Introduction
Surgery alone has not been effective in improving
the prognosis of advanced esophageal cancer, despite recent
advances in surgical techniques and perioperative management. Even
following curative resection by esophagectomy with extended 3-field
lymphadenectomy, cancer recurs in ∼50% of patients (1). Thus, it is likely that systemic
micrometastases are present outside the surgical field at the time
of diagnosis. To improve the prognosis of advanced esophageal
cancer, neoadjuvant chemotherapy (NACT), administered to eradicate
systemic micrometastases, followed by surgical resection, is a
promising treatment strategy. Recent studies have reported
successful results with NACT (2,3).
NACT has been shown to improve the prognosis of responders;
however, non-responders suffer from the side effects in addition to
losing valuable time seeking alternative treatments (2,4,5). As
demonstrated by certain studies, the prognosis of non-responders
may be worse than that of patients undergoing primarily surgical
treatment (2,4,5).
This is partly due to therapy-induced adverse events, selection of
chemotherapy-resistant, more biologically aggressive tumors and
delay of surgical treatment. Disease progression during ineffective
chemotherapy may also be a factor contributing to the poor survival
of non-responders. Therefore, prediction of the response to
chemotherapy prior to treatment or early during the course of
therapy, is critical. Despite intensive efforts to identify
predictors of response prior to chemotherapy, there are currently
no clear candidate predictors that may be applicable in daily
practice (6–8).
In this study, we retrospectively attempted to
identify criteria for discontinuing NACT after the first cycle,
based on the response as evaluated by computed tomography (CT).
Patients with advanced squamous cell carcinoma of the thoracic
esophagus received 2 cycles of cisplatin-based chemotherapy as NACT
and their response was evaluated by CT following the completion of
each cycle.
Materials and methods
Patient eligibility
Between January, 2000 and December, 2008, a total of
988 patients with squamous cell carcinoma of the thoracic esophagus
underwent esophagectomy at our hospitals. All patients underwent
esophageal fiberscopy and CT scan for tumor staging, according to
the 6th edition of the TNM classification (9). Patients satisfying the following
criteria were enrolled in this study: i) no prior treatment for
esophageal cancer; ii) ≤80 years of age; iii) a performance status
(Eastern Cooperative Oncology Group) of 0 or 1; iv) tumor depth of
T3 or less; v) no lymph node metastasis or curatively resectable
lymph node metastases, including N1 or M1 LYM (cervical or celiac
nodes); vi) 2 cycles of NACT comprising 5-fluorouracil (5-FU),
adriamycin and cisplatin (FAP therapy) or only 1 cycle of NACT (FAP
therapy) due to ineffectiveness; vii) primary tumors that were
measurable by CT scan (>10 mm in diameter); viii) adequate organ
function (leukocyte count at least in the lower limit of the normal
range; platelet count of at least 100,000/mm3; total
bilirubin level of ≤2.0 mg/dl; aspartate and alanine
aminotransferase levels ≤2.5 times the upper limit of the normal
range; and serum creatinine ≤1.5 times the upper limit of the
normal range); and ix) CT scans with 5-mm slices prior to NACT and
following each cycle of chemotherapy.
The study protocol was approved by the Human Ethics
Review Committee of Osaka Medical Center for Cancer and
Cardiovascular Diseases, Kinki University and Osaka University
Graduate School of Medicine. Written informed consent was obtained
from each patient.
Neoadjuvant chemotherapy and evaluation
of the response to chemotherapy
The regimen of FAP therapy was as follows: cisplatin
at a dose of 70 mg/m2 and adriamycin at a dose of 35
mg/m2 were administered by a drip infusion on day 1.
5-FU was administered at a dose of 700 mg/m2 by
continuous infusion on days 1–7. Two cycles of chemotherapy were
administered, separated by a 3-week interval (10,11).
Patients underwent CT scans with 5-mm slices prior to chemotherapy
and 2 weeks after the completion of each cycle. The
chemotherapeutic response was evaluated by monitoring the area of
the primary tumor. The largest area of the primary tumor was
measured bidimensionally, using the greatest diameter and the
greatest perpendicular distance. The reduction rate was calculated
as: (tumor area prior to treatment - tumor area following
treatment)/tumor area prior to treatment. Patients with >50%
decrease in the size of the primary tumor after 2 cycles of
chemotherapy were defined as responders. Patients with >20%
decrease in the size of the primary tumor after the first cycle of
chemotherapy were defined as early responders and the remaining
patients as early non-responders.
Surgery and pathological findings
Patients were scheduled for surgery ∼4 weeks after
the last day of chemotherapy. Surgical therapy consisted of en bloc
esophagectomy via right thoracotomy with 2- or 3-field
lymphadenectomy and reconstruction using the stomach, jejunum or
colon. Pathological T stage was determined according to the TNM
classification (9). The
pathological response of the primary tumor was defined according to
the Japanese Classification of Esophageal Cancer, 10th edition
(12) as: grade 3, complete
disappearance of cancer cells; grade 2, >2/3 disappearance;
grade 1, <2/3 disappearance; grade 0, no therapeutic effect.
Statistical analysis
Statistical analyses were performed using Stat View
5.0J software (SAS Institute, Inc., Cary, NC, USA). Differences in
continuous variables were evaluated using the Student’s t-test. The
association between two non-continuous parameters was evaluated
using the Chi-square test. Univariate and multivariate survival
analyses were performed using Cox’s proportional hazards regression
model. Survival was calculated by the Kaplan-Meier method and
assessed by the log-rank test. A two-tailed p<0.05 was
considered to indicate a statistically significant difference.
Results
Patient and tumor characteristics
A total of 103 patients with esophageal cancer
received 2 cycles of NACT. The clinical characteristics of these
patients are presented in Table I.
The majority of the patients were clinically node-positive, since
this was used as an indication for NACT. There were 17 patients
with clinical stage II disease, 60 with stage III disease and 26
with stage IV disease. The median follow-up period was 25.7
months.
 | Table IClinical characteristics of patients
who received 2 cycles of neoadjuvant chemotherapy. |
Table I
Clinical characteristics of patients
who received 2 cycles of neoadjuvant chemotherapy.
| Variables | n |
|---|
| Gender | |
| Male | 84 |
| Female | 19 |
| Age (years) | 64.4±8.5 |
| Location | |
| Upper | 7 |
| Middle | 55 |
| Lower | 41 |
| cTa | |
| T1 | 2 |
| T2 | 25 |
| T3 | 76 |
| cNa | |
| N0 | 3 |
| N1 | 74 |
| M1 LYM | 26 |
| cStagea | |
| IIA | 3 |
| IIB | 14 |
| III | 60 |
| IV | 26 |
Chemotherapeutic response and reduction
rate of the primary tumor in each cycle
Based on a 50% reduction rate as the definition of
response, 52 patients were classified as responders and the
remaining 51 as non-responders. Responders had a significantly
improved progression-free survival (PFS) compared to non-responders
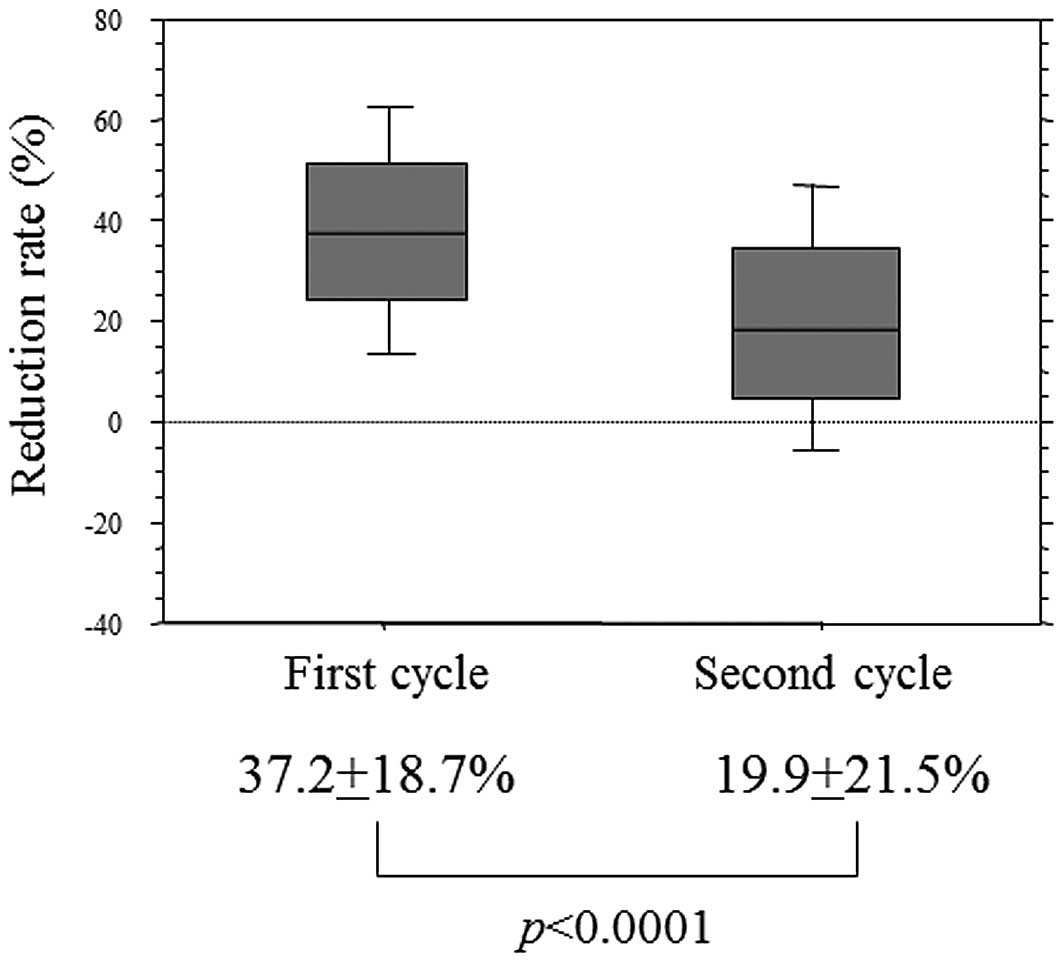
(p<0.0001, data not shown). The reduction rate after the first
cycle was 37.2±18.7% and that after the second cycle was
significantly lower (19.9±21.5%; p<0.0001) (Fig. 1).
Evaluation of early response using
CT
To avoid repetition of ineffective therapy, we
attempted to establish criteria for discontinuing NACT after the
first cycle, based on the response as evaluated by CT. Of the 103
patients, 82 were early responders and 21 were early
non-responders, using a 20% decrease in the primary tumor size
after the first cycle as the definition of early response. The
reduction rate of the second cycle was 23.4±22.0% in early
responders and 6.4±12.3% in early non-responders (p=0.001).
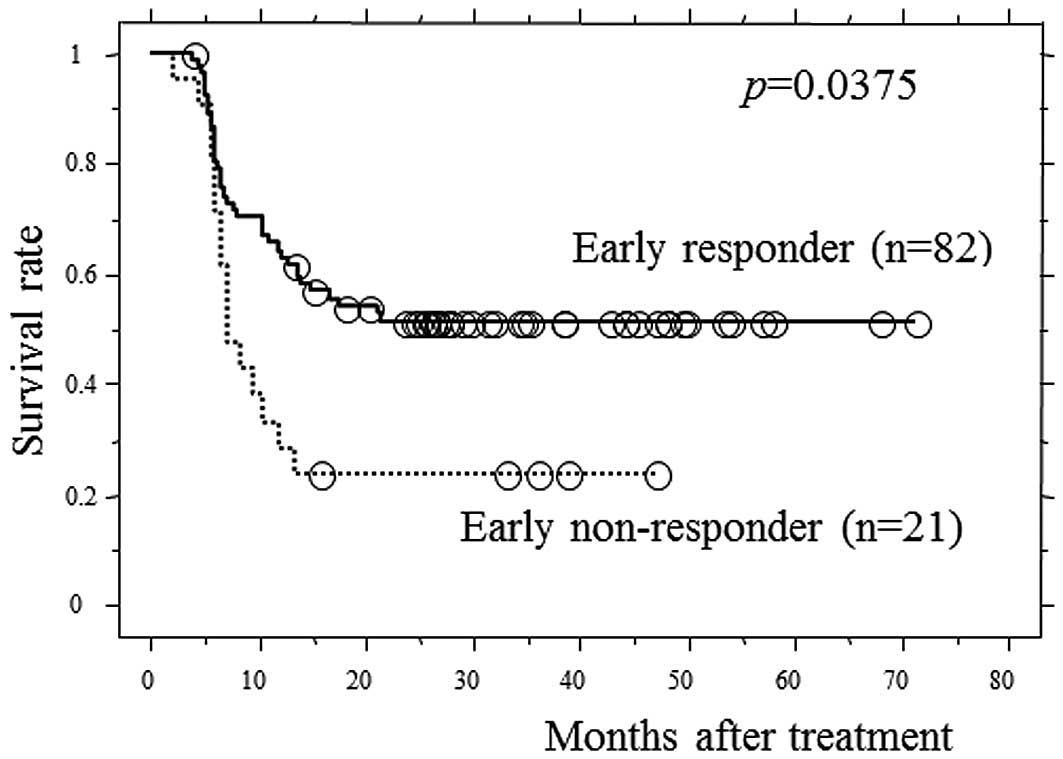
Fig. 2 shows the PFS curves
according to early response status. The 3-year PFS rate of early
responders and early non-responders was 53.2 and 22.2%,
respectively, and early responders exhibited significantly higher
survival rates, compared to early non-responders (p=0.0375).
Table II lists the baseline and
pathological characteristics of early responders vs. early
non-responders. Females had a higher early response rate compared
to males (p=0.0011). Early responders had a more favorable
pathological T stage, pathological tumor response and number of
metastatic lymph nodes, compared to early nonresponders (p=0.023,
0.009 and 0.041, respectively).
 | Table IIBaseline and pathological
characteristics of early non-responders and early responders. |
Table II
Baseline and pathological
characteristics of early non-responders and early responders.
| Variables | Early non-responders
(n=21) | Early responders
(n=82) | P-value |
|---|
| Baseline
characteristics | | | |
| Gender | | | |
| Male | 21 | 63 | 0.0011 |
| Female | 0 | 19 | |
| Age (years) | | | |
| <70 | 17 | 60 | 0.51 |
| ≥70 | 4 | 22 | |
| Location | | | |
| Upper | 2 | 5 | 0.73 |
| Middle | 12 | 43 | |
| Lower | 7 | 34 | |
| cTa | | | |
| T1-2 | 5 | 22 | 0.78 |
| T3 | 16 | 60 | |
| cNa | | | |
| N0–1 | 13 | 64 | 0.13 |
| M1 LYM | 8 | 18 | |
| Reduction rate after
the second cycle of chemotherapy | 6.4±12.3% | 23.4±22.0% | 0.001 |
| Pathological
characteristics | | | |
| pTa | | | |
| T0–2 | 4 | 38 | 0.023 |
| T3–4 | 17 | 44 | |
| Pathological
response of the primary tumorb | | | |
| Grade 0–1 | 19 | 57 | 0.009 |
| Grade 2–3 | 0 | 22 | |
| Number of
metastatic lymph nodes | 5.5±5.4 | 3.1±4.3 | 0.041 |
Prognostic significance of early
response
Among the clinical characteristics available prior
to the initiation of the second cycle of chemotherapy, including
gender (male/female), age (<70/>70 years), tumor location
(upper/middle, lower esophagus), T stage (T1–2/T3), N stage
(N0-1/M1 LYM) and early response status (early responder/early
non-responder), T1-2 and early responder status were significantly
correlated with higher PFS rates in a univariate analysis (p=0.031
and 0.032, respectively; Table
III). A multivariate analysis using T stage and early responder
status demonstrated that the two factors were independently
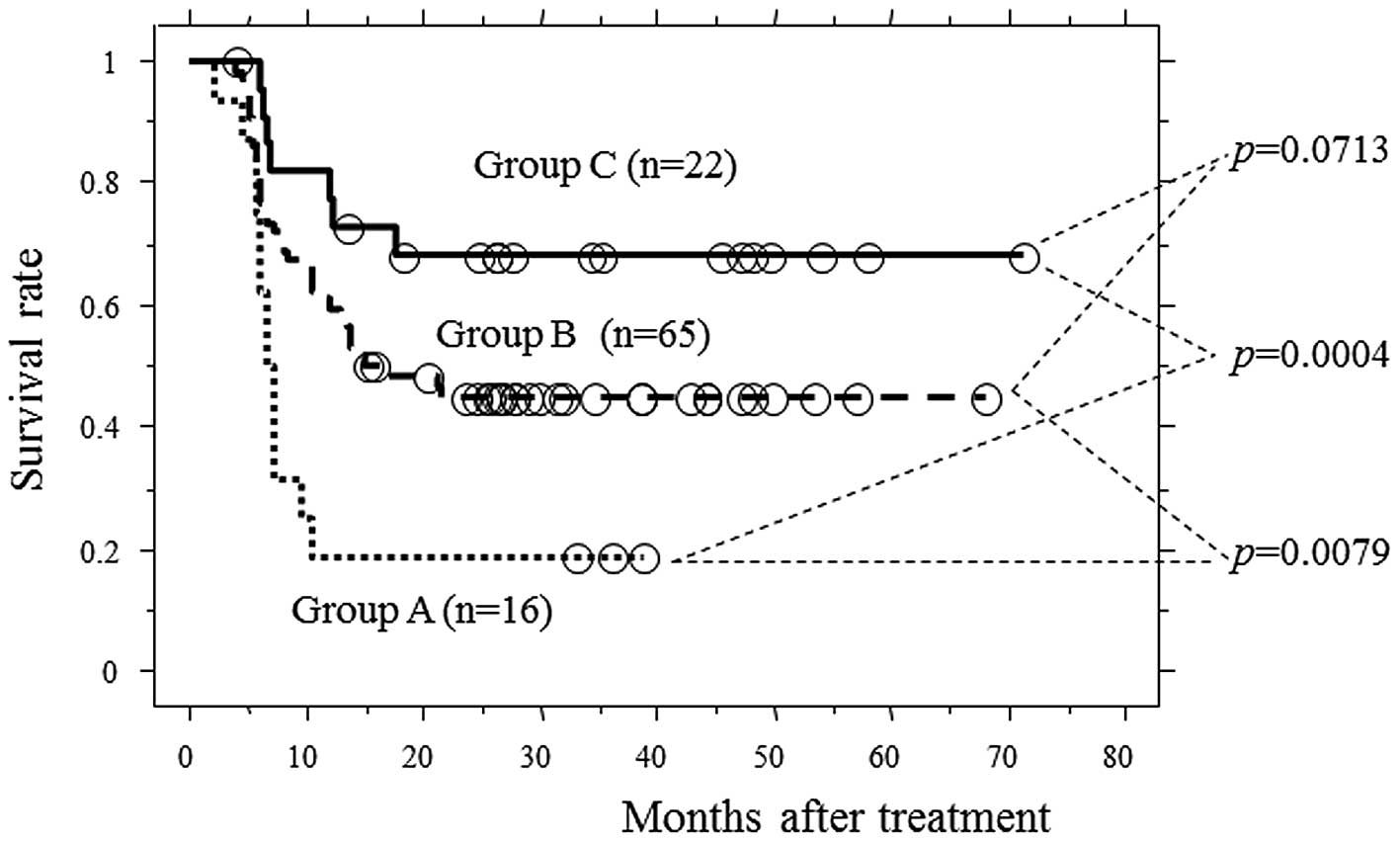
associated with PFS (p=0.028 and 0.0062, respectively; Table III). PFS curves among patients with
both unfavorable factors (group A; T3 and early non-responders),
those with no unfavorable factors (group C; T1-2 and early
responders) and the remaining patients (group B; others) were
examined. Group A had a significantly worse PFS when compared to
groups B and C (p=0.0079 and 0.0004, respectively; Fig. 3) .
 | Table IIIUnivariate and multivariate analysis
of progression-free survival. |
Table III
Univariate and multivariate analysis
of progression-free survival.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|---|
| Variables | P-value | HR | 95% CI | P-value |
|---|
| Gender
(male/female) | 0.76 | N.I. | | |
| Age (<70/≥70
years) | 0.14 | N.I | | |
| Location
(upper/middle, lower) | 0.3 | N.I | | |
| cT (T1-2/T3) | 0.031 | 2.16 | 1.08–4.29 | 0.028 |
| cN (N0–1/M1
LYM) | 0.57 | N.I. | | |
| Early response
status (early responder/early non-responder) | 0.032 | 2.28 | 1.26–4.12 | 0.0062 |
Significance of the second cycle of
chemotherapy in group A patients
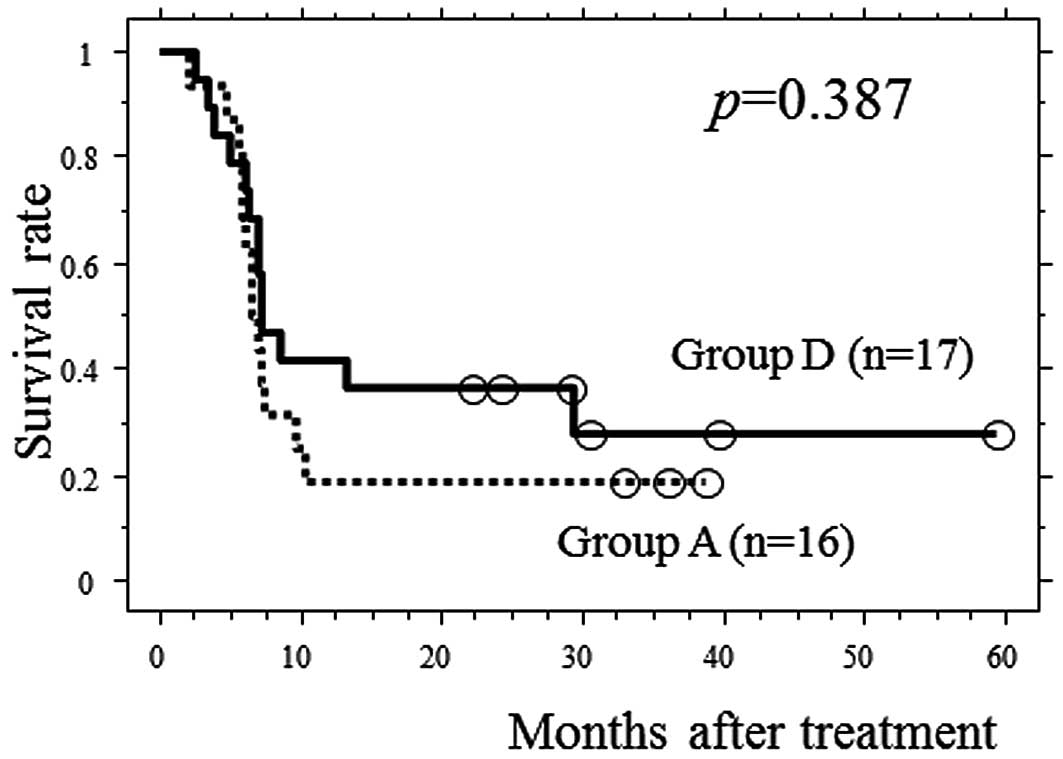
Twenty-five patients discontinued NACT after the
first cycle due to ineffectiveness and underwent esophagectomy.
Among these patients, 17 had clinical T3 tumors and exhibited
<20% decrease in the size of the primary tumor following the
first cycle of NACT (group D). To investigate the significance of
the second cycle of chemotherapy in group A patients, we compared
PFS between group A and group D patients. No significant
differences in the baseline clinical factors and the reduction rate
after the first cycle of chemotherapy between the 2 groups were
observed (Table IV). No
significant difference were identified in PFS between the 2 groups
(Fig. 4).
 | Table IVClinical characteristics of group A
and group D patients. |
Table IV
Clinical characteristics of group A
and group D patients.
| Variables | Group Ab (n=16) | Group Dc (n=17) | P-value |
|---|
| Gender | | | |
| Male | 16 | 14 | 0.24 |
| Female | 0 | 3 | |
| Age (years) | | | |
| <70 | 14 | 14 | 0.99 |
| ≥70 | 2 | 3 | |
| Location | | | |
| Upper | 1 | 1 | 0.27 |
| Middle | 9 | 5 | |
| Lower | 6 | 11 | |
| cTa | | | |
| T3 | 16 | 17 | |
| cNa | | | |
| N0–1 | 10 | 14 | 0.81 |
| M1 LYM | 6 | 3 | |
| Reduction rate of
the first cycle of NACT | 11.2±7.4% | 5.6±13.4% | 0.16 |
Discussion
Multiple cycles of cisplatin-based chemotherapy is a
standard protocol for NACT for advanced esophageal cancer. In order
to avoid the repetition of ineffective therapy in non-responders,
it is important to establish criteria for discontinuing NACT after
the first cycle. In early non-responders (patients with <20%
decrease in the size of the primary tumor after the first cycle of
chemotherapy), the reduction rate after the second cycle of
chemotherapy, PFS and pathological factors (pathological T stage,
pathological response of the primary tumor and number of metastatic
lymph nodes) were significantly worse. Therefore, evaluation of
early response with CT may be a useful method for identifying
patients likely to have unfavorable outcomes after a number of
courses of NACT. Among the clinical variables available prior to
administration of the second cycle of NACT, clinical T3 stage and
early non-responder status were independent unfavorable prognostic
factors, and patients with the two factors exhibited significantly
poorer PFS. Moreover, there was no significant difference between
the prognosis of patients who had both unfavorable factors and
received 1 or 2 cycles of NACT. Therefore, in patients with both
unfavorable prognostic factors, the second cycle of NACT should be
avoided, and patients should undergo salvage therapies, such as
alternative chemotherapy regimens, chemoradiotherapy, or immediate
surgery. Such an individualized approach may improve prognosis by
reducing the length of time during which a patient receives
ineffective therapy.
Previous studies have demonstrated that metabolic
response as measured by positron emission tomography (PET) may help
differentiate between responding and non-responding esophageal
cancers early in the course of therapy (13–15).
Weber et al(15) reported
that changes in tumor metabolic activity after 14 days of NACT, as
assessed using PET, were significantly correlated with
histopathological response and survival rates. However, CT is more
prevalent and PET is associated with issues regarding the
complexity of the technology and the absence of standardization for
metabolic imaging.
Among baseline factors, clinical T stage was
significantly correlated with prognosis, as opposed to clinical N
stage. Clinical T stage was significantly correlated with
pathological T stage (p=0.0001). There was no correlation between
clinical N stage and the number of metastatic lymph nodes. This may
be partly attributed to the fact that we administered NACT mainly
to clinically node-positive esophageal cancer patients. The rate of
early response was significantly higher in females compared to
males. Overall response (after the second cycle of NACT) was also
significantly higher in females (p=0.0011, data not shown). The
response to chemotherapeutic agents may be partly determined by
drug concentration in the tumor environment (16,17).
Investigators have previously suggested that gender-specific
pharmacokinetics exist for certain chemotherapeutic agents. Milano
et al(18) reported that
the capacity to clear 5-FU is lower in women than in men. Dobbs
et al(19) demonstrated
that, among patients with normal liver function, men exhibit a
higher rate of doxorubicin clearance compared to women. Higher
response to chemotherapy in females observed in this study may
partly be due to the higher blood concentrations of
chemotherapeutic agents.
The reduction rate after the second cycle was
significantly worse compared to that after the first cycle. This
was consistent with a previous study (20). It is likely that tumors are
heterogeneous and the first cycle eliminates only the sensitive
tumor cells, sparing resistant tumor cells (21). Another reason is that the first
cycle may eliminate tumor cells located around the tumor vessels
with a high drug concentration and the second cycle may kill tumors
distant from the tumor vessel with a low drug concentration. The
efficacy of chemotherapy may deteriorate as the number of cycles
increases. When administering multiple cycles of NACT, physicians
should evaluate chemotherapeutic response following completion of
each cycle of chemotherapy.
Early non-responders were defined as the patients
with <20% decrease in the size of the primary tumor after the
first cycle of chemotherapy. When the cut-off value was set at 30%,
the reduction rate after the second cycle chemotherapy, PFS,
pathological T stage and pathological response were significantly
worse in early non-responders, although there was no significant
difference in the number of metastatic lymph nodes between the 2
groups. When the cut-off value was set at 10%, there was no
significant difference in PFS between the 2 groups. The relatively
low threshold of 20% may be appropriate, since it ensures that all
patients who potentially benefit from NACT receive further
treatment.
In conclusion, this study has demonstrated that the
reduction rate of the primary tumor as evaluated by CT after the
first cycle of NACT may aid physicians in determining whether to
administer the second cycle. In patients with T3 tumors and <20%
decrease in the size of the primary tumor after the first cycle of
chemotherapy, NACT should be discontinued after the first
cycle.
References
|
1.
|
Akiyama H, Tsurumaru M, Udagawa H and
Kajiyama Y: Radical lymph node dissection for cancer of the
thoracic esophagus. Ann Surg. 220:364–372. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Medical Research Council Oesophageal
Cancer Working Group: Surgical resection with or without
preoperative chemotherapy in oesophageal cancer: a randomised
controlled trial. Lancet. 359:1727–1733. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ando N, Kato H, Igaki H, et al: A
randomized trial comparing postoperative adjuvant chemotherapy with
cisplatin and 5-fluorouracil versus preoperative chemotherapy for
localized advanced squamous cell carcinoma of the thoracic
esophagus (JCOG9907). Ann Surg Oncol. 19:1968–1974. 2012.
View Article : Google Scholar
|
|
4.
|
Kelsen DP, Ginsberg R, Pajak TF, et al:
Chemotherapy followed by surgery compared with surgery alone for
localized esophageal cancer. N Engl J Med. 339:1979–1984. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Yano M, Takachi K, Doki Y, et al:
Preoperative chemotherapy for clinically node-positive patients
with squamous cell carcinoma of the esophagus. Dis Esophagus.
19:158–163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kishi K, Doki Y, Yano M, et al: Reduced
MLH1 expression after chemotherapy is an indicator for poor
prognosis in esophageal cancers. Clin Cancer Res. 9:4368–4375.
2003.PubMed/NCBI
|
|
7.
|
Motoori M, Takemasa I, Yamasaki M, et al:
Prediction of the response to chemotherapy in advanced esophageal
cancer by gene expression profiling of biopsy samples. Int J Oncol.
37:1113–1120. 2010.PubMed/NCBI
|
|
8.
|
Luthra R, Wu TT, Luthra MG, et al: Gene
expression profiling of localized esophageal carcinomas:
association with pathologic response to preoperative
chemoradiation. J Clin Oncol. 24:259–267. 2006. View Article : Google Scholar
|
|
9.
|
Sobin LH and Wittekind CH: TNM
Classification of Malignant Tumors. 6th edition. Wiley-Liss; New
York, NY: 2002
|
|
10.
|
Motoori M, Yano M, Yasuda T, et al:
Chemotherapy-induced toxicities and treatment efficacy in advanced
esophageal cancer treated with neoadjuvant chemotherapy followed by
surgery. Esophagus. 8:81–87. 2011. View Article : Google Scholar
|
|
11.
|
Matsuyama J, Doki Y, Yasuda T, et al: The
effect of neoadjuvant chemotherapy on lymph node micrometastases in
squamous cell carcinomas of the thoracic esophagus. Surgery.
141:570–580. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Japanese Society for Esophageal Diseases:
Guidelines for the Clinical and Pathologic Studies on Carcinoma of
the Esophagus. 10th edition. Kanehara Syuppan; Tokyo: 2007
|
|
13.
|
Wieder HA, Brücher BL, Zimmermann F, et
al: Time course of tumor metabolic activity during
chemoradiotherapy of esophageal squamous cell carcinoma and
response to treatment. J Clin Oncol. 22:900–908. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Lordick F, Ott K, Krause BJ, et al: PET to
assess early metabolic response and to guide treatment of
adenocarcinoma of the oesophagogastric junction: the MUNICON phase
II trial. Lancet Oncol. 8:797–805. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Weber WA, Ott K, Becker K, et al:
Prediction of response to preoperative chemotherapy in
adenocarcinomas of the esophagogastric junction by metabolic
imaging. J Clin Oncol. 19:3058–3065. 2001.PubMed/NCBI
|
|
16.
|
Gamelin EC, Danquechin-Dorval EM, et al:
Relationship between 5-fluorouracil (5-FU) dose intensity and
therapeutic response in patients with advanced colorectal cancer
receiving infusional therapy containing 5-FU. Cancer. 77:441–451.
1996. View Article : Google Scholar
|
|
17.
|
Di Paolo A, Lencioni M, Amatori F, et al:
5-fluorouracil pharmacokinetics predicts disease-free survival in
patients administered adjuvant chemotherapy for colorectal cancer.
Clin Cancer Res. 14:2749–2755. 2008.PubMed/NCBI
|
|
18.
|
Milano G, Etienne MC, Cassuto-Viguier E,
et al: Influence of sex and age on fluorouracil clearance. J Clin
Oncol. 10:1171–1175. 1992.PubMed/NCBI
|
|
19.
|
Dobbs NA, Twelves CJ, Gillies H, et al:
Gender affects doxorubicin pharmacokinetics in patients with normal
liver biochemistry. Cancer Chemother Pharmacol. 36:473–476. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Akita H, Doki Y, Miyata H, et al: Clinical
significance of the second cycle response to cisplatin-based
chemotherapy as preoperative treatment for esophageal squamous cell
carcinoma. J Surg Oncol. 93:401–409. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Mathieu A, Remmelink M, D’Haene N, et al:
Development of a chemoresistant orthotopic human nonsmall cell lung
carcinoma model in nude mice: analyses of tumor heterogenity in
relation to the immunohistochemical levels of expression of
cyclooxygenase-2, ornithine decarboxylase, lung-related resistance
protein, prostaglandin E synthetase, and
glutathione-S-transferase-alpha (GST)-alpha, GST-mu, and GST-pi.
Cancer. 101:1908–1918. 2004.
|