Spandidos Publications style
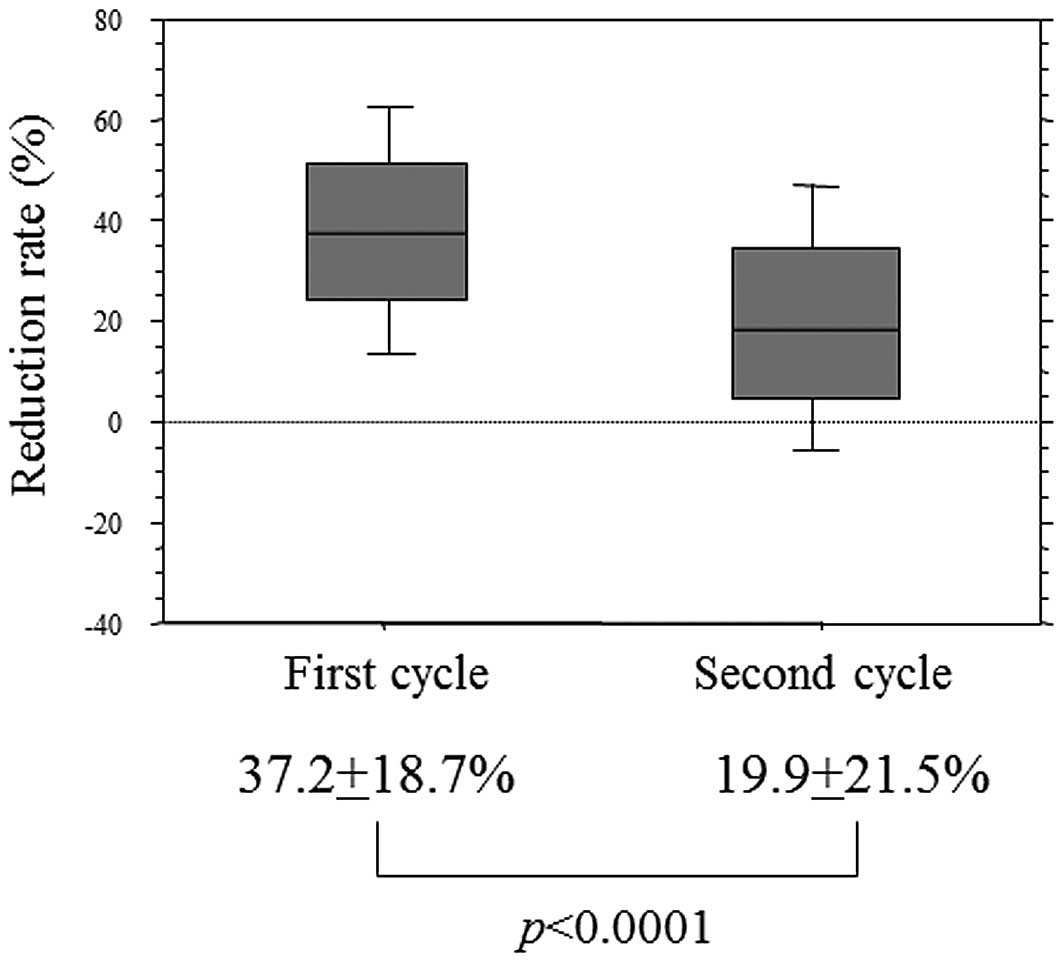
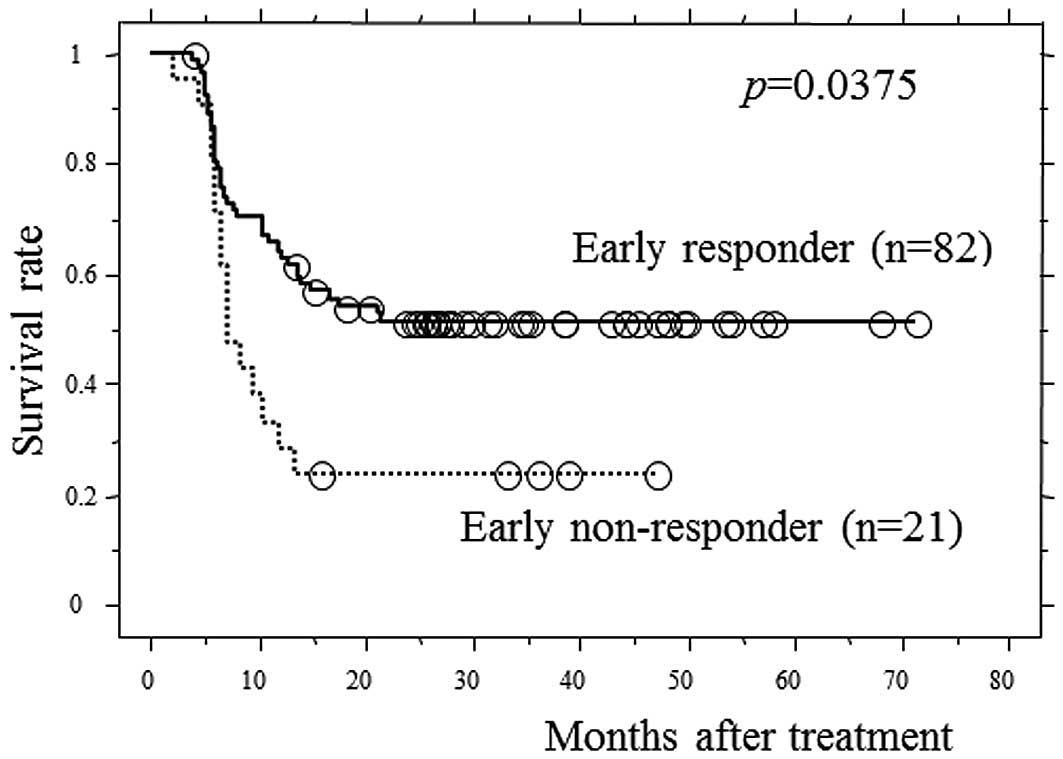
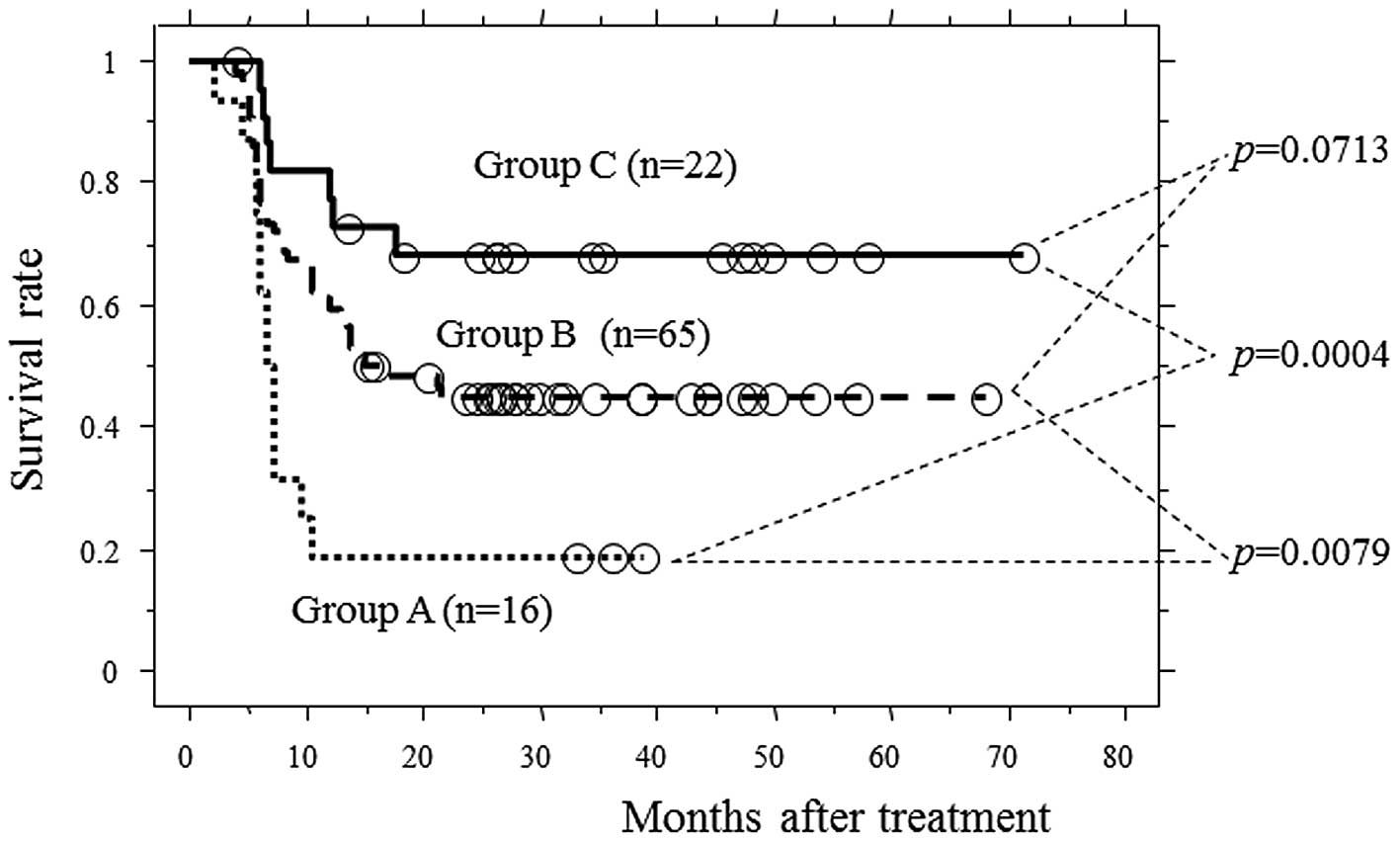
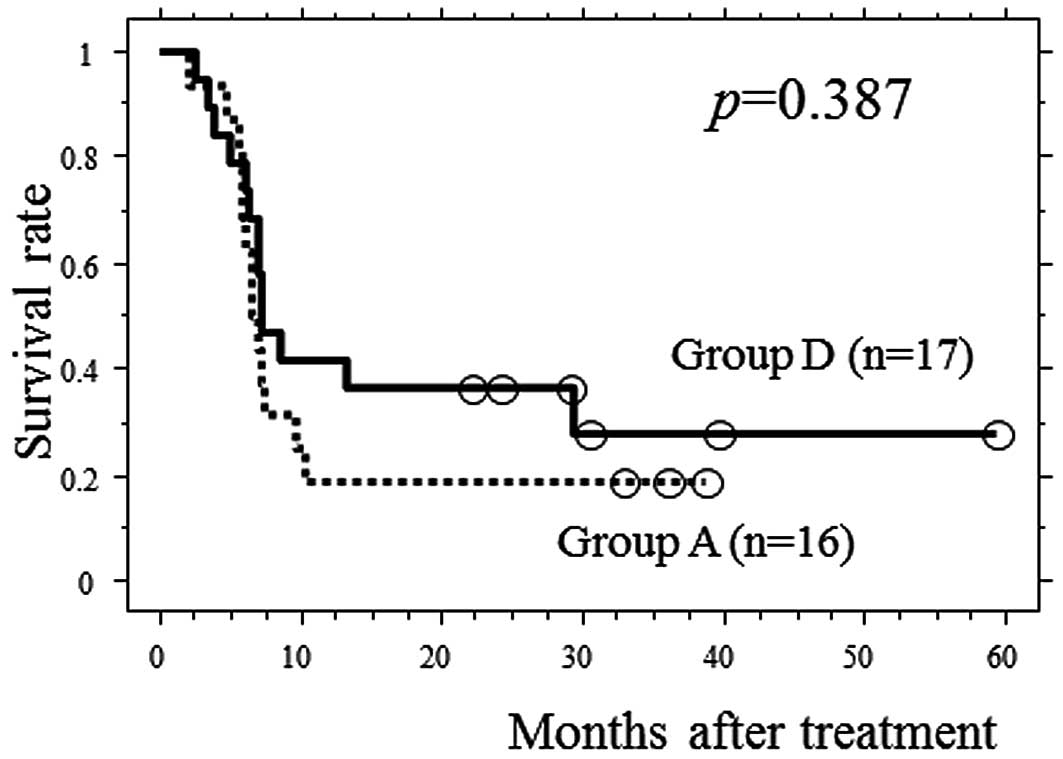
Motoori M, Yano M, Yasuda T, Miyata H, Peng Y, Yamasaki M, Shiraishi O, Tanaka K, Ishikawa O, Shiozaki H, Shiozaki H, et al: Early response to neoadjuvant chemotherapy in advanced esophageal cancer evaluated by computed tomography predicts the utility of a second cycle of chemotherapy. Mol Clin Oncol 1: 521-526, 2013.
APA
Motoori, M., Yano, M., Yasuda, T., Miyata, H., Peng, Y., Yamasaki, M. ... Doki, Y. (2013). Early response to neoadjuvant chemotherapy in advanced esophageal cancer evaluated by computed tomography predicts the utility of a second cycle of chemotherapy. Molecular and Clinical Oncology, 1, 521-526. https://doi.org/10.3892/mco.2013.89
MLA
Motoori, M., Yano, M., Yasuda, T., Miyata, H., Peng, Y., Yamasaki, M., Shiraishi, O., Tanaka, K., Ishikawa, O., Shiozaki, H., Doki, Y."Early response to neoadjuvant chemotherapy in advanced esophageal cancer evaluated by computed tomography predicts the utility of a second cycle of chemotherapy". Molecular and Clinical Oncology 1.3 (2013): 521-526.
Chicago
Motoori, M., Yano, M., Yasuda, T., Miyata, H., Peng, Y., Yamasaki, M., Shiraishi, O., Tanaka, K., Ishikawa, O., Shiozaki, H., Doki, Y."Early response to neoadjuvant chemotherapy in advanced esophageal cancer evaluated by computed tomography predicts the utility of a second cycle of chemotherapy". Molecular and Clinical Oncology 1, no. 3 (2013): 521-526. https://doi.org/10.3892/mco.2013.89