Introduction
Although the survival of patients with gastric
cancer has improved due to the recent advances in treatment, the
prognosis of locally advanced or metastatic cancer remains poor
(1–3). A proportion of the patients develop
recurrences even after curative resection, possibly reflecting the
presence of residual cancer cells and micrometastases that had not
been detected by the currently available diagnostic technology
(4, 5). Therefore, the accurate evaluation of
microscopic residual disease may lead to more appropriate
therapeutic strategies and improvement in survival.
Epithelial-to-mesenchymal transition (EMT) is a
critical process during which the adhesion and migration properties
of cancer cells change dramatically (6, 7).
During EMT, the cells lose epithelial polarity and acquire a
spindle-shaped, highly motile fibroblastoid phenotype. Various
transcription factors are known to trigger EMT (8–10),
including zinc-finger E-box binding homeobox 1 (ZEB1), a central
EMT mediator (11, 12). ZEB1 reportedly affects cancer
progression by regulating EMT in gastric, breast, prostate, ovarian
and colorectal cancers (13–20).
In gastric cancer, carcinoembryonic antigen (CEA)
mRNA levels in peritoneal washing have been reported to be
potential predictors of peritoneal recurrence (21, 22). Kodera et al reported that
the combination of CEA and cytokeratin-20 in peritoneal washes may
more accurately predict prognosis (23). ZEB1 expression has also been
recently reported as a novel biomarker in cancer tissue that may
independently predict overall survival (13, 14,
24). We recently reported on a
significant correlation between ZEB1 expression and diffuse
phenotype in gastric cancer (24).
Okugawa et al reported that ZEB1 was an independent
predictor of peritoneal dissemination in gastric cancer patients
and was expressed in disseminated cancer cells in the peritoneum in
the same pattern as that seen in the primary lesions (13). Therefore, we hypothesized that the
ZEB1 mRNA levels in peritoneal washing (pZEB1) in conjunction with
peritoneal washing cytology may predict intraperitoneal recurrence
and prognosis.
This study investigated the association of pZEB1
with clinicopathological parameters and prognosis and the potential
of pZEB1 as a predictive marker. To the best of our knowledge, this
is the first report on the clinical implication of pZEB1 in gastric
cancer.
Materials and methods
Patients
We enrolled 107 consecutive gastric cancer patients
who underwent surgical procedures that included collection of
peritoneal washing samples at the left subphrenic area at the
beginning of surgery, between January, 2005 and August, 2010 at the
Department of Gastroenterological Surgery, Nagoya University
Hospital, Nagoya, Aichi, Japan. All the patients had histologically
confirmed gastric cancer. Of the 107 patients, 4 had received
chemotherapy prior to surgery, 2 of whom achieved a complete
response. All the patients had been staged according to the Union
for International Cancer Control staging criteria for gastric
cancer (7 th edition, 2009) as follows: 2 patients had stage 0; 12
had stage IA; 11 had stage IB; 7 had stage IIA; 12 had stage IIB; 8
had stage IIIA; 10 had stage IIIB; 10 had stage IIIB; 10 had stage
IIIC; and 35 had stage IV disease. Overall, 72 patients underwent
curative resection, 35 patients underwent non-curative resection,
of whom 2 patients did not receive gastrectomy due to disseminated
cancer. All the patients underwent gastrectomy with D2
lymphadenectomy when potentially curative R0 resection was planned.
The median follow-up period was 41.9 months (range, 1–106 months).
This study was approved by the Ethics Committee of our hospital and
signed informed consent was obtained from all the participating
patients.
Peritoneal washes
At the beginning of each surgery, 100–200 ml saline
was introduced into the left subphrenic area and aspirated soon
after gentle stirring. Half of each fluid sample was sent for
routine cytopathology with conventional Papanicolaou and Giemsa
staining, whereas the other half was used to measure ZEB1 mRNA
levels. The sample was centrifuged at 540 × g for 5 min to collect
intact cells, rinsed with phosphate-buffered saline, dissolved in
ISOGEN-LS RNA extraction buffer (Nippon Gene, Tokyo, Japan) and
stored immediately in liquid nitrogen at-80 ˚C until analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from each of the frozen
samples with the RNeasy mini kit (Qiagen, Hilden, Germany)
according to manufacturer's instructions. cDNA was synthesized
using the QuantiTect Reverse Transcription kit (Qiagen, Hilden,
Germany) and amplified by PCR primers as follows: ZEB1: 5′
-TGCACTGAGTGTGGAAAAGC-3′ (forward) and 5′ -TGGTGATGCTGAAAGAGACG-3′
(reverse), which amplify a 237-bp product. RNA expression was
determined using the real-time quantitative PCR method. To quantify
and demonstrate the integrity of the isolated RNA,
glyceraldehyde-3-phophate dehydrogenase was also analyzed with
RT-qPCR using the primer set 5′ -AACGGCTCCGGCATGTGCAA-3′ (forward)
and 5′ -GGCTCCTGTGCAGAGAAAGC-3′ (reverse). All the PCR reactions
were performed as follows: 1 cycle at 50˚C for 2 min, 1 cycle at
95˚C for 10 min, followed by 40 cycles at 95˚C for 15 sec and at
60˚C for 60 sec. Real-time detection of the emission intensity of
SYBR-Green was performed with an ABI prism 7000 Sequence Detector
(Perkin-Elmer Applied Biosystems, Foster City, California, USA).
qPCR was performed at least 3 times, including a negative
no-template control.
Statistical analysis
Correlations between pZEB1 expression and
clinicopathological variables were analyzed by the χ2
and Fisher's exact tests. Disease-specific survival (DSS) and
disease-free survival (DFS) were calculated using the Kaplan-Meier
method and differences in survival curves were analyzed using the
log-rank test. The Cox proportional hazards model was used for
multivariate analysis, after relevant prognostic variables had been
defined by univariate analysis. Data were analyzed using JMP v10
software (JMP, SAS Institute, Cary, North Carolina, USA). P<0.05
was considered to indicate statistically significant
differences.
Results
Patient demographics
The 107 subjects in this study included 83 men and
24 women, with a median age of 63 years (range, 20–84 years)
(Table I). Of the 107 patients, 45
underwent total gastrectomy, 57 distal gastrectomy, 3 proximal
gastrectomy, 1 gastrojejunostomy and 1 exploratory laparotomy.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
Characteristics | Patient no. |
|---|
| Age, years | |
| (mean ± SD) | 63±13.5 |
| Gender | |
|
Male | 83 |
|
Female | 24 |
| Operative
method | |
|
TGX | 45 |
|
DGX | 57 |
|
PGX | 3 |
|
Gastrojejunostomy | 1 |
|
Exploratory laparotomy | 1 |
| UICC stage | |
| 0 | 2 |
| IA | 12 |
| IB | 11 |
|
IIA | 7 |
|
IIB | 12 |
|
IIIA | 8 |
|
IIIB | 10 |
|
IIIC | 10 |
| IV | 35 |
Correlation between pZEB1 and
clinicopathological factors
pZEB1 was technically detectable in all 107 patients
by qPCR. The values ranged from 3.0×10−6 to
7.0×10−3 µg/µ l (median, 1.2×10− µg/µ l). The
pZEB1 cut-off point was set at the top quartile, which was
3.5×10−4 µg/µl. Accordingly, patients with low pZEB1
expression (<3.5×10−4 µg/µ l) were assigned to the
pZEB1Low group (n=80), whereas those with high
expression (≥3.5×10−4 µg/µ l) were assigned to the
pZEB1High group (n=27).
The analysis of pZEB1 expression and various
clinicopathological factors (Table
II) revealed that pZEB1 was correlated with pathological T
stage (P=0.03) and vascular involvement (P=0.03), but not with
gender, age, tumor size, histological type, lymphatic vessel
involvement, lymph node metastasis, liver metastasis, peritoneal
dissemination, peritoneal washing cytology, or TNM stage.
 | Table II.Correlation between
clinicopathological variables and pZEB1 expression in patients with
gastric cancer. |
Table II.
Correlation between
clinicopathological variables and pZEB1 expression in patients with
gastric cancer.
| Variables | pZEB1Low
(n=80) |
pZEB1High (n=27) | P-value |
|---|
| Gender | | |
0.30 |
|
Male | 64 | 19 | |
|
Female | 16 | 8 | |
| Age, years | | |
0.61 |
|
≥65 | 46 | 14 | |
|
<65 | 34 | 13 | |
| Tumor size, cm | | |
0.78 |
| ≥5 | 39 | 13 | |
|
<5 | 41 | 12 | |
| Histological
type | | |
0.38 |
|
Diffuse | 52 | 20 | |
|
Intestinal | 28 | 7 | |
| Pathological T
stage | | |
0.03a |
|
pT1/2 | 30 | 4 | |
|
pT3/4 | 50 | 23 | |
| Vascular
involvement | | |
0.03a |
|
Present | 37 | 18 | |
|
Absent | 42 | 7 | |
| Lymphatic vessel
involvement | | |
0.20 |
|
Present | 64 | 23 | |
|
Absent | 15 | 2 | |
| Lymph node
metastasis | | |
0.45 |
|
Present | 52 | 19 | |
|
Absent | 28 | 7 | |
| Liver
metastasis | | |
0.16 |
|
Present | 7 | 5 | |
|
Absent | 73 | 22 | |
| Peritoneal
dissemination | | |
0.22 |
|
Present | 10 | 6 | |
|
Absent | 70 | 21 | |
| Peritoneal washing
cytology | | |
0.46 |
|
Present | 18 | 8 | |
|
Absent | 62 | 19 | |
| TNM stage | | |
0.16 |
|
I/II | 36 | 8 | |
|
III/IV | 44 | 19 | |
Patient survival by pZEB1
expression
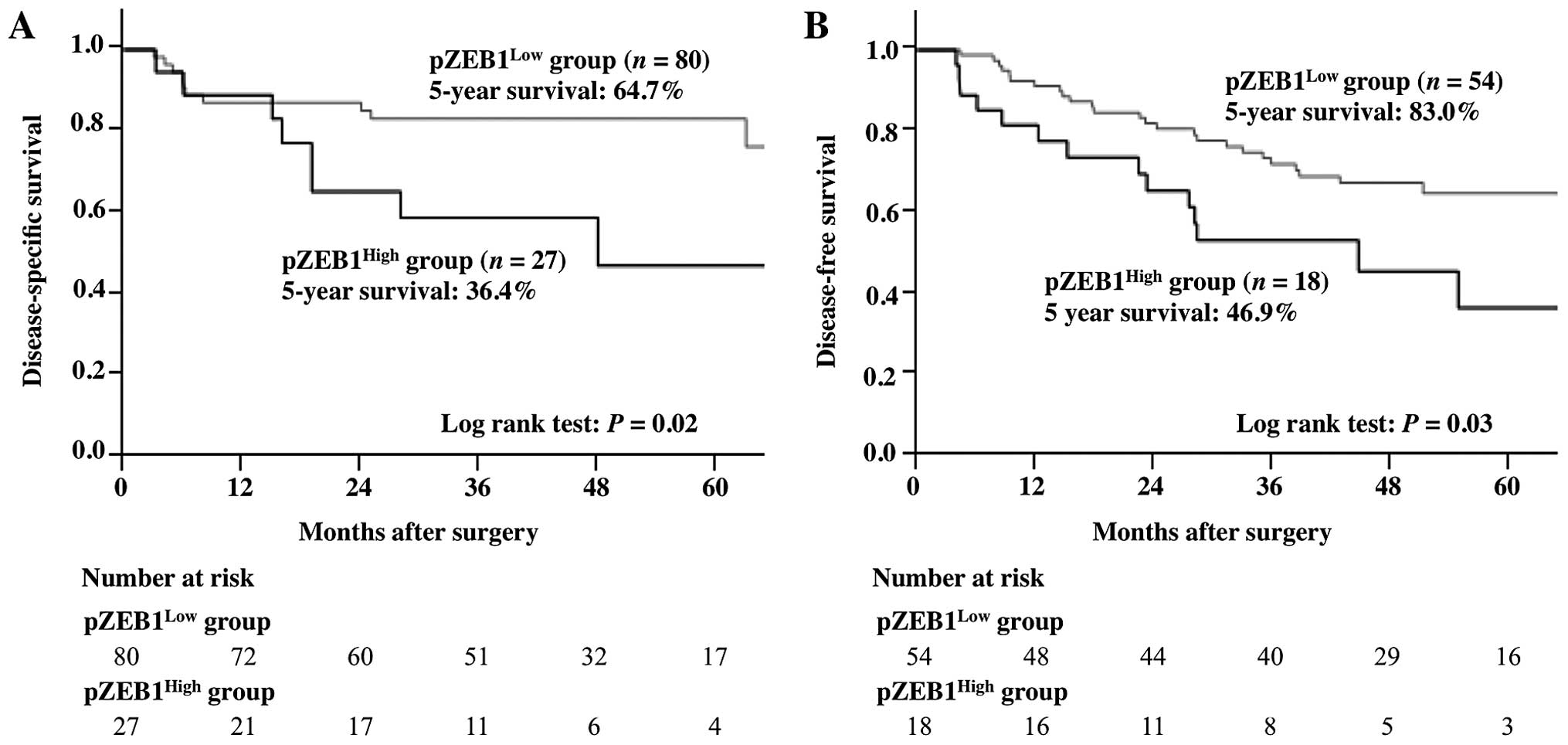
The survival curves of patients with gastric cancer
by pZEB1 expression are presented in Fig. 1. DSS was significantly lower in
patients with pZEB1High expression compared to those
with pZEB1Low expression. The 5-year DSS was 36.4% in
the pZEB1High group and 64.7% in the pZEB1Low
group (P=0.02), whereas the 5-year DFS was 46.9%, in the
pZEB1High group and 83.0% in the pZEB1Low
group (P=0.03).
The patients were next subclassified into 4 groups
according to negative or positive peritoneal washing cytology (CY0
and CY1, respectively) as follows: CY0/pZEB1Low,
CY0/pZEB1High, CY1/pZEB1Low and
CY1/pZEB1High. In the CY0 group, DSS was significantly
lower in the pZEB1High group compared to that in the
pZEB1Low group. The 5-year survival rate was 48.7% in
the CY0/pZEB1High group and 82.0% in the
CY0/pZEB1Low group (P=0.01). In the CY1 group, DSS was
also lower among patients with pZEB1High expression
compared to those with pZEB1Low expression. The 5-year
survival rate was 0% in the CY1/pZEB1High group and 9.3%
in the CY1/pZEB1Low group (P=0.13) (Fig. 2).
pZEB1 as a predictor of recurrence
after surgery
Among the 18 patients who developed recurrences
after surgery, 10 patients had pZEB1Low expression and 8
had pZEB1High expression. The recurrence rate in the
pZEB1High group (8/27) was significantly higher compared
to that in the pZEB1Low group (10/80; P=0.03, Table IIIA). Of these 18 patients 6
developed lymph node metastases, 6 peritoneal metastases, 5 liver
metastases and 1 lung metastasis. Of the 6 patients with recurrent
peritoneal metastases, 4 were in the pZEB1High group
(Table IIIB).
 | Table III.Correlation of pZEB1 expression
status with recurrence of gastric cancer and recurrence site. |
Table III.
Correlation of pZEB1 expression
status with recurrence of gastric cancer and recurrence site.
| A, Correlation of
pZEB1 expression with recurrence |
|---|
|
|---|
| Recurrence | pZEB1Low
(n=54) |
pZEB1High (n=18) | P-value |
|---|
| Yes | 10 | 8 |
0.03a |
| No | 44 | 10 | |
|
| B, Correlation of
pZEB1 expression with recurrence site |
|
| Recurrence
site | No. |
pZEB1Low/High | |
|
| Lymph nodes | 6 | 4/2 | |
| Peritoneum | 6 | 2/4 | |
| Liver | 5 | 3/2 | |
| Lung | 1 | 1/0 | |
The characteristics of the 18 patients with
pZEB1High and CY0, excluding those with stage IV
disease, are summarized in Table
IV. Among these, 8 patients ultimately developed recurrent
metastases (4 in the peritoneum, 2 in the liver and 2 in the lymph
nodes).
 | Table IV.Characteristics of patients with
pZEB1High expression excluding those with s tage IV
disease. |
Table IV.
Characteristics of patients with
pZEB1High expression excluding those with s tage IV
disease.
| Patients | Age (yrs) | Gender | DFS | Recurrence
site | T stage |
Metastasisa | Histology |
|---|
| 1 | 62 | F | 48 | Peritoneum | T4a | N3a | Diffuse |
| 2 | 60 | F | 28 | Peritoneum | T4a | N1 | Diffuse |
| 3 | 55 | M | 3.2 | Peritoneum | T4a | N0 | Diffuse |
| 4 | 55 | M | 19 | Peritoneum | T2 | N0 | Diffuse |
| 5 | 63 | M | 15 | Liver | T3 | N3b | Intestinal |
| 6 | 61 | M | 6 | Liver | T3 | N2 | Intestinal |
| 7 | 71 | M | 16 | Lymph node | T3 | N2 | Diffuse |
| 8 | 75 | F | 19 | Lymph node | T4a | N3a | Diffuse |
| 9 | 56 | M | 70 | None | T3 | N1 | Diffuse |
| 10 | 70 | M | 9.5 | None | T3 | N0 | Intestinal |
| 11 | 71 | F | 69 | None | T2 | N0 | Diffuse |
| 12 | 67 | M | 27 | None | T1a | N0 | Intestinal |
| 13 | 52 | M | 31 | None | T3 | N0 | Diffuse |
| 14 | 72 | M | 45 | None | T4a | N1 | Diffuse |
| 15 | 74 | M | 35 | None | T1b | N0 | Intestinal |
| 16 | 65 | M | 58 | None | T4a | N1 | Intestinal |
| 17 | 35 | F | 50 | None | T4a | N2 | Diffuse |
| 18 | 59 | M | 43 | None | T2 | N0 | Diffuse |
Prognostic factors of gastric cancer
patients by univariate and multivariate analysis
The univariate analysis using the Cox proportional
hazards model identified 9 prognostic factors, namely tumor size, T
stage, histological type, lymph node metastasis, lymphatic vessel
involvement, vascular involvement, peritoneal metastasis, liver
metastasis and pZEB1 expression (Table
V). However, in the multivariate analysis of these parameters,
pZEB1 was not identified as an independent predictor of DSS.
 | Table V.Univariate and multivariate analysis
of clinicopathological factors for disease-specific survival. |
Table V.
Univariate and multivariate analysis
of clinicopathological factors for disease-specific survival.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Gender
(female) | 1.3 | 0.6-2.5 | 0.52 | | | |
| Age (≥65
years) | 1.0 | 0.6-2.0 | 0.89 | | | |
| Tumor size (≥5
cm) | 2.3 | 1.2-4.6 | 0.01a | 1.1 | 0.5-2.5 | 0.76 |
| Pathological T
stage (pT3/4) | 8.4 | 3.0-34.9 |
<0.001a | 4.4 | 1.1-24.8 | 0.04a |
| Histological type
(diffuse) | 2.3 | 1.1-5.4 | 0.02a | 1.3 | 0.5-3.5 | 0.57 |
| Lymph node
metastasis | 4.2 | 1.8-12.4 |
<0.001a | 2.2 | 0.7-10.1 | 0.22 |
| Lymphatic vessel
involvement | 4.7 | 1.4-28.9 | 0.008a | 0.4 | 0.05-3.8 | 0.40 |
| Vascular
involvement | 3.9 | 1.9-8.7 |
<0.001a | 2.0 | 0.8-5.3 | 0.13 |
| Peritoneal
metastasis | 10.6 | 5.2-21.2 |
<0.001a | 4.1 | 1.8-9.4 | 0.001a |
| Liver
metastasis | 5.2 | 2.2-11.1 |
<0.001a | 2.9 | 0.9-7.9 | 0.06 |
|
pZEB1High | 2.1 | 1.1-4.0 | 0.03a | 1.0 | 0.4-2.1 | 0.98 |
Discussion
EMT is a process through which epithelial cells
attain fibroblastic characteristics, which enable them to invade
neighboring tissues (25, 26). ETM is regulated by several
transcription factors, including Snail, Slug, Twist,
CarB-box-binding factor, mesenchyme forkhead 1, Kr ü ppel-like
factor and ZEB1 (26–29).
ZEB1 is reportedly a key player in cancer
progression (17, 30–32).
In particular, high expression of ZEB1 in endometrial and
colorectal cancers and hepatocellular carcinoma has been associated
with poor prognosis (15, 33, 34). In gastric cancer, ZEB1 expression
in cancer tissues has been identified as an independent prognostic
factor (13, 14). We have also reported a correlation
between high ZEB1 expression and diffuse pathological cancer type
(24). However, the diffuse type
is a known risk factor for peritoneal recurrence in gastric cancer,
which supports the findings of Okugawa et al (13), who reported that high ZEB1
expression is an independent factor for peritoneal
carcinomatosis.
Comparisons of the expression of EMT markers in the
primary tumor and corresponding lymph node metastases have been
performed for several cancer types (35, 36,
37). These studies demonstrated
that the expression of EMT markers in mature metastatic lymph nodes
was lower compared to that in the primary lesions; therefore it was
hypothesized that mesenchymal-to-epithelial transition (MET), the
reverse phenomenon of EMT, may occur at secondary metastatic sites
before the metastasized cells develop into clinically significant
metastatic lesions. However, Okugawa et al (13) observed through immunostaining that
ZEB1 expression in the peritoneal metastatic sites exhibited the
same pattern as that observed in the primary lesions. The role of
EMT and MET in the development of peritoneal metastasis may be
different from that of nodal metastasis and it may be of value to
investigate the EMT status of intraperitoneal cancer cells that
likely develop into visible peritoneal deposits. To the best of our
knowledge, there are no available studies investigating pZEB1 in
gastric cancer patients.
The major finding in this study was that pZEB1
expression was significantly associated with DSS and DFS in
patients with gastric cancer. Furthermore, pZEB1 may be a more
sensitive diagnostic tool for poor prognosis compared to
conventional peritoneal washing cytology, as the RT-qPCR more
sensitively detects intraperitoneal free cancer cells and also
because positive pZEB1 reflects the capability of the primary tumor
to disseminate ZEB1-positive mesenchymally transformed cells into
the peritoneal cavity as well as through the hematogeneous and
lymphatic metastatic pathways. Although ZEB1 expression in the
primary lesion is already known as an independent prognostic factor
(13, 14, 24), pZEB1 expression may also represent
a novel marker of a poorer prognosis.
However, our results failed to demonstrate
statistical correlations between pZEB1 and peritoneal dissemination
and peritoneal recurrence. As stated above, although local ZEB1
production by cancer cells in the peritoneal cavity is the most
important factor in pZEB1 expression, the primary pZEB1-high tumor
may disseminate metastatic and ZEB1-producing carcinoma cells to
any other sites in the body, leading to various other types of
metastasis and consequent cancer-related death. Thus, pZEB1 may be
correlated with poor prognosis, but not necessarily with peritoneal
dissemination. There is also a possibility that a proportion of the
patients did actually harbor peritoneal recurrence, but its
manifestation was preceded by other types of metastasis that were
clinically more relevant. Further investigation is required to
elucidate the mechanisms underlying pZEB1 expression in a large
population with a long-term follow-up.
In conclusion, pZEB1 may be a predictive marker for
poor prognosis or tumor aggressiveness in gastric cancer, similar
to ZEB1 expression in primary lesions. pZEB1 may add valuable
information to conventional peritoneal washing cytology and, thus,
help with the selection of candidates for more aggressive
chemotherapies.
References
|
1
|
Macdonald JS, Smalley SR, Benedetti J, et
al: Chemoradiotherapy after surgery compared with surgery alone for
adenocarcinoma of the stomach or gastroesophageal junction. N Engl
Med. 345:725–730. 2001. View Article : Google Scholar
|
|
2
|
Cunningham D, Allum WH, Stenning SP, et
al: Perioperative chemotherapy versus surgery alone for resectable
gastroesophageal cancer. N Engl Med. 355:11–20. 2006. View Article : Google Scholar
|
|
3
|
Sakuramoto S, Sasako M, Yamaguchi T, et
al: Adjuvant chemotherapy for gastric cancer with S-1, an oral
fluoropyrimidine. N Engl Med. 357:1810–1820. 2007. View Article : Google Scholar
|
|
4
|
Allum W, Garofalo A, Degiuli M and
Schuhmacher C: The first European Union Network of Excellence for
Gastric Cancer conference, Rome, Italy, April 2008. Gastric Cancer.
12:56–65. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yonemura Y, Elnemr A, Endou Y, et al:
Multidisciplinary therapy for treatment of patients with peritoneal
carcinomatosis from gastric cancer. World J Gastrointest Oncol.
2:85–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gotzmann J, Mikula M, Eger A, et al:
Molecular aspects of epithelial cell plasticity: implications for
local tumor invasion and metastasis. Mutat Res. 566:9–20. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cavallaro U and Christofori G: Cell
adhesion and signalling by cadherins and Ig-CAMs in cancer. Nature
Rev Cancer. 4:118–132. 2004. View
Article : Google Scholar
|
|
9
|
Tomita K, van Bokhoven A, van Leenders GJ,
et al: Cadherin switching in human prostate cancer progression.
Cancer Res. 60:3650–3654. 2000.PubMed/NCBI
|
|
10
|
Rieger-Christ KM, Cain JW, Braasch JW, et
al: Expression of classic cadherins type I in urothelial neoplastic
progression. Hum Pathol. 32:18–23. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Christiansen JJ and Rajasekaran AK:
Reassessing epithelial to mesenchymal transition as a prerequisite
for carcinoma invasion and metastasis. Cancer Res. 66:8319–8326.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klymkowsky MW and Savagner P:
Epithelial-mesenchymal transition: a cancer researcher's conceptual
friend and foe. Am J Pathol. 174:1588–1593. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okugawa Y, Toiyama Y, Tanaka K, et al:
Clinical significance of z inc finger E-box b inding homeobox 1
(ZEB1) in human gastric cancer. J Surg Oncol. 106:280–285. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jia B, Liu H, Kong Q, et al:
Overexpression of ZEB1 associated with metastasis and invasion in
patients with gastric carcinoma. Mol Cell Biochem. 366:223–229.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang GJ, Zhou T, Tian HP, et al: High
expression of ZEB1 correlates with liver metastasis and poor
prognosis in colorectal cancer. Oncol Lett. 5:564–568.
2013.PubMed/NCBI
|
|
16
|
Spaderna S, Schmalhofer O, Hlubek F, et
al: A transient, EMT-linked loss of basement membranes indicates
metastasis and poor survival in colorectal cancer.
Gastroenterology. 131:830–840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eger A, Aigner K, Sonderegger S, et al:
Delta EF1 is a transcriptional repressor of E-cadherin and
regulates epithelial plasticity in breast cancer cells. Oncogene.
24:2375–2385. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohira T, Gemmill RM, Ferguson K, et al:
WNT7a induces E-cadherin in lung cancer cells. Proc Natl Acad Sci
USA. 100:10429–10434. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chua HL, Bhat-Nakshatri P, Clare SE, et
al: NF-kappa B represses E-cadherin expression and enhances
epithelial to mesenchymal transition of mammary epithelial cells:
potential involvement of ZEB-1 and ZEB-2. Oncogene. 26:711–724.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Graham TR, Zhau HE, Odero-Marah VA, et al:
Insulin-like growth factor-I-dependent up-regulation of ZEB1 drives
epithelial-to-mesenchymal transition in human prostate cancer
cells. Cancer Res. 68:2479–2488. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abe N, Watanabe T, Toda H, et al:
Prognostic significance of carcinoembryonic antigen levels in
peritoneal washes in patients with gastric cancer. Am J Surg.
181:356–361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ito S, Nakanishi H, Kodera Y, et al:
Prospective validation of quantitative CEA mRNA detection in
peritoneal washes in gastric carcinoma patients. Br J Cancer.
93:986–992. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kodera Y, Nakanishi H, Ito S, et al:
Prognostic significance of intraperitoneal cancer cells in gastric
carcinoma: detection of cytokeratin 20 mRNA in peritoneal washes,
in addition to detection of carcinoembryonic antigen. Gastric
Cancer. 8:142–148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murai T, Yamada S, Fuchs BC, et al:
Epithelial-to-mesenchymal transition predicts prognosis in clinical
gastric cancer. J Surg Oncol. 109:684–689. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmalhofer O, Brabletz S and Brabletz T:
E-cadherin, beta-catenin and ZEB1 in malignant progression of
cancer. Cancer Metastasis Rev. 28:151–166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Wever O, Pauwels P, De Craene B, et al:
Molecular and pathological signatures of epithelial-mesenchymal
transitions at the cancer invasion front. Histochem Cell Biol.
130:481–494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Waldmann J, Feldmann G, Slater EP, et al:
Expression of the zinc-finger transcription factor Snail in
adrenocortical carcinoma is associated with decreased survival. Br
J Cancer. 99:1900–1907. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Iwatsuki M, Mimori K, Yokobori T, et al:
Epithelial-mesenchymal transition in cancer development and its
clinical significance. Cancer Sci. 101:293–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rosivatz E, Becker I, Specht K, et al:
Differential expression of the epithelial-mesenchymal transition
regulators Snail, SIP1 and Twist in gastric cancer. Am J Pathol.
161:1881–1891. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Spaderna S, Schmalhofer O, Wahlbuhl M, et
al: The transcriptional repressor ZEB1 promotes metastasis and loss
of cell polarity in cancer. Cancer Res. 68:537–544. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Drake JM, Strohbehn G, Bair TB, et al:
ZEB1 enhances transendothelial migration and represses the
epithelial phenotype of prostate cancer cells. Mol Biol Cell.
20:2207–2217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takeyama Y, Sato M, Horio M, et al:
Knockdown of ZEB 1, a master epithelial-to-mesenchymal transition
(EMT) gene, suppresses anchorage-independent cell growth of lung
cancer cells. Cancer Lett. 296:216–224. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Singh M, Spoelstra NS, Jean A, et al: ZEB1
expression in type I vs type II endometrial cancers: a marker of
aggressive disease. Mod Pathol. 21:912–923. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhou YM, Cao L, Li B, et al:
Clinicopathological significance of ZEB1 protein in patients with
hepatocellular carcinoma. Ann Surg Oncol. 19:1700–1706. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kurahara H, Takao S, Maemura K, et al:
Epithelial-mesenchymal transition and mesenchymal-epithelial
transition via regulation of ZEB-1 and ZEB-2 expression in
pancreatic cancer. J Surg Oncol. 105:655–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Toll A, Masferrer E, Hernández-Ruiz ME, et
al: Epithelial to mesenchymal transition markers are associated
with an increased metastatic risk in primary cutaneous squamous
cell carcinomas but are attenuated in lymph node metastases. J
Dermatol Sci. 72:93–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aokage K, Ishii G, Ohtaki Y, et al:
Dynamic molecular changes associated with epithelial-mesenchymal
transition and subsequent mesenchymal-epithelial transition in the
early phase of metastatic tumor formation. Int J Cancer.
128:1585–1595. 2011. View Article : Google Scholar : PubMed/NCBI
|