process of reactivation of
dormant PCa cells in bone tissue involves a complex interplay of
various molecular mechanisms. Initially, these dormant cells reside
in a quiescent state within the bone microenvironment, often
shielded from systemic therapies. Upon reactivation, several key
factors contribute to this transition. Inflammatory cytokines, such
as IL-6 and TGF-β, released from the bone microenvironment can
stimulate the dormant cells to re-enter the cell cycle (35,36). Additionally, the interaction
between PCa cells and osteoblasts creates a conducive niche that
promotes cell survival and proliferation. This is often mediated by
the activation of signaling pathways, including the AKT and ERK
pathways, which enhance cell motility and invasiveness (37,38). Furthermore, the expression of
specific adhesion molecules allows cancer cells to better anchor
within the bone matrix, facilitating their continued growth
(39). Understanding these
processes is crucial for developing targeted therapies aimed at
preventing or delaying the reactivation of dormant PCa cells,
thereby improving patient outcomes in cases of bone metastasis.
After PCa bone metastasis, the balance between
osteoclast absorption and osteoblast formation is altered as the
original bone structure and the function are reconstructed
(Fig. 1). Following PCa bone
metastasis, the equilibrium between osteoclast-mediated bone
resorption and osteoblast-mediated bone formation is markedly
altered, leading to the disruption of normal bone structure and
function (40). The presence of
PCa cells in the bone microenvironment triggers osteoclastogenesis,
primarily through the release of factors such as RANKL and
parathyroid hormone-related peptide (41,42). These factors promote the
differentiation and activity of osteoclasts, resulting in increased
bone resorption. Concurrently, the activity of osteoblasts is often
suppressed due to the local TME and inflammatory cytokines such as
IL-6 and TNF-α, which impair new bone formation (43). This abnormal remodeling not only
depletes bone mass but also manifests as osteolytic lesions,
further compromising skeletal integrity (44). Understanding these mechanisms is
critical, as they provide potential therapeutic targets to restore
the balance between osteoclasts and osteoblasts, thereby addressing
bone metastasis and improving patient outcomes in PCa. Effective
interventions could include RANKL inhibitors or agents that enhance
osteoblast activity, offering a promising approach to manage the
skeletal complications associated with PCa metastasis.
PCa promotes the growth and survival of tumor cells
in the bone environment through numerous molecular mechanisms, and
recruit bystander dormant cells to participate in bone metastasis.
This process involves molecular communication between tumor cells
and bone tissue. PCa is characterized by the use of cytokines
released by bone tissue during the proliferation and migration of
tumor cells, thereby establishing an environment for the growth of
PCa cells in bone tissue, and then breaking the balance between
osteoclasts and osteoblasts to achieve the outcome of bone
destruction (45). The process of
PCa bone metastasis is regulated by the genes of tumor cells to
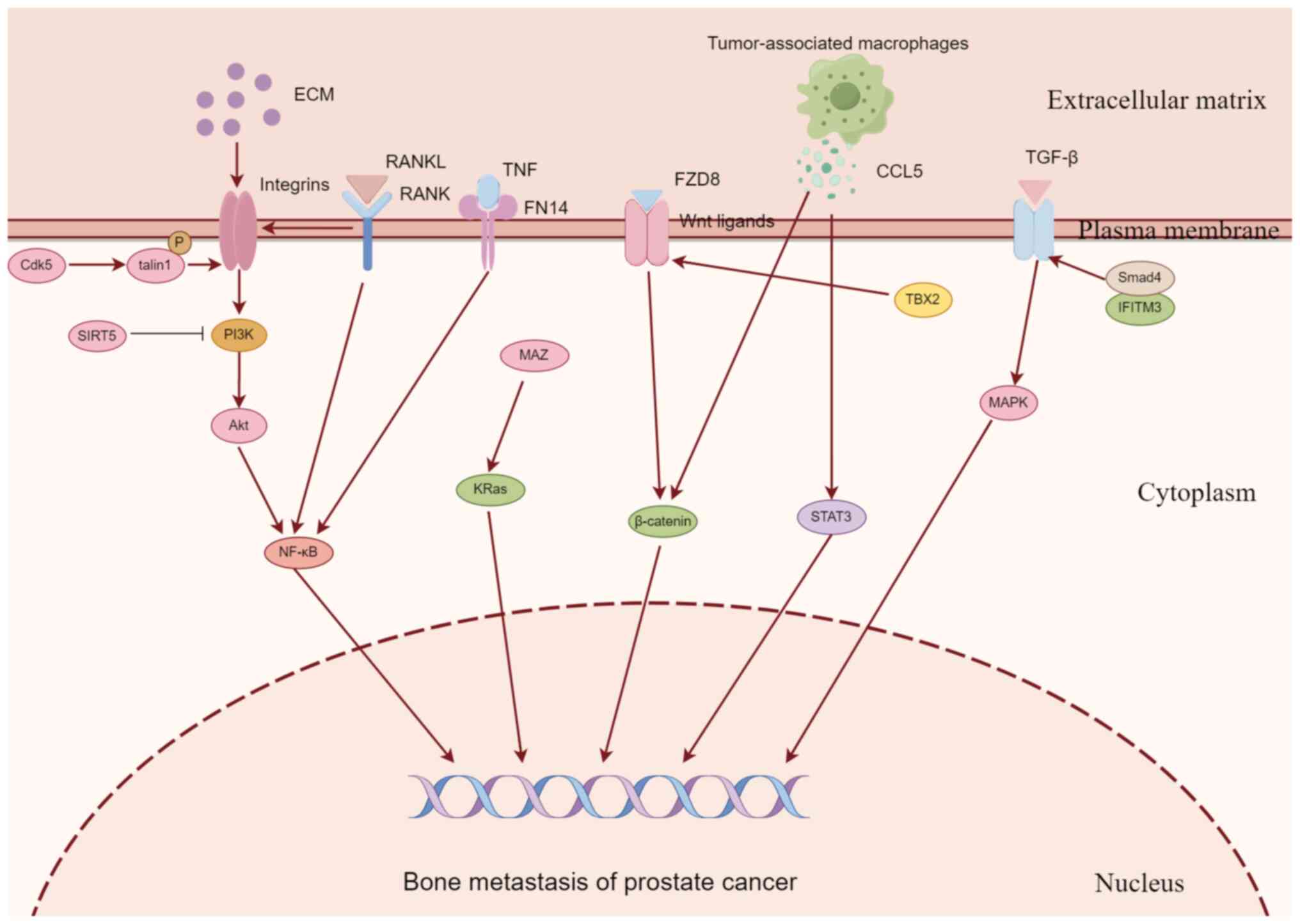
promote its proliferation and metastasis, which is controlled by a
variety of molecules and signaling pathways (Fig. 2). In recent years, numerous
studies have explored the relevant signaling pathways (Table I), and a number of potential
therapeutic targets have been identified.
The NF-κB pathway plays a role in controlling
various biological processes, such as inflammation and immune
responses (46). Abnormal
regulation of the NF-κB pathway may facilitate the growth,
infiltration and spread of tumors (47). Research has indicated that NF-κB
expression is upregulated during PCa progression, leading to an
increased cell cycle progression and proliferation rate (48). In addition, NF-κB resists cell
death and enhances metastatic capacity, especially bone metastatic
capacity (48). PCa bone
metastasis is significantly influenced by the PI3K/AKT pathway,
which activates NF-κB and leads to the stimulation of RANKL,
parathyroid hormone-like hormone and BMP-2 expression (49).
A study revealed that elevated levels of RANKL in
PCa cells had a notable impact on the promotion of PCa bone
metastasis. Ziaee and Chung (50)
used PCa bone metastasis cell lines overexpressing RANKL as a model
to study the molecular mechanism of increased adhesion between PCa
cells and collagen. The findings indicated that RANKL strongly
attached to the Escherichia coli framework through
upregulating integrin α2 expression. The interaction between PCa
and E. coli mediated by RANKL via integrin α2 may be a key
molecular event in PCa bone metastasis. In PCa, FN14 (TNFRSF12A),
which belongs to the TNF receptor family, has been shown to play a
role in bone metastasis in PCa. A study has shown that inhibition
of FN14 can significantly impede the spread of PCa cells to bone
(51). PCa bone metastasis
exhibited upregulation of FN14 in >50% of cases. It was also
found that FN14 expression was negatively correlated with AR
signaling output (51). The
findings of this research indicate that FN14 facilitates the spread
of PCa by activating the NF-κB signaling pathway, implying that
FN14 could potentially serve as a viable target for treating CRPC.
Further research has discovered that hepatocyte growth factor and
VEGF-A enhanced the levels of RANKL and macrophage-colony
stimulating factor (M-CSF), which are crucial elements in the
generation of osteoclasts (52).
Transcriptional activation of cellular-mesenchymal epithelial
transition factor (c-Met) by insulin-like growth factor-1
additionally enhances the expression of RANKL and M-CSF.
Suppression of c-Met and vascular endothelial growth factor
receptor 2 (VEGFR2) in osteoblasts led to a decrease in the levels
of RANKL and M-CSF, resulting in a reduction in tumor-induced
osteolysis (52). These findings
indicate that genes that enhance the NF-κB signaling pathway may
hold promise in the management of PCa bone metastasis.
Nonetheless, there are genes that hinder the spread
of PCa to the bones by suppressing the NF-κB signaling pathway,
thereby exerting a safeguarding effect on the advancement of PCa.
Sirtuin 5 (SIRT5) is a NAD(+) dependent deacetylase that is
considered a key regulator of a variety of cancer types (53). Choi et al (53) found that SIRT5 levels were
significantly reduced in PC-3M cell lines. The differentially
expressed proteins between parental and SIRT5 knockout PC-3 cells
were further analyzed by proteomics. IL-1β expression and
PI3K/AKT/NF-ĸB signaling were significantly increased in SIRT5
knockout cells. Finally, a co-immunoprecipitation experiment
confirmed that SIRT5 could combine with PI3K to inhibit PCa bone
metastasis by inhibiting the PI3K/AKT/NF-κB signaling pathway.
Over the past few decades, integrins have been
crucial in facilitating cell adhesion and signaling, with research
confirming their diverse roles in the development of tumors
(54-56). Integrins, upon binding to the
extracellular matrix (ECM), arrange the cytoskeleton and trigger
intracellular signaling, thereby controlling intricate cellular
activities such as viability, growth and movement (57,58). Integrins and RTKs must collaborate
to ensure the activation of the pro-mitosis and pro-survival
PI3K/AKT signaling pathway via Ras extracellular signaling
(59). Research has indicated
that integrin β1 becomes activated in the metastatic cells of PCa,
leading to an increase in the spread of PCa to lymph nodes and bone
(59). Adaptor proteins termed
talins control the signaling of adhesion plaques by connecting
integrins to the cytoskeleton. Talins have a direct interaction
with integrins and are essential for activating integrins (60). Jin et al (60) demonstrated the significant
involvement of talin1 in the activation of integrin β1 through
knockdown experiments. The research verified that the expression of
p35, which activates cyclin-dependent kinase 5 (Cdk5), and the
activity of Cdk5 are heightened in cancer cells (including PCa)
that have spread to other parts of the body. Furthermore, it has
been established that the kinase activity of Cdk5 is accountable
for the phosphorylation of talin1 and the subsequent activation of
integrin β1. Furthermore, platelet-responsive protein-2 (TSP-2)
functions as a secreted glycoprotein in stromal cells, facilitating
cellular attachment to the ECM and participating in numerous
physiological and pathological processes (61). Chen et al (61) discovered that the levels of TSP-2
increase as PCa advances, particularly in cases of metastatic PCa.
It was also demonstrated that TSP-2 augmented the expression of
matrix metallopeptidase 2 by attaching to integrin ανβ3,
consequently amplifying the migratory capacity of PCa cells. Hence,
TSP-2 is expected to be a promising target for the treatment of PCa
bone metastasis.
The Wnt family proteins and β-catenin are essential
for the regulation of numerous carcinogenic processes (62,63). Bone metastasis is common in PCa
and is mostly regulated by Wnt ligands and/or β-catenin. Li et
al (64) discovered that
frizzled class receptor 8 (FZD8) expression was notably increased
in PCa cell lines and tissues that had spread to the bones.
Clinical tumor progression and bone metastasis was positively
correlated with elevated FZD8 expression. Furthermore, the
excessive expression of FZD8 was observed to enhance the movement,
infiltration and stem-like characteristics of PCa cells in
vitro by activating the conventional Wnt/β-catenin signaling
pathway. Crucially, the inhibition of FZD8 led to a significant
reduction in the development of PCa bone metastasis in vivo.
These results uncovered a new bone metastasis pathway in PCa and
FZD8 was proposed as a promising target for treating PCa bone
metastasis.
T-Box transcription factor 2 (TBX2) exerts a
negative control on the cell cycle inhibitor, p21, and holds
significance in embryogenesis. Recent research has emphasized the
involvement of TBX2 in the spread of PCa to the bones. Nandana
et al (65) found that
transplanting TBX2-knockdown human PCa cell lines into mice reduced
tumor invasion and the spread of cancer cells to bone tissue.
Furthermore, the inhibition of endogenous TBX2 not only suppressed
the growth of tumor cells but also hindered bone remodeling in a
mouse tibial model, leading to a significant decrease in the
ability of PCa cells to colonize the bone. TBX2 plays a trans-role
by promoting the transcription of classic WNT (WNT3A) promoters.
Findings indicate that TBX2 serves as a new therapeutic objective
preceding WNT3A, and the use of WNT3A inhibitors could potentially
lead to the development of innovative medications to address the
spread of PCa to associated skeletal issues. A crucial aspect of
PCa bone metastasis is the increased G1/S phase transition due to
reduced protein levels of p16INK4a (p16) (65). Ubiquitin binding enzyme 2S (UBE2S)
was discovered to break down p16 via K11-linked ubiquitination,
consequently enhancing the transition from G1 to S phase in both
in vivo and in vitro PCa cells (66). Moreover, UBE2S additionally
enhanced the migration and invasion of tumor cells in PCa bone
metastasis by stabilizing β-catenin via K11-linked ubiquitination.
The findings of this research validate that UBE2S has a
cancer-promoting function in the spread of PCa to the bones and
indicate that targeting UBE2S could have multiple benefits in
treating PCa metastasis.
The presence of PCa stem cells (PCSCs) is crucial in
the advancement and spread of PCa, posing a challenge to
effectively treating the disease (67,68). Tumor-associated macrophages (TAMs)
are the most abundant immune cell population in the TME (69). Examining the systematic
interactions and network communication among PCSCs and TAMs can aid
in identifying crucial targets to hinder PCSCs and prevent
metastasis. Huang et al (70) demonstrated that TAMs secrete
chemokine ligand 5 (CCL5), which has a significant impact on the
migration, invasion and EMT of PCa cells and the self-renewal of
PCSCs. Additional research revealed that TAMs/CCL5 facilitated the
self-renewal of PCSCs and the metastasis of PCa through activation
of the β-catenin/STAT3 signaling pathway. The findings of this
research offer a justification for the exploration of TAMs/CCL5 as
a promising molecular focal point in the eradication of PCSCs and
the hindrance of metastatic PCa.
Furthermore, when PCa spreads to the bone, the fresh
surroundings can trigger epigenetic reprogramming and alteration of
the stemness of cancer cells, ultimately enhancing the ability of
cancer cells to adapt to the bone environment and potentially
resulting in the development of secondary tumor metastasis. RNA
binding motif 3 (RBM3), functioning as a protein that responds to
stress, has the ability to withstand the remodeling of the
microenvironment in PCa, particularly when it comes to bone
metastasis (71).
Methyltransferase 3 increases the methylation of N6-methyladenosine
on catenin β1 (CTNNβ1) mRNA, as induced by RBM3. Consequently, this
alteration results in a decrease in the stability of CTNNβ1 mRNA
and consequent deactivation of the Wnt signaling pathway,
ultimately impedes the remodeling of PCa cells by osteoblasts
(71).
According to previous research, the Ras signaling
pathway is crucial in the development of bone metastasis in
individuals with PCa (75). MYC
associated zinc finger protein (MAZ) is an oncogene implicated in
the advancement and spread of numerous cancer types (75). Yang et al (76) used real-time fluorescence
quantitative PCR and immunohistochemistry to detect the expression
of MAZ in PCa tissues with and without bone metastasis. The
findings indicated that the MAZ expression level was elevated in
PCa tissues with bone metastasis compared with those without bone
metastasis, and there was a further increase in MAZ expression in
metastatic bone tissues. Additionally, poor overall survival was
positively associated with high MAZ expression levels. The
enhancement of MAZ expression can augment the invasiveness and
migratory capacity of PCa cells in vitro, whereas the
suppression of MAZ can impede the ability of PCa cells to
metastasize to the bone in vivo (76). The findings additionally
demonstrated that MAZ enhances the spread of PCa to the bones by
activating the KRas pathway. The MAZ/KRas signaling axis has a
significant role in enhancing the spread of PCa to the bones,
indicating that MAZ could be a valuable therapeutic option for
treating PCa bone metastasis.
Fibroblast growth factor receptor 1 (FGFR1) has been
found to control cell proliferation, cell differentiation, cell
migration and cell survival through Ras/MAPK signaling pathways
(77). Labanca et al
(78) investigated FGFR1 in the
pathogenesis of PCa bone metastasis. The experimental evidence
demonstrated that the expression of FGFR1 led to the development of
bone metastasis and was notably abundant in the bone metastasis of
CRPC, thus affirming its crucial role in promoting metastasis in
PCa. Furthermore, PCa bone metastases exhibited an upregulation of
FGFR1 expression, and potential genes associated with FGFR1-induced
metastasis were discovered.
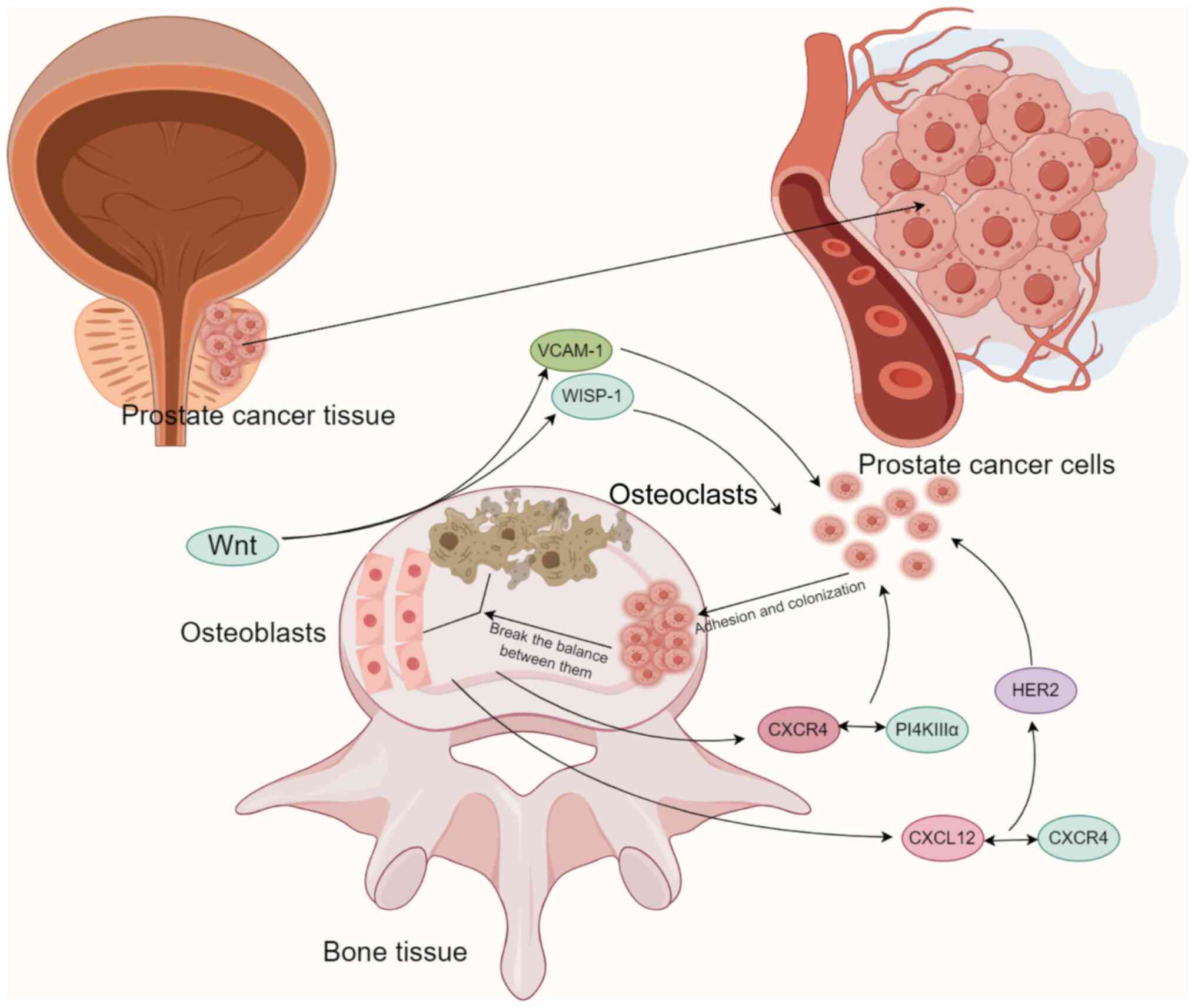
Numerous investigations have also examined the
involvement of bone tissue in the metastasis of PCa (Table II). These studies have revealed
that protein molecules associated with bone tissue facilitate the
attachment and establishment of PCa cells via pertinent signaling
pathways (Fig. 3). The secretion
of cytokines by bone tissue plays a crucial role in the onset and
progression of PCa bone metastasis. The function of WNT-induced
secreted protein 1 (WISP-1)/vascular adhesion molecule-1 (VCAM-1)
in enhancing the movement of PCa cells in humans has been
explained. Tai et al (79)
discovered that medium conditioned by osteoblast conditioned medium
(OBCM) prompted the movement and increased the expression of VCAM-1
in human PCa cells (PC3 and DU145). The introduction of WISP-1
shRNA into osteoblasts decreased PCa migration and the expression
of VCAM-1 induced by OBCM. Activation of PCa with OBCM or WISP-1
resulted in an elevation of the phosphorylation of focal adhesion
kinase (FAK) and p38. The migration and VCAM-1 expression of PCa
cells were promoted by osteoblast-derived WISP-1, which decreased
the expression of microRNA-126 through the integrin αvβ1, FAK and
p38 signaling pathways. Chang et al (80) discovered that WISP-1 controlled
the process of bone mineralization by stimulating the production of
bone morphogenetic protein 2, bone morphogenetic protein 4 (BMP4)
and osteopontin within osteoblasts. Additionally, it was discovered
that osteoblast-derived WISP-1 has a crucial function in
controlling the attachment of PCa cells to osteoblasts via the
VCAM-1/integrin α4β1 mechanism. WISP-1 is expected to be a crucial
target for PCa bone metastasis therapy.
Chemokine signaling in the bone environment plays a
crucial role in the progression of PCa, as supported by a
significant amount of evidence (22,81,82). Therapeutic strategies targeting
chemokines provide promising treatment options for bone metastasis.
The complexity of these signaling pathways is due to their
generation by various cell types, such as stromal cells and tumor
cells within the prostate tumor-bone microenvironment (83). The growth, invasion and bone
marrow metastasis of PCa cells are regulated by the signaling of
the chemokine receptor, chemokine C-X-C-primitive receptor 4
(CXCR4). In PCa cells, the binding of CXCR4 to the adaptor protein,
tetratricopeptide repeat domain 7, leads to the generation of
phosphatidylinositol 4-phosphate on the plasma membrane. The
interaction between CXCR4 and PI4KIIIα through the chemokine
signaling axis facilitates the proliferation of PCa bone metastasis
(84). Likewise, research has
indicated that the interaction between CXCL12 and CXCR4 activates
human epidermal growth factor receptor-2 and facilitates the growth
of tumors within the bone (85).
The initial development of PCa is assisted by the bone marrow
environment and inhibiting the CXCL12/CXCR4 pathway of this
environment and its subsequent signaling significantly impacts the
early formation of tumors in the bone microenvironment, while
advanced bone tumors are only responsive to inhibitors of growth
factor receptors (85). In the
bone metastasis of PCa, TGF-β derived from the bone triggers the
acetylation of Krüppel-like factor 5 (KLF5). This acetylated form
of KLF5 activates CXCR4, leading to the secretion of IL-11. This
secretion then stimulates the Sonic hedgehog/IL-6 paracrine
signaling pathway, resulting in the generation of osteoclasts and
the formation of bone metastatic lesions (86). Furthermore, it has been shown that
growth differentiation factor-15(GDF15) enhances osteoblast
activity and stimulates the development of PCa in the bone by
inducing the secretion of CCL2 and RANKL from osteoblasts and
attracting osteoblasts to initiate osteoclastogenesis (25).
In addition, studies have confirmed that the tumor
immunosuppressive microenvironment is also a major factor in
promoting PCa bone metastasis (14,87). Yin et al (88) reported that basic helix-loop-helix
family member e22 (BHLHE22) is upregulated in bone metastasis and
drives the immunosuppressed bone TME. Specifically, BHLHE22
facilitates the elevated production of colony stimulating factor 2,
resulting in the infiltration of immune-suppressing neutrophils and
monocytes and the extension of the immune-suppressing T cells
condition. These findings uncovered a mechanism by which PCa bone
metastasis suppresses the immune system and offer a possible
treatment strategy for individuals with bone metastasis from
PCa.
The communication between cancer cells and bone
tissue is also significant in the development of PCa metastasis to
the bones, and extensive research has been conducted in this area
(Table III). The spread of PCa
to the bone is a common occurrence, yet the underlying reasons for
this specific preference are still not fully understood. PCa bone
metastasis was discovered to be facilitated by the interaction of
receptor for advanced glycation end-products (RAGE; a cell surface
receptor expressed by malignant cells in advanced PCa) and
proteinase 3 (PR3) within the bone marrow microenvironment
(89). The interaction between
RAGE and PR3 was discovered to facilitate the migration of PCa
cells to the bone marrow. In vitro, PR3 attaches to RAGE
located on the surface of PCa cells and stimulates the movement of
tumor cells by activating and phosphorylating a non-proteolytic
signal transduction cascade involving ERK1/2 and JNK1. In animal
models of experimental metastasis, overexpression of RAGE on human
PCa cells is enough to facilitate migration to the bone marrow for
a brief duration. The findings of this research demonstrated the
role of the interaction between RAGE-PR3 in the bone metastasis,
which occurs during the progression of PCa, and have significant
implications for the prognosis and treatment of PCa. Furthermore,
growth factor progranulin (PGRN) was discovered as a potential
associate for prostate stem cell antigen (PSCA) in PCa cells.
Research has indicated that the NF-κB/integrin α4 pathway is
responsible for the promotion of PCa cell metastasis by PSCA/PGRN,
as it facilitates the adhesion of these cells to BMECs (90). These results indicated that
targeting PSCA/PGRN may have potential as a therapeutic approach
for the spread of PCa, particularly to the bones.
Recently, exosomes have been linked to the
communication between PCa cells and the microenvironment of bone
metastasis (91). Research has
indicated that the exosomal enzyme, phospholipase D (PLD) variant
1/2, facilitates the breakdown of phosphatidylcholine into
phosphatidic acid, thereby controlling the advancement of tumors
and their spread to other parts of the body (91). Borel et al (92) demonstrated for the first time that
phospholipase D2 (PLD2) is present in the exosomes of C4-2B and
PC-3 cells. Exosomes derived from C4-2B cells stimulate ERK 1/2
phosphorylation, leading to enhanced proliferation and
differentiation of osteoblast models. This activation also results
in increased activity of tissue non-specific alkaline phosphatase
and the upregulation of osteogenic differentiation markers. Thus,
PLD2 can be regarded as a proficient contributor to the formation
of PCa bone metastasis through exosomes released by tumor cells.
Moreover, the presence of RANKL receptor activator demonstrated the
ability of extracellular vesicles (EVs) derived from metastatic PCa
cells to enhance the development of osteoclasts (93). Through the characterization of EVs
and the screening of functional small interfering RNA, it was
discovered that cub domain containing protein 1 (CDCP1), a
transmembrane protein, functions as a stimulator of
osteoclastogenesis. Furthermore, the expression of CDCP1 is
increased on EVs derived from the plasma of patients with PCa who
have developed bone metastasis (93). These findings clarify the impact
of EVs derived from the metastatic cells of PCa on the creation of
osteoclasts, a process that is aided by the presence of CDCP1 on
the EVs. The findings of this research therefore indicate that the
presence of CDCP1 on EVs could potentially serve as a valuable
indicator for identifying bone metastasis in individuals with
PCa.
PCa bone metastasis triggers the conversion of
endothelial cells into osteoblasts (EC-OSBs) through the secretion
of BMP4 by tumor tissue, which induce interstitial reprogramming
and promote the progression of PCa (94). Yu et al (94) discovered that the signaling
pathway responsible for this process is inhibited by the
BMP4-induced phosphorylated-Smad1/Notch/hairy enhancer-of-split
related with YRPW motif 1 (Hey1) pathway, leading to a decrease in
endothelial cell migration and tube formation. Furthermore, BMP4
was observed to enhance the expression of tenascin C (TNC) in
EC-OSB cells via the Smad1/Notch/Hey1 signaling pathway (95). The migration of PCa cells is
facilitated by TNC via the integrin α5β1. The findings of these
studies indicate that tumor-induced interstitial reprogramming
produces TNC, which promotes the spread of PCa. This implies that
targeting TNC could be a potential approach for PCa treatment.
Spondin 2, a specific diagnostic marker for PCa, enhances the
expression of Osterix and Runx2 in osteoblasts, and this mechanism
is strongly linked to the stimulation of the PI3K/AKT/mTOR pathway.
Furthermore, the involvement of Spondin 2 in the promotion of
osteogenesis caused by PCa relies on the integrin receptor α5β1
(96). These findings indicate
that Spondin 2 facilitates the generation of bone by activating the
PI3K/AKT/mTOR pathway during the advancement of PCa.
Given the difficulties in managing PCa bone
metastasis, the current clinical approach emphasizes symptom
control, the development of novel targeted medications and the
prevention of SREs. According to the aforementioned research,
molecules associated with bone tissue and PCa cells are anticipated
to serve as a novel focus for combating PCa bone metastasis.
Numerous clinical studies have been conducted in recent years
(Table IV), most of which have
been published on ClinicalTrials.gov. By analyzing the published
research results, it was found that targeting molecules related to
bone metastasis of PCa has a certain value in the treatment of PCa.
However, due to the low survival time of the subjects and severe
side effects, some studies have not shown significant efficacy.
A clinical study conducted by Amgen (NCT00321620)
compared denosumab with zoledronic acid in the treatment of bone
metastasis in hormone-resistant PCa. For the first time, a
non-inferiority analysis was performed on the timing of SREs in the
study, Kaplan-Meier comparisons of median survival and dispersion
of denosumab vs. zoledronic acid treatment were 629.0
(573.00-757.00) vs. 521.0 (456.0-592.0) days (97). The time of the first SRE after
treatment was also compared (NCT00330759). The median time to the
first SREs for denosumab and zoledronic acid treatment was 625.0
(456.00-NA) vs. 496.0 (371.00-589.00) days (98). Since then, a phase 3 clinical
study of denosumab for the treatment of advanced PCa (NCT01419717)
has found a serious adverse event rate of 45/128 (35.16%) for
denosumab. These studies suggest that denosumab is more effective
than zoledronic acid in the treatment of prostate bone metastasis,
but with higher side effects.
Researchers are also trying to apply immune
checkpoint inhibitors to the treatment of PCa bone metastasis. A
related clinical study was carried out at Sidney Kimmel
Comprehensive Cancer Center at Johns Hopkins (NCT02601014). In this
study, enzalutamide plus nivolumab and ipilimumab was compared with
nivolumab and ipilimumab for 3 years and the resulting overall
survival time was 14.2 (8.5-NA) vs. 8.2 (5.5-10.4) months (99). To evaluate the safety and
tolerability of durvalumab plus tremelimumab in patients with
mCRPC, a clinical study was conducted by M.D. Anderson Cancer
Center (NCT03204812). It was found that the median overall survival
time of patients treated with durvalumab plus tremelimumab was 28.1
(14.5-37.3) months (100). In
addition, the efficacy of vaccine therapy and pembrolizumab in the
treatment of patients with hormone-resistant metastatic PCa
(NCT02499835) was investigated, and the 6-month progression-free
survival rate was 45% (101).
The data of this study indicated an improved median
progression-free survival time compared with the 3.7 months
reported by the study treating with sipuleucel-T alone (102). These studies indicate that
immune checkpoint inhibitors have certain value in the treatment of
bone metastasis of PCa, especially in combination with chemotherapy
drugs.
At present, inhibitors targeting small molecules are
widely used in the application of tumor targeted therapy and have
achieved notable efficacy. A dual kinase inhibitor of c-Met and
VEGFR-2 has been shown to reduce the growth of PCa in bone, and
there is evidence that it inhibits osteoblast activity (103,104). Some researchers have utilized
cabozantinib for the treatment of PCa bone metastasis. Cabozantinib
is a tyrosine kinase inhibitor that inhibits a variety of receptors
such as VEGFR2, c-Met, Kit, Axl and fms related receptor tyrosine
kinase 3 (105). A study
evaluated the effect of cabozantinib vs. prednisone on overall
survival in previously treated patients with mCRPC with bone
metastasis (NCT01605227). The overall survival time of the
cabozantinib and prednisone groups was 11.0 (10.09-11.63) vs. 9.8
(9.00-11.53) months and the progression-free survival time was 5.6
(5.49-5.62) vs. 2.8 (2.79-2.86) months (106). The University of Michigan Rogel
Cancer Center conducted a trial on cabozantinib (XL184) in mCRPC
(NCT01428219). The results showed that the amount of
progression-free patients at 12 weeks was 77.3%. These studies have
demonstrated that cabozantinib can significantly improve the
progression-free survival in patients with PCa and bone metastasis,
suggesting that it still has a positive therapeutic prospect.
In addition, there have been a number of studies on
the application of endothelin A receptor antagonists in the bone
metastasis of PCa. AstraZeneca conducted a Phase 3 clinical study
of ZD4054 (Zibotentan) in patients with PCa and bone metastasis
(NCT00554229). However, there was no statistically significant
difference in the overall survival and progression-free survival of
patients compared with the placebo. The therapeutic value of
Dovitinib (NCT01994590), sunitinib (NCT00299741) and Tandutinib
(NCT00390468) in the treatment of PCa bone metastasis have also
been investigated through clinical studies. Most of the treatment
results did not achieve significant survival benefits and had a
high number of serious side effects.
The treatment of mCRPC through targeted therapy for
prostate specific membrane antigen (PSMA) has made significant
progress in recent years. The use of 177Lu-PSMA-617 and
225Ac-PSMA-617 Radioligand therapy (RLT) in treating patients with
mCRPC has demonstrated positive biochemical responses. As a salvage
treatment option, this treatment option enhances patient survival
rates and minimizes treatment side effects (107-111). Sadaghiani et al (107) systematically evaluated the
effectiveness of RLT targeting PSMA in CRPC. According to the study
results, prostate specific antigen (PSA) decreased in more than
half of the patients after RLT treatment compared with the control
group. In a meta-analysis of patients with mCRPC, Kim and Kim
(108) found that PSA decreased
in two-thirds of patients and >50% in one-third of patients
after the first cycle of Lu-PSMA-617 RLT. In addition, over the
past decade, various targeted nanoparticles have been developed for
the diagnosis and treatment of bone metastases in PCa. In these
bone-targeting nanoparticles, ligands such as bisphosphonates,
peptides rich in aspartic acid and synthetic polymers were grafted
onto nanoparticles, such as poly (lactic-co-glycolic acid), for
bone targeting (112). At
present, nanomaterials such as liposomal doxorubicin
(Doxil®) and albumin/paclitaxel nanoparticles
(Abraxane®) have entered clinical studies (113).
Furthermore, while conventional treatments have
demonstrated efficacy in eradicating non-stem cell cancer cells,
they have not been as effective in targeting dormant cancer stem
cells (CSCs) (114,115). CSCs express high levels of
ATP-binding transporters that induce active drug efflux and block
drug uptake. In this context, P-glycoproteins and multidrug
resistance-associated proteins 1 and 2 are often upregulated in
CSCs (116,117). CSCs in PCa have been shown to be
resistant to radiation therapy, which may be related to the
activation of Chk1 and Chk2 (118). Radiotherapy induces cancer cells
to produce reactive oxygen species (ROS) leading to cancer cell
death in the treatment of PCa with bone metastasis. Nonetheless,
the exposure of CSCs to radiation has been found to elicit only
modest increases in ROS levels, consequently diminishing the extent
of DNA damage incurred (119).
There is growing evidence that CSC surface markers, including CXCR4
and epithelial cell adhesion molecule (EpCAM), are involved in
chemotherapy resistance. Specifically, inhibition of CXCR4 by
AMD3100 can improve the chemotherapy efficiency of docetaxel
(120) and knocking down EpCAM
in PCa cell lines can increase chemical sensitivity (121). The dormancy of CSCs is also an
important factor in drug resistance. Strategies to identify dormant
CSCs will benefit therapies targeting this cancer subgroup. New
treatment strategies should be adapted to effectively identify
CSCs, such as those expressing high levels of CD44. It has been
shown that inhibition of CD44 expression in PCSCs significantly
reduces the progression and metastasis of PCa (122).
An increasing number of studies have indicated that
the spread of PCa to the bones is the primary determinant of
prognosis for individuals with PCa (123,124). The presence of bone metastasis
holds immense clinical importance in the diagnosis and treatment of
patients. The molecular mechanisms reported thus far provide clues
for targeted therapy for bone metastasis. Bone metastasis in PCa
involves the participation of multiple molecules and pathways. It
has been shown that NF-κB, Wnt/β-catenin, TGF-β, Ras and other
signaling pathways promote the migration and metastasis of PCa
cells (49,64,73,76). Previous studies have confirmed
that NF-κB and Wnt/β-catenin signaling pathways are more involved
in PCa bone metastasis, and the molecules involved in regulation
will therefore be more promising targets for PCa bone metastasis
therapy, such as RANKL (50),
SIRT5 (53), FN14 (51), Cdk5 (60) and FZD8 (64). Bone tissue-related protein
molecules (WISP-1, CXCL12/CXCR4, BHLHE22, KLF5 and GDF15)
facilitate the adhesion and colonization of PCa cells in bone
tissue (25,79,85,86,88). In addition, the molecular signal
interaction between PCa tissue and bone tissue leads to the
directed metastasis of PCa cells (89,90,92,94). These molecular mechanisms offer
valuable insights into the prevention and management of bone
metastasis. The U.S. Food and Drug Administration (FDA) has also
approved targeted therapy for treating bone metastasis (125). In addition, existing clinical
studies have applied the aforementioned molecular mechanisms to
develop targeted drugs and have achieved efficacy in the initial
clinical studies. However, at present, the molecular mechanisms of
PCa bone metastasis, such as those in other tumors, are still
incompletely understood, especially the molecular interactions.
This leads to the problem of drug resistance to current tumor
targeted therapies (126).
Therefore, more basic and clinical studies are needed to reveal the
molecular mechanisms of bone metastasis. It is necessary to explore
the molecular interaction mechanism to identify the specific
molecules involved in bone metastasis to develop more precise
targeted drugs.
Epigenetic reprogramming enhances the adaptability
of PCa cells in the bone environment. Epigenetic regulators that
control key epigenetic changes, including histone modification and
DNA methylation, have been suggested to play key roles in the
dysregulation of transcription in cancer cells. Among the
epigenetic regulators, lysine-specific demethylase 1A (LSD1) is a
histone modifying enzyme responsible for the demethylation of the
histone H3 lysine 4. LSD1 has been reported to interact with the AR
and act as an active regulator of AR signaling in PCa (127-129). LSD1 has been identified as a
potential oncogene and therapeutic target for several cancer types
(130,131). Liang et al (132) reported that LSD1-mediated
deinherited reprogramming in CRPC, which activated the cell cycle
gene, centromere protein E, to drive PCa progression. Homeobox B13
(HOXB13) is a homeodomain transcription factor that plays an
important role in the regulation of AR activity and
androgen-dependent PCa growth (133). Lu et al (133) reported the interaction between
HOXB13 and histone deacetylase 3, which is disrupted by the HOXB13
G84E mutation. The mutation was found to be associated with
early-onset PCa. HOXB13 deletion or G84E mutation leads to lipid
accumulation in PCa cells, which promotes cell motility and
xenograft tumor metastasis. These studies suggest the potential
value of epigenetic regulatory factors in the treatment of bone
metastases in PCa.
The heterogeneity of tumor cells determines the
biological function of the tumor (134). The breakthrough method,
single-cell sequencing, has revealed the genetic and functional
heterogeneity of tumor cells (135). The heterogeneity of bone
metastasis and the identification of associated cell subsets will
be resolved with this methodology, which may lead to new findings
at the cellular therapeutic level. In the future, patients with
bone metastases should be subgrouped and treatments should be
selected on the basis of specific molecular characteristics.
Moreover, the molecular mechanism underlying PCa bone metastasis
has gradually become clear, which will also aid in the targeted
treatment of PCa bone metastasis.
In addition, the immunosuppressive TME has an
important role in the progression of PCa. Therefore, ameliorating
the immunosuppressive TME is an important strategy in the treatment
of bone metastases in PCa. For instance, the cancer vaccine
represented by sipuleucel-T is approved by the U.S. FDA for the
treatment of asymptomatic or mildly mCRPC (136). Immune checkpoint inhibitors have
also achieved initial efficacy in the treatment of mCRPC, with an
effective disease control rate (137,138). In addition, adoptive
immunotherapy involving chimeric antigen receptor (CAR)-T cells has
shown good tumor-killing efficacy in preclinical studies of PCa. At
present, more clinical studies are being conducted, and most of the
reported research results have shown suitable tolerance, with a
tumor immune response induced by CAR-T cells (139-141).
Multiple molecules and related pathways are involved
in PCa bone metastasis, and drugs targeting key molecules or
pathways involved in bone metastasis are being discovered and
validated in PCa. The targeting of key molecules involved in PCa
bone metastasis represents a new approach for treating PCa. As an
increasing number of important targets have been discovered,
targeted drugs for the treatment of bone metastasis will be widely
used in the near future.
Not applicable.
YX and ZZ made substantial contributions to
conception and design for the manuscript. GZ and JC performed
acquisition, analysis and interpretation of data. ZZ, YuL, YaL and
AT performed editing, drafting and writing of the manuscript. Data
authentication is not applicable. All authors read and approved the
final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This research was funded by Shandong Province Medical and Health
Science and Technology Development Plan Project (grant no.
202304051613) and Science and Technology Program of Yantai
Affiliated Hospital of Binzhou Medical University (grant no.
YTFY2024KYQD01).
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salji M, Hendry J, Patel A, Ahmad I, Nixon
C and Leung HY: Peri-prostatic fat volume measurement as a
predictive tool for castration resistance in advanced prostate
cancer. Eur Urol Focus. 4:858–866. 2018. View Article : Google Scholar
|
|
4
|
Yang L, Jin M, Park SJ, Seo SY and Jeong
KW: SETD1A promotes proliferation of castration-resistant prostate
cancer cells via FOXM1 transcription. Cancers (Basel). 12:17362020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chi JT, Lin PH, Tolstikov V, Oyekunle T,
Chen EY, Bussberg V, Greenwood B, Sarangarajan R, Narain NR,
Kiebish MA and Freedland SJ: Metabolomic effects of androgen
deprivation therapy treatment for prostate cancer. Cancer Med.
9:3691–3702. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu Z, Zou H, Wang H, Li Q and Yu D:
Identification of key gene signatures associated with bone
metastasis in castration-resistant prostate cancer using
co-expression analysis. Front Oncol. 10:5715242021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee S, Mendoza TR, Burner DN, Muldong MT,
Wu CCN, Arreola-Villanueva C, Zuniga A, Greenburg O, Zhu WY,
Murtadha J, et al: Novel dormancy mechanism of castration
resistance in bone metastatic prostate cancer organoids. Int J Mol
Sci. 23:32032022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clézardin P, Coleman R, Puppo M, Ottewell
P, Bonnelye E, Paycha F, Confavreux CB and Holen I: Bone
metastasis: Mechanisms, therapies, and biomarkers. Physiol Rev.
101:797–855. 2021. View Article : Google Scholar
|
|
9
|
Clarke NW, Hart CA and Brown MD: Molecular
mechanisms of metastasis in prostate cancer. Asian J Androl.
11:57–67. 2009. View Article : Google Scholar
|
|
10
|
Talreja DB: Importance of antiresorptive
therapies for patients with bone metastases from solid tumors.
Cancer Manag Res. 4:287–297. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Coleman RE: Clinical features of
metastatic bone disease and risk of skeletal morbidity. Clin Cancer
Res. 12:6243s–6249s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nørgaard M, Jensen AØ, Jacobsen JB, Cetin
K, Fryzek JP and Sørensen HT: Skeletal related events, bone
metastasis and survival of prostate cancer: A population based
cohort study in Denmark (1999 to 2007). J Urol. 184:162–167. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang X: Interactions between cancer cells
and bone microenvironment promote bone metastasis in prostate
cancer. Cancer Commun (Lond). 39:762019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang J, La Manna F, Bonollo F, Sampson N,
Alberts IL, Mingels C, Afshar-Oromieh A, Thalmann GN and
Karkampouna S: Tumor microenvironment mechanisms and bone
metastatic disease progression of prostate cancer. Cancer Lett.
530:156–169. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh DK, Patel VG, Oh WK and
Aguirre-Ghiso JA: Prostate cancer dormancy and reactivation in bone
marrow. J Clin Med. 10:26482021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bedeschi M, Marino N, Cavassi E, Piccinini
F and Tesei A: Cancer-associated fibroblast: Role in prostate
cancer progression to metastatic disease and therapeutic
resistance. Cells. 12:8022023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim JM, Lin C, Stavre Z, Greenblatt MB and
Shim JH: Osteoblast-osteoclast communication and bone homeostasis.
Cells. 9:20732020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mughees M, Kaushal JB, Sharma G, Wajid S,
Batra SK and Siddiqui JA: Chemokines and cytokines: Axis and allies
in prostate cancer pathogenesis. Semin Cancer Biol. 86:497–512.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gartrell BA, Coleman R, Efstathiou E,
Fizazi K, Logothetis CJ, Smith MR, Sonpavde G, Sartor O and Saad F:
Metastatic prostate cancer and the bone: Significance and
therapeutic options. Eur Urol. 68:850–858. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ban J, Fock V, Aryee DNT and Kovar H:
Mechanisms, diagnosis and treatment of bone metastases. Cells.
10:29442021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng X, He G, Liu J, Luo F, Peng X, Tang
S, Gao Z, Lin Q, Keller JM, Yang T and Keller ET: Recent advances
in bone-targeted therapies of metastatic prostate cancer. Cancer
Treat Rev. 40:730–738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baci D, Bruno A, Cascini C, Gallazzi M,
Mortara L, Sessa F, Pelosi G, Albini A and Noonan DM:
Acetyl-L-carnitine downregulates invasion (CXCR4/CXCL12, MMP-9) and
angiogenesis (VEGF, CXCL8) pathways in prostate cancer cells:
Rationale for prevention and interception strategies. J Exp Clin
Cancer Res. 38:4642019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Midavaine É, Côté J and Sarret P: The
multifaceted roles of the chemokines CCL2 and CXCL12 in osteophilic
metastatic cancers. Cancer Metastasis Rev. 40:427–445. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cioni B, Nevedomskaya E, Melis MHM, van
Burgsteden J, Stelloo S, Hodel E, Spinozzi D, de Jong J, van der
Poel H, de Boer JP, et al: Loss of androgen receptor signaling in
prostate cancer-associated fibroblasts (CAFs) promotes CCL2- and
CXCL8-mediated cancer cell migration. Mol Oncol. 12:1308–1323.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siddiqui JA, Seshacharyulu P, Muniyan S,
Pothuraju R, Khan P, Vengoji R, Chaudhary S, Maurya SK, Lele SM,
Jain M, et al: GDF15 promotes prostate cancer bone metastasis and
colonization through osteoblastic CCL2 and RANKL activation. Bone
Res. 10:62022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Y, He Y, Butler W, Xu L, Chang Y, Lei
K, Zhang H, Zhou Y, Gao AC, Zhang Q, et al: Targeting cellular
heterogeneity with CXCR2 blockade for the treatment of
therapy-resistant prostate cancer. Sci Transl Med. 11:eaax04282019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Singh R, Kapur N, Mir H, Singh N, Lillard
JW Jr and Singh S: CXCR6-CXCL16 axis promotes prostate cancer by
mediating cytoskeleton rearrangement via Ezrin activation and αvβ3
integrin clustering. Oncotarget. 7:7343–7353. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Connell B, Kopach P, Ren W, Joshi R, Naber
S, Zhou M and Mathew P: Aberrant integrin αv and α5 expression in
prostate adenocarcinomas and bone-metastases is consistent with a
bone-colonizing phenotype. Transl Androl Urol. 9:1630–1638. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Massagué J and Obenauf AC: Metastatic
colonization by circulating tumour cells. Nature. 529:298–306.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Quayle L, Ottewell PD and Holen I: Bone
metastasis: Molecular mechanisms implicated in tumour cell dormancy
in breast and prostate cancer. Curr Cancer Drug Targets.
15:469–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yumoto K, Eber MR, Wang J, Cackowski FC,
Decker AM, Lee E, Nobre AR, Aguirre-Ghiso JA, Jung Y and Taichman
RS: Axl is required for TGF-β2-induced dormancy of prostate cancer
cells in the bone marrow. Sci Rep. 6:365202016. View Article : Google Scholar
|
|
32
|
Kobayashi A, Okuda H, Xing F, Pandey PR,
Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C, et al:
Bone morphogenetic protein 7 in dormancy and metastasis of prostate
cancer stem-like cells in bone. J Exp Med. 208:2641–2655. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Park M, Cho YJ, Kim B, Ko YJ, Jang Y, Moon
YH, Hyun H and Lim W: RANKL immunisation inhibits prostate cancer
metastasis by modulating EMT through a RANKL-dependent pathway. Sci
Rep. 11:121862021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ren D, Dai Y, Yang Q, Zhang X, Guo W, Ye
L, Huang S, Chen X, Lai Y, Du H, et al: Wnt5a induces and maintains
prostate cancer cells dormancy in bone. J Exp Med. 216:428–449.
2019. View Article : Google Scholar :
|
|
35
|
Ruppender N, Larson S, Lakely B, Kollath
L, Brown L, Coleman I, Coleman R, Nguyen H, Nelson PS, Corey E, et
al: Cellular adhesion promotes prostate cancer cells escape from
dormancy. PLoS One. 10:e01305652015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rojas A, Liu G, Coleman I, Nelson PS,
Zhang M, Dash R, Fisher PB, Plymate SR and Wu JD: IL-6 promotes
prostate tumorigenesis and progression through autocrine
cross-activation of IGF-IR. Oncogene. 30:2345–2355. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Danilucci TM, Santos PK, Pachane BC,
Pisani GFD, Lino RLB, Casali BC, Altei WF and Selistre-de-Araujo
HS: Recombinant RGD-disintegrin DisBa-01 blocks integrin
αvβ3 and impairs VEGF signaling in
endothelial cells. Cell Commun Signal. 17:272019. View Article : Google Scholar
|
|
38
|
Hashemi M, Taheriazam A, Daneii P,
Hassanpour A, Kakavand A, Rezaei S, Hejazi ES, Aboutalebi M,
Gholamrezaie H, Saebfar H, et al: Targeting PI3K/Akt signaling in
prostate cancer therapy. J Cell Commun Signal. 17:423–443. 2023.
View Article : Google Scholar :
|
|
39
|
Cooper CR and Pienta KJ: Cell adhesion and
chemotaxis in prostate cancer metastasis to bone: A minireview.
Prostate Cancer Prostatic Dis. 3:6–12. 2000. View Article : Google Scholar
|
|
40
|
Yin JJ, Pollock CB and Kelly K: Mechanisms
of cancer metastasis to the bone. Cell Res. 15:57–62. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang Y, Liang J, Liu P, Wang Q, Liu L and
Zhao H: The RANK/RANKL/OPG system and tumor bone metastasis:
Potential mechanisms and therapeutic strategies. Front Endocrinol
(Lausanne). 13:10638152022. View Article : Google Scholar
|
|
42
|
Wong SK, Mohamad NV, Giaze TR, Chin KY,
Mohamed N and Ima-Nirwana S: Prostate cancer and bone metastases:
The underlying mechanisms. Int J Mol Sci. 20:25872019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim SW, Kim JS, Papadopoulos J, Choi HJ,
He J, Maya M, Langley RR, Fan D, Fidler IJ and Kim SJ: Consistent
interactions between tumor cell IL-6 and macrophage TNF-α enhance
the growth of human prostate cancer cells in the bone of nude
mouse. Int Immunopharmacol. 11:862–872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Baldessari C, Pipitone S, Molinaro E,
Cerma K, Fanelli M, Nasso C, Oltrecolli M, Pirola M, D'Agostino E,
Pugliese G, et al: Bone metastases and health in prostate cancer:
From pathophysiology to clinical implications. Cancers (Basel).
15:15182023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vičić I and Belev B: The pathogenesis of
bone metastasis in solid tumors: A review. Croat Med J. 62:270–282.
2021. View Article : Google Scholar
|
|
46
|
Yu H, Lin L, Zhang Z, Zhang H and Hu H:
Targeting NF-κB pathway for the therapy of diseases: Mechanism and
clinical study. Signal Transduct Target Ther. 5:2092020. View Article : Google Scholar
|
|
47
|
Verzella D, Fischietti M, Capece D,
Vecchiotti D, Del Vecchio F, Cicciarelli G, Mastroiaco V, Tessitore
A, Alesse E and Zazzeroni F: Targeting the NF-κB pathway in
prostate cancer: A promising therapeutic approach? Curr Drug
Targets. 17:311–320. 2016. View Article : Google Scholar
|
|
48
|
Al-Rashidi RR, Noraldeen SAM, Kareem AK,
Mahmoud AK, Kadhum WR, Ramírez-Coronel AA, Iswanto AH, Obaid RF,
Jalil AT, Mustafa YF, et al: Malignant function of nuclear
factor-kappaB axis in prostate cancer: Molecular interactions and
regulation by non-coding RNAs. Pharmacol Res. 194:1067752023.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhu W, Hu X, Xu J, Cheng Y, Shao Y and
Peng Y: Effect of PI3K/Akt signaling pathway on the process of
prostate cancer metastasis to bone. Cell Biochem Biophys.
72:171–177. 2015. View Article : Google Scholar
|
|
50
|
Ziaee S and Chung LW: Induction of
integrin α2 in a highly bone metastatic human prostate cancer cell
line: Roles of RANKL and AR under three-dimensional suspension
culture. Mol Cancer. 13:2082014. View Article : Google Scholar
|
|
51
|
Yin J, Liu YN, Tillman H, Barrett B,
Hewitt S, Ylaya K, Fang L, Lake R, Corey E, Morrissey C, et al:
AR-regulated TWEAK-FN14 pathway promotes prostate cancer bone
metastasis. Cancer Res. 74:4306–4317. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lee C, Whang YM, Campbell P, Mulcrone PL,
Elefteriou F, Cho SW and Park SI: Dual targeting c-met and VEGFR2
in osteoblasts suppresses growth and osteolysis of prostate cancer
bone metastasis. Cancer Lett. 414:205–213. 2018. View Article : Google Scholar
|
|
53
|
Choi SY, Jeon JM, Na AY, Kwon OK, Bang IH,
Ha YS, Bae EJ, Park BH, Lee EH, Kwon TG, et al: SIRT5 directly
inhibits the PI3K/AKT pathway in prostate cancer cell lines. Cancer
Genomics Proteomics. 19:50–59. 2022. View Article : Google Scholar :
|
|
54
|
Chen JR, Zhao JT and Xie ZZ:
Integrin-mediated cancer progression as a specific target in
clinical therapy. Biomed Pharmacother. 155:1137452022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hamidi H and Ivaska J: Every step of the
way: Integrins in cancer progression and metastasis. Nat Rev
Cancer. 18:533–548. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li M, Wang Y, Li M, Wu X, Setrerrahmane S
and Xu H: Integrins as attractive targets for cancer therapeutics.
Acta Pharm Sin B. 11:2726–2737. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Giancotti FG and Ruoslahti E: Integrin
signaling. Science. 285:1028–1032. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hynes RO: Integrins: Versatility,
modulation, and signaling in cell adhesion. Cell. 69:11–25. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cooper J and Giancotti FG: Integrin
signaling in cancer: Mechanotransduction, stemness, epithelial
plasticity, and therapeutic resistance. Cancer Cell. 35:347–367.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jin JK, Tien PC, Cheng CJ, Song JH, Huang
C, Lin SH and Gallick GE: Talin1 phosphorylation activates β1
integrins: A novel mechanism to promote prostate cancer bone
metastasis. Oncogene. 34:1811–1821. 2015. View Article : Google Scholar
|
|
61
|
Chen PC, Tang CH, Lin LW, Tsai CH, Chu CY,
Lin TH and Huang YL: Thrombospondin-2 promotes prostate cancer bone
metastasis by the up-regulation of matrix metalloproteinase-2
through down-regulating miR-376c expression. J Hematol Oncol.
10:332017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Krishnamurthy N and Kurzrock R: Targeting
the Wnt/beta-catenin pathway in cancer: Update on effectors and
inhibitors. Cancer Treat Rev. 62:50–60. 2018. View Article : Google Scholar
|
|
63
|
Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L, Wu
C, Wang C and Ye L: Wnt/β-catenin signaling in cancers and targeted
therapies. Signal Transduct Target Ther. 6:3072021. View Article : Google Scholar
|
|
64
|
Li Q, Ye L, Zhang X, Wang M, Lin C, Huang
S, Guo W, Lai Y, Du H, Li J, et al: FZD8, a target of p53, promotes
bone metastasis in prostate cancer by activating canonical
Wnt/β-catenin signaling. Cancer Lett. 402:166–176. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nandana S, Tripathi M, Duan P, Chu CY,
Mishra R, Liu C, Jin R, Yamashita H, Zayzafoon M, Bhowmick NA, et
al: Bone metastasis of prostate cancer can be therapeutically
targeted at the TBX2-WNT signaling axis. Cancer Res. 77:1331–1344.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Peng S, Chen X, Huang C, Yang C, Situ M,
Zhou Q, Ling Y, Huang H, Huang M, Zhang Y, et al: UBE2S as a novel
ubiquitinated regulator of p16 and β-catenin to promote bone
metastasis of prostate cancer. Int J Biol Sci. 18:3528–3543. 2022.
View Article : Google Scholar :
|
|
67
|
Tang DG: Understanding and targeting
prostate cancer cell heterogeneity and plasticity. Semin Cancer
Biol. 82:68–93. 2022. View Article : Google Scholar :
|
|
68
|
Wolf I, Gratzke C and Wolf P: Prostate
cancer stem cells: Clinical aspects and targeted therapies. Front
Oncol. 12:9357152022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Pittet MJ, Michielin O and Migliorini D:
Clinical relevance of tumour-associated macrophages. Nat Rev Clin
Oncol. 19:402–421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Huang R, Wang S, Wang N, Zheng Y, Zhou J,
Yang B, Wang X, Zhang J, Guo L, Wang S, et al: CCL5 derived from
tumor-associated macrophages promotes prostate cancer stem cells
and metastasis via activating β-catenin/STAT3 signaling. Cell Death
Dis. 11:2342020. View Article : Google Scholar
|
|
71
|
Zhang S, Lv C, Niu Y, Li C, Li X, Shang Y,
Zhang Y, Zhang Y, Zhang Y and Zeng Y: RBM3 suppresses stemness
remodeling of prostate cancer in bone microenvironment by
modulating N6-methyladenosine on CTNNB1 mRNA. Cell Death Dis.
14:912023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Meng X, Vander Ark A, Daft P, Woodford E,
Wang J, Madaj Z and Li X: Loss of TGF-β signaling in osteoblasts
increases basic-FGF and promotes prostate cancer bone metastasis.
Cancer Lett. 418:109–118. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu X, Chen L, Fan Y, Hong Y, Yang X, Li
Y, Lu J, Lv J, Pan X, Qu F, et al: IFITM3 promotes bone metastasis
of prostate cancer cells by mediating activation of the TGF-β
signaling pathway. Cell Death Dis. 10:5172019. View Article : Google Scholar
|
|
74
|
Yan Z, Jin S, Wei Z, Huilian H, Zhanhai Y,
Yue T, Juan L, Jing L, Libo Y and Xu L: Discoidin domain receptor 2
facilitates prostate cancer bone metastasis via regulating
parathyroid hormone-related protein. Biochim Biophys Acta.
1842:1350–1363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lin SR, Mokgautsi N and Liu YN: Ras and
Wnt interaction contribute in prostate cancer bone metastasis.
Molecules. 25:23802020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yang Q, Lang C, Wu Z, Dai Y, He S, Guo W,
Huang S, Du H, Ren D and Peng X: MAZ promotes prostate cancer bone
metastasis through transcriptionally activating the KRas-dependent
RalGEFs pathway. J Exp Clin Cancer Res. 38:3912019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Eswarakumar VP, Lax I and Schlessinger J:
Cellular signaling by fibroblast growth factor receptors. Cytokine
Growth Factor Rev. 16:139–149. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Labanca E, Yang J, Shepherd PDA, Wan X,
Starbuck MW, Guerra LD, Anselmino N, Bizzotto JA, Dong J,
Chinnaiyan AM, et al: Fibroblast growth factor receptor 1 drives
the metastatic progression of prostate cancer. Eur Urol Oncol.
5:164–175. 2022. View Article : Google Scholar
|
|
79
|
Tai HC, Chang AC, Yu HJ, Huang CY, Tsai
YC, Lai YW, Sun HL, Tang CH and Wang SW: Osteoblast-derived
WNT-induced secreted protein 1 increases VCAM-1 expression and
enhances prostate cancer metastasis by down-regulating miR-126.
Oncotarget. 5:7589–7598. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Chang AC, Chen PC, Lin YF, Su CM, Liu JF,
Lin TH, Chuang SM and Tang CH: Osteoblast-secreted WISP-1 promotes
adherence of prostate cancer cells to bone via the VCAM-1/integrin
α4β1 system. Cancer Lett. 426:47–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T,
Chen Y, Han X and Wu K: The CXCL8-CXCR1/2 pathways in cancer.
Cytokine Growth Factor Rev. 31:61–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hao Q, Vadgama JV and Wang P: CCL2/CCR2
signaling in cancer pathogenesis. Cell Commun Signal. 18:822020.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Johnson CS and Cook LM: Osteoid
cell-derived chemokines drive bone-metastatic prostate cancer.
Front Oncol. 13:11005852023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Govindarajan B, Sbrissa D, Pressprich M,
Kim S, Vaishampayan U, Cher ML and Chinni S: Adaptor proteins
mediate CXCR4 and PI4KA crosstalk in prostate cancer cells and the
significance of PI4KA in bone tumor growth. Res Sq [Preprint]:
rs.3.rs-2590830. 2023.
|
|
85
|
Conley-LaComb MK, Semaan L, Singareddy R,
Li Y, Heath EI, Kim S, Cher ML and Chinni SR: Pharmacological
targeting of CXCL12/CXCR4 signaling in prostate cancer bone
metastasis. Mol Cancer. 15:682016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang B, Li Y, Wu Q, Xie L, Barwick B, Fu
C, Li X, Wu D, Xia S, Chen J, et al: Acetylation of KLF5 maintains
EMT and tumorigenicity to cause chemoresistant bone metastasis in
prostate cancer. Nat Commun. 12:17142021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhang Z, Karthaus WR, Lee YS, Gao VR, Wu
C, Russo JW, Liu M, Mota JM, Abida W, Linton E, et al: Tumor
microenvironment-derived NRG1 promotes antiandrogen resistance in
prostate cancer. Cancer Cell. 38:279–296.e9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yin C, Wang M, Wang Y, Lin Q, Lin K, Du H,
Lang C, Dai Y and Peng X: BHLHE22 drives the immunosuppressive bone
tumor microenvironment and associated bone metastasis in prostate
cancer. J Immunother Cancer. 11:e0055322023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kolonin MG, Sergeeva A, Staquicini DI,
Smith TL, Tarleton CA, Molldrem JJ, Sidman RL, Marchiò S,
Pasqualini R and Arap W: Interaction between tumor cell surface
receptor RAGE and proteinase 3 mediates prostate cancer metastasis
to bone. Cancer Res. 77:3144–3150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhao Z, Li E, Luo L, Zhao S, Liu L, Wang
J, Kang R and Luo J: A PSCA/PGRN-NF-κB-integrin-α4 axis promotes
prostate cancer cell adhesion to bone marrow endothelium and
enhances metastatic potential. Mol Cancer Res. 18:501–513. 2020.
View Article : Google Scholar
|
|
91
|
Geng X, Chang B and Shan J: Role and
correlation of exosomes and integrins in bone metastasis of
prostate cancer. Andrologia. 54:e145502022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Borel M, Lollo G, Magne D, Buchet R,
Brizuela L and Mebarek S: Prostate cancer-derived exosomes promote
osteoblast differentiation and activity through phospholipase D2.
Biochim Biophys Acta Mol Basis Dis. 1866:1659192020. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Urabe F, Kosaka N, Yamamoto Y, Ito K,
Otsuka K, Soekmadji C, Egawa S, Kimura T and Ochiya T: Metastatic
prostate cancer-derived extracellular vesicles facilitate
osteoclastogenesis by transferring the CDCP1 protein. J Extracell
Vesicles. 12:e123122023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yu G, Shen P, Lee YC, Pan J, Song JH, Pan
T, Lin SC, Liang X, Wang G, Panaretakis T, et al: Multiple pathways
coordinating reprogramming of endothelial cells into osteoblasts by
BMP4. iScience. 24:1023882021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lee YC, Lin SC, Yu G, Zhu M, Song JH,
Rivera K, Pappin DJ, Logothetis CJ, Panaretakis T, Wang G, et al:
Prostate tumor-induced stromal reprogramming generates tenascin C
that promotes prostate cancer metastasis through YAP/TAZ
inhibition. Oncogene. 41:757–769. 2022. View Article : Google Scholar :
|
|
96
|
Wang H, Zhang M, Lu W and Yuan C: Prostate
cancer cell-derived spondin 2 boosts osteogenic factor levels in
osteoblasts via the PI3K/AKT/mTOR pathway. Oncol Rep. 49:232023.
View Article : Google Scholar
|
|
97
|
Fizazi K, Carducci M, Smith M, Damião R,
Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, et al:
Denosumab versus zoledronic acid for treatment of bone metastases
in men with castration-resistant prostate cancer: A randomised,
double-blind study. Lancet. 377:813–822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Henry D, Vadhan-Raj S, Hirsh V, von Moos
R, Hungria V, Costa L, Woll PJ, Scagliotti G, Smith G, Feng A, et
al: Delaying skeletal-related events in a randomized phase 3 study
of denosumab versus zoledronic acid in patients with advanced
cancer: An analysis of data from patients with solid tumors.
Support Care Cancer. 22:679–687. 2014. View Article : Google Scholar
|
|
99
|
Shenderov E, Boudadi K, Fu W, Wang H,
Sullivan R, Jordan A, Dowling D, Harb R, Schonhoft J, Jendrisak A,
et al: Nivolumab plus ipilimumab, with or without enzalutamide, in
AR-V7-expressing metastatic castration-resistant prostate cancer: A
phase-2 nonrandomized clinical trial. Prostate. 81:326–338. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Subudhi SK, Siddiqui BA, Aparicio AM,
Yadav SS, Basu S, Chen H, Jindal S, Tidwell RSS, Varma A,
Logothetis CJ, et al: Combined CTLA-4 and PD-L1 blockade in
patients with chemotherapy-naïve metastatic castration-resistant
prostate cancer is associated with increased myeloid and neutrophil
immune subsets in the bone microenvironment. J Immunother Cancer.
9:e0029192021. View Article : Google Scholar
|
|
101
|
McNeel DG, Eickhoff JC, Wargowski E,
Johnson LE, Kyriakopoulos CE, Emamekhoo H, Lang JM, Brennan MJ and
Liu G: Phase 2 trial of T-cell activation using MVI-816 and
pembrolizumab in patients with metastatic, castration-resistant
prostate cancer (mCRPC). J Immunother Cancer. 10:e0041982022.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, et al: Sipuleucel-T immunotherapy for castration-resistant
prostate cancer. N Engl J Med. 363:411–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Su P, Zhang M and Kang X: Targeting c-Met
in the treatment of urologic neoplasms: Current status and
challenges. Front Oncol. 13:10710302023. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Azad AA, Beardsley EK, Hotte SJ, Ellard
SL, Klotz L, Chin J, Kollmannsberger C, Mukherjee SD and Chi KN: A
randomized phase II efficacy and safety study of vandetanib
(ZD6474) in combination with bicalutamide versus bicalutamide alone
in patients with chemotherapy naïve castration-resistant prostate
cancer. Invest New Drugs. 32:746–752. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Maroto P, Porta C, Capdevila J, Apolo AB,
Viteri S, Rodriguez-Antona C, Martin L and Castellano D:
Cabozantinib for the treatment of solid tumors: A systematic
review. Ther Adv Med Oncol. 14:175883592211071122022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Smith M, De Bono J, Sternberg C, Le Moulec
S, Oudard S, De Giorgi U, Krainer M, Bergman A, Hoelzer W, De Wit
R, et al: Phase III Study of cabozantinib in previously treated
metastatic castration-resistant prostate cancer: COMET-1. J Clin
Oncol. 34:3005–3013. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Sadaghiani MS, Sheikhbahaei S, Werner RA,
Pienta KJ, Pomper MG, Gorin MA, Solnes LB and Rowe SP:
177 Lu-PSMA radioligand therapy effectiveness in
metastatic castration-resistant prostate cancer: An updated
systematic review and meta-analysis. Prostate. 82:826–835. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Kim YJ and Kim YI: Therapeutic responses
and survival effects of 177Lu-PSMA-617 radioligand therapy in
metastatic castrate-resistant prostate cancer: A meta-analysis.
Clin Nucl Med. 43:728–734. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Thang SP, Violet J, Sandhu S, Iravani A,
Akhurst T, Kong G, Ravi Kumar A, Murphy DG, Williams SG, Hicks RJ
and Hofman MS: Poor outcomes for patients with metastatic
castration-resistant prostate cancer with low prostate-specific
membrane antigen (PSMA) expression deemed ineligible for
177Lu-labelled PSMA radioligand therapy. Eur Urol Oncol.
2:670–676. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Ma J, Li L, Liao T, Gong W and Zhang C:
Efficacy and safety of 225Ac-PSMA-617-targeted alpha
therapy in metastatic castration-resistant prostate cancer: A
systematic review and meta-analysis. Front Oncol. 12:7966572022.
View Article : Google Scholar
|
|
111
|
Ballal S, Yadav MP, Sahoo RK, Tripathi M,
Dwivedi SN and Bal C: 225 Ac-PSMA-617-targeted alpha
therapy for the treatment of metastatic castration-resistant
prostate cancer: A systematic review and meta-analysis. Prostate.
81:580–591. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Gao X, Li L, Cai X, Huang Q, Xiao J and
Cheng Y: Targeting nanoparticles for diagnosis and therapy of bone
tumors: Opportunities and challenges. Biomaterials. 265:1204042021.
View Article : Google Scholar
|
|
113
|
Chen G, Arns S and Young RN: Determination
of the rat in vivo pharmacokinetic profile of a bone-targeting
dual-action pro-drug for treatment of osteoporosis. Bioconjug Chem.
26:1095–1103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Bighetti-Trevisan RL, Sousa LO, Castilho
RM and Almeida LO: Cancer stem cells: Powerful targets to improve
current anticancer therapeutics. Stem Cells Int. 2019:96180652019.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Garcia-Mayea Y, Mir C, Masson F, Paciucci
R and LLeonart ME: Insights into new mechanisms and models of
cancer stem cell multidrug resistance. Semin Cancer Biol.
60:166–180. 2020. View Article : Google Scholar
|
|
116
|
Riganti C, Contino M, Guglielmo S, Perrone
MG, Salaroglio IC, Milosevic V, Giampietro R, Leonetti F, Rolando
B, Lazzarato L, et al: Design, biological evaluation, and molecular
modeling of tetrahydroisoquinoline derivatives: Discovery of a
potent p-glycoprotein ligand overcoming multidrug resistance in
cancer stem cells. J Med Chem. 62:974–986. 2019. View Article : Google Scholar
|
|
117
|
Cho Y and Kim YK: Cancer stem cells as a
potential target to overcome multidrug resistance. Front Oncol.
10:7642020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wang X, Ma Z, Xiao Z, Liu H, Dou Z, Feng X
and Shi H: Chk1 knockdown confers radiosensitization in prostate
cancer stem cells. Oncol Rep. 28:2247–2254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Mei W, Lin X, Kapoor A, Gu Y, Zhao K and
Tang D: The contributions of prostate cancer stem cells in prostate
cancer initiation and metastasis. Cancers (Basel). 11:4342019.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Domanska UM, Timmer-Bosscha H, Nagengast
WB, Oude Munnink TH, Kruizinga RC, Ananias HJ, Kliphuis NM, Huls G,
De Vries EG, de Jong IJ and Walenkamp AM: CXCR4 inhibition with
AMD3100 sensitizes prostate cancer to docetaxel chemotherapy.
Neoplasia. 14:709–718. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Ni J, Cozzi P, Beretov J, Duan W, Bucci J,
Graham P and Li Y: Epithelial cell adhesion molecule (EpCAM) is
involved in prostate cancer chemotherapy/radiotherapy response in
vivo. BMC Cancer. 18:10922018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Yao L and Zhang X: Interaction between
prostate cancer stem cells and bone microenvironment regulates
prostate cancer bone metastasis and treatment resistance. J Cancer.
13:2757–2767. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Klaff R, Varenhorst E, Berglund A, Hedlund
PO, Sjöberg F and Sandblom G; SPCG-5 Study Group: Clinical
presentation and predictors of survival related to extent of bone
metastasis in 900 prostate cancer patients. Scand J Urol.
50:352–359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Fizazi K, Massard C, Smith M, Rader M,
Brown J, Milecki P, Shore N, Oudard S, Karsh L, Carducci M, et al:
Bone-related parameters are the main prognostic factors for overall
survival in men with bone metastases from castration-resistant
prostate cancer. Eur Urol. 68:42–50. 2015. View Article : Google Scholar
|
|
125
|
Zhang J, Sun J, Bakht S and Hassan W:
Recent development and future prospects of molecular targeted
therapy in prostate cancer. Curr Mol Pharmacol. 15:159–169.
2022.
|
|
126
|
Liang XW, Liu B, Chen JC, Cao Z, Chu FR,
Lin X, Wang SZ and Wu JC: Characteristics and molecular mechanism
of drug-tolerant cells in cancer: A review. Front Oncol.
13:11774662023. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Cai C, He HH, Gao S, Chen S, Yu Z, Gao Y,
Chen S, Chen MW, Zhang J, Ahmed M, et al: Lysine-specific
demethylase 1 has dual functions as a major regulator of androgen
receptor transcriptional activity. Cell Rep. 9:1618–1627. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Yatim A, Benne C, Sobhian B,
Laurent-Chabalier S, Deas O, Judde JG, Lelievre JD, Levy Y and
Benkirane M: NOTCH1 nuclear interactome reveals key regulators of
its transcriptional activity and oncogenic function. Mol Cell.
48:445–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wissmann M, Yin N, Müller JM, Greschik H,
Fodor BD, Jenuwein T, Vogler C, Schneider R, Günther T, Buettner R,
et al: Cooperative demethylation by JMJD2C and LSD1 promotes
androgen receptor-dependent gene expression. Nat Cell Biol.
9:347–353. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Lynch JT, Harris WJ and Somervaille TC:
LSD1 inhibition: A therapeutic strategy in cancer? Expert Opin Ther
Targets. 16:1239–1249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Maes T, Mascaró C, Ortega A, Lunardi S,
Ciceri F, Somervaille TC and Buesa C: KDM1 histone lysine
demethylases as targets for treatments of oncological and
neurodegenerative disease. Epigenomics. 7:609–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Liang Y, Ahmed M, Guo H, Soares F, Hua JT,
Gao S, Lu C, Poon C, Han W, Langstein J, et al: LSD1-mediated
epigenetic reprogramming drives CENPE expression and prostate
cancer progression. Cancer Res. 77:5479–5490. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lu X, Fong KW, Gritsina G, Wang F, Baca
SC, Brea LT, Berchuck JE, Spisak S, Ross J, Morrissey C, et al:
HOXB13 suppresses de novo lipogenesis through HDAC3-mediated
epigenetic reprogramming in prostate cancer. Nat Genet. 54:670–683.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Meacham CE and Morrison SJ: Tumour
heterogeneity and cancer cell plasticity. Nature. 501:328–337.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Tanay A and Regev A: Scaling single-cell
genomics from phenomenology to mechanism. Nature. 541:331–338.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Apostolopoulos V, Thalhammer T, Tzakos AG
and Stojanovska L: Targeting antigens to dendritic cell receptors
for vaccine development. J Drug Deliv. 2013:8697182013. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Kwon ED, Drake CG, Scher HI, Fizazi K,
Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R,
Mahammedi H, et al: Ipilimumab versus placebo after radiotherapy in
patients with metastatic castration-resistant prostate cancer that
had progressed after docetaxel chemotherapy (CA184-043): A
multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol.
15:700–712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Fizazi K, Drake CG, Beer TM, Kwon ED,
Scher HI, Gerritsen WR, Bossi A, den Eertwegh AJMV, Krainer M,
Houede N, et al: Final analysis of the ipilimumab versus placebo
following radiotherapy phase III trial in postdocetaxel metastatic
castration-resistant prostate cancer identifies an excess of
long-term survivors. Eur Urol. 78:822–830. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Hillerdal V, Ramachandran M, Leja J and
Essand M: Systemic treatment with CAR-engineered T cells against
PSCA delays subcutaneous tumor growth and prolongs survival of
mice. BMC Cancer. 14:302014. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Zhou JE, Yu J, Wang Y, Wang H, Wang J,
Wang Y, Yu L and Yan Z: ShRNA-mediated silencing of PD-1 augments
the efficacy of chimeric antigen receptor T cells on subcutaneous
prostate and leukemia xenograft. Biomed Pharmacother.
137:1113392021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Wang D, Shao Y, Zhang X, Lu G and Liu B:
IL-23 and PSMA-targeted duo-CAR T cells in prostate cancer
eradication in a preclinical model. J Transl Med. 18:232020.
View Article : Google Scholar : PubMed/NCBI
|