Introduction
Lung cancer is a leading cause of cancer-related
mortality worldwide and is expected to remain a major health
problem (1). The morbidity and
mortality rates of lung cancer in China are the highest among all
the malignant tumors. The majority of patients are diagnosed at an
advanced stage, at which the cancer is inoperable. Thus,
chemotherapy has become the primary treatment. However, the adverse
effects of certain agents, which lead to failure to complete the
scheduled regimen, extension of chemotherapy intervals or reduction
of the recommended dosage, have limited their clinical application.
Therefore, it is extremely important to investigate and identify
effective chemotherapy agents with low toxicity.
Nedaplatin [NDP; cis-diamine (glycolate)
platinum II] is a second-generation platinum analog, synthesized by
Shionogi & Co. Ltd. (Osaka, Japan). NDP has a higher aqueous
solubility than cisplatin (DDP), and was found to be highly
effective against solid tumors, in preclinical studies (2–4). Koshiyama
et al (5) reported that the
mean tumor inhibition rate for NDP was equal to or higher than that
for DDP in 15 cervical (70.7 vs. 63.9%), 65 ovarian (61.7 vs.
54.8%) and 57 endometrial (52.1 vs. 47.7%) carcinoma patients.
Compared to DDP, NDP-induced emesis and nephrotoxicity are
substantially reduced, bypassing the requirement for hydration
therapy for renal protection (6). The
dose-limiting toxicity of NDP is characterized by
thrombocytopenia.
Numerous cancers, including nasopharyngeal cancer,
NSCLC, esophageal cancer, urothelial carcinoma and cervical cancer,
have been reported to be effective to NDP-based chemotherapy in
clinical studies (7–13). However, the majority of recent studies
have focused on the therapeutic effect of NDP on esophageal cancer,
although this type of cancer does not respond well to
platinum-based chemotherapy. Limited studies have addressed the
effect of NDP on the treatment of lung cancer. Sasaki et al
(14) reported that NDP shows
equivalent antitumor activity to DDP against lung cancer cell lines
in vitro. Furuse et al (15) reported that a combination of NDP and
vindesin (VDS) was a safe and effective regimen for the treatment
of NSCLC, generating antitumor effects equivalent to that of the
DDP/VDS regimen. Thus far, no study has compared the survival
benefit between NDP and DDP in the treatment of NSCLC.
In the last decade, NDP-based chemotherapy has been
extensively used in Chinese NSCLC patients (16). The present study reports a
retrospective study comparing the efficacy of NDP and DDP in the
treatment of NSCLC. In the study, a retrospective analysis based on
392 patients diagnosed with NSCLC revealed that NDP-based
chemotherapy increased the median survival time (MST) of NSCLC
patient compared to DDP. The observed survival benefit is due to
the reduced toxicity of NDP, which allows patients to tolerate more
cycles of chemotherapy.
Patients and methods
Eligibility criteria
A total of 966 patients diagnosed with NSCLC at the
Cancer Center of Daping Hospital at the Third Medical University
(Chongqing, China), in the period between January 2003 and December
2007 were retrospectively reviewed. Every patient was evaluated for
age, gender, smoking status, stage, histology type, chemotherapy
regimen, overall chemotherapy cycles and other treatments.
Eligibility criteria for the study were as follows: Histological or
cytological confirmation of NSCLC, previously untreated with
chemotherapy, at least two cycles of platinum-based therapy (DDP-
or NDP-based chemotherapy), no surgical treatment of the primary
site and no changing to a different platinum agent or to a
non-platinum regimen in a subsequent treatment. Based on the above
criteria, a total of 392 NSCLC patients were selected. Among them,
202 patients received DDP-based chemotherapy and 190 patients
received NDP-based chemotherapy. Table
I shows that the two patient groups were not significantly
different in terms of demographics, disease severity and treatment
regimen.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variables | DDP, n (%)
(n=202) | NDP, n (%)
(n=190) | χ2 | P-value |
|---|
| Gender |
|
|
|
|
|
Female | 61 (30.2) | 50 (26.3) | 0.727 | 0.394 |
| Male | 141 (69.8) | 140 (73.7) |
|
|
| Age, years |
|
|
|
|
|
<60 | 110 (54.5) | 103 (54.2) | 0.002 | 0.961 |
| ≥60 | 92 (45.5) | 87 (45.8) |
|
|
| Smoking status |
|
|
|
|
|
Non-smoker | 105 (52.0) | 89 (46.8) | 1.034 | 0.309 |
| Current
smoker | 97 (48.0) | 101 (53.2) |
|
|
| Histology type |
|
|
|
|
| Sq | 74 (37.6) | 58 (30.5) | 2.192 | 0.139 |
|
Non-Sq | 128 (62.4) | 132 (69.5) |
|
|
| Stage |
|
|
|
|
| I–II | 18 (8.9) | 22 (11.6) | 4.371 | 0.112 |
| III | 118 (58.4) | 91 (47.9) |
|
|
| IV | 66 (32.7) | 77 (40.5) |
|
|
| Regimen |
|
|
|
|
| GP | 24 (11.9) | 30 (15.8) | 39.706 | <0.001 |
| TP | 114 (56.4) | 90 (47.4) |
|
|
| DP | 24 (11.9) | 61 (32.1) |
|
|
| CAP | 22 (10.9) | 3 (1.6) |
|
|
| NP | 18 (8.9) | 6 (3.2) |
|
|
| Cycles |
|
|
|
|
|
2–3 | 114 (56.4) | 70 (36.8) | 20.206 | <0.001 |
|
4–5 | 70 (34.7) | 78 (41.1) |
|
|
| ≥6 | 18 (8.9) | 42 (22.1) |
|
|
| Other
treatments |
|
|
|
|
|
Radiotherapy | 162 (80.2) | 137 (72.1) | 1.796 | 0.180 |
| Target
therapy | 11 (5.4) | 16 (8.4) |
|
|
Clinical data from these patients were acquired and
stored according to protocols approved by the local ethics
committee.
Treatment schedule
The patients received one of the following
combination chemotherapies by intravenous injection: Gemcitabine +
platinum (GP), paclitaxel + platinum (TP), navelbine + platinum
(NP), docetaxel + platinum (DP) and cyclophosphamide + doxorubicin
+ platinum (CAP). In each regimen, the platinum-based compound was
either DDP or NDP. The dose of gemcitabine was 1000
mg/m2 on days 1 and 8; docetaxel was 75 mg/m2
on day 1; paclitaxel was 135–175 mg/m2 on day 1;
navelbine was 25 mg/m2 on days 1 and 8; cyclophosphamide
was 600 mg/m2 on day 1; doxorubicin was 50
mg/m2 on day 1; and DDP and NDP were 80 mg/m2
on day 1.
All the patients received dexamethasone and the
5-hydroxytryptamine receptor antagonist on days 1, 2 and 3, or days
8 and 9, to prevent chemotherapy-induced nausea and vomiting.
Dexamethasone was also used prior to the administration of
paclitaxel, to prevent allergic reaction. Hydration with 3 to 6 l
of intravenous fluids and mannitol was conducted before and at the
day of the administration of DDP. All the chemotherapy regimens
were repeated every 21–28 days. Chemotherapy was continued until
unacceptable toxicity was observed, or until the patient refused
further treatment.
Evaluation
The overall survival time and toxicities observed
were analyzed in each patient group. Overall survival time was
calculated from the first day of chemotherapy until the last
follow-up or until the patient succumbed. For patients with longer
survival times, follow-up was discontinued at 5 years. The severity
of all the toxicities associated with chemotherapy was assessed
according to the National Cancer Institute Common Terminology
Criteria for Adverse Events (version 3.0) grading system (17). Anemia, neutropenia, thrombocytopenia,
nausea/vomiting, anorexia, renal toxicity, neurotoxicity and weight
loss were the symptoms of toxicities that were evaluated.
Statistical analysis
Statistical analysis was performed using a
statistical software package (SPSS for Windows, version 13.0; SPSS,
Chicago, IL, USA). Survival curves were estimated using the
Kaplan-Meier method, with censoring to correct for loss to
follow-up. Survival difference was analyzed by the log-rank test.
Multivariate analysis was performed with the Cox proportional
hazards model. To calculate statistical significance between
categorical variables, χ2 or the Fisher exact test were
used. Pearson correlation analysis was used to assess the
association between the two groups. Two-tailed P-values were
assessed and P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
The retrospective study analyzed a total number of
392 patients diagnosed with NSCLC between January 2003 and December
2007. Among them, 202 had received DDP-based chemotherapy and 190
had received NDP-based chemotherapy. The patient characteristics
are shown in Table I. No statistical
difference was observed between the two groups with regard to
gender, age, smoking status, histology type and stage, as analyzed
by the Pearson χ2 test (P<0.05).
All the enrolled patients had received at least two
cycles of chemotherapy. The mean chemotherapy duration was 3.3
cycles in the DDP and 4.1 cycles in the NDP group. The number of
patients receiving >4 chemotherapy cycles was 88/202 (43.6%) for
the DDP group and 120/190 (63.2%) for the NDP group
(χ2=20.206, P<0.001, Table
I).
Enrolled patients had undergone multiple regimens as
the first-line regimen, including GP, TP, DP, NP and CAP. Although
there were significant differences between the DDP and NDP groups
(χ2=39.706, P<0.001), ~50% of patients had received
the TP regimen in each group (Table
I).
Certain patients received radiotherapy or targeted
therapy in the subsequent treatment, but there was no statistical
difference between the percentages of patients receiving this
therapy in the two groups (Table
I).
Survival
Overall survival (OS) was considered from the start
of treatment to the date of data analysis or the date of loss from
follow-up for the remaining patients. The median follow-up time was
28 months (range, 4–60 months). As a result, the MST was 15 months
[95% confidence interval (CI), 13.4–16.6] for the DDP group and 20
months (95% CI, 17.0–23.0) for the NDP group. Statistical analysis
indicated that the NSCLC patients treated with NDP survived
significantly longer than those with DDP (χ2=5.189,
P=0.023) (Table II and Fig. 1). Multivariate analyses showed that
the type of platinum agent used was an independent predictive
factor for the overall survival time of NSCLC patients [hazard
ratio (HR), 0.764; 95% CI, 0.606–0.963; P=0.022] (Table II). The 1-, 2- and 3-year overall
survival rates were 62.4, 25.7 and 15.8% for the DDP group, and
78.9, 38.9 and 16.8% for the NDP group, respectively. A statistical
difference was observed between the two groups in the 1- and 2-year
overall survival rates (χ2=13.904, P<0.001;
χ2=7.827, P=0.005, respectively).
 | Table II.Association between patient
characteristics and overall survival time. |
Table II.
Association between patient
characteristics and overall survival time.
| Variables | n | MST, months (95%
CI) | P-value (Univariate
analysis) | HR (95% CI) | P-value
(Multivariate analysis) |
|---|
| Gender |
|
|
|
|
|
|
Female | 111 | 16 (13.1–18.9) | 0.380 | 0.952
(0.694–1.307) | 0.761 |
|
Male | 281 | 17 (13.1–18.9) |
|
|
| Age, years |
|
|
|
|
|
|
<60 | 212 | 18 (16.2–19.8) | 0.066 | 0.994
(0.791–1.249) | 0.958 |
|
≥60 | 180 | 15 (12.7–17.3) |
|
|
|
| Smoking status |
|
|
|
|
|
|
Non-smoker | 194 | 18 (15.6–20.4) | 0.241 | 0.829
(0.616–1.115) | 0.214 |
| Current
smoker | 198 | 6 (13.9–18.1) |
|
|
|
| Histology type |
|
|
|
|
|
| Sq | 132 | 18 (15.7–20.3) | 0.102 | 0.898
(0.682–1.164) | 0.416 |
|
Non-Sq | 260 | 16 (14.0–18.0) |
|
|
|
| Stage |
|
|
|
|
|
|
I–II | 40 | 36 (29.5–50.5) | <0.001 | 2.099
(1.756–2.510) | <0.001 |
|
III | 209 | 17 (14.8–19.2) |
|
|
|
| IV | 143 | 13 (11.8–14.2) |
|
|
|
| Regimen |
|
|
|
|
| GP | 54 | 18
(16.8.0–23.2) | 0.060 | 0.952
(0.866–1.046) | 0.304 |
| TP | 204 | 15 (12.6–17.4) |
|
|
|
| DP | 85 | 17 (15.4–18.6) |
|
|
|
CAP | 25 | 14 (9.4–18.6) |
|
|
|
| NP | 24 | 18 (12.8–23.2) |
| 0.539
(0.451–0.643) | <0.001 |
| Cycles |
|
|
|
|
|
2–3 | 184 | 12 (10.6–13.4) | <0.001 |
|
|
|
4–5 | 148 | 19 (17.7–24.3) |
|
|
| ≥6 | 60 | 23 (19.8–32.2) |
|
|
| Platinum |
|
|
|
|
|
|
DDP | 202 | 15 (13.4–16.6) | 0.023 | 0.764
(0.607–0.962) | 0.022 |
|
NDP | 190 | 20 (17.0–2.30) |
|
|
|
From Table II, the
chemotherapy cycle number was an independent predictive factor for
the overall survival time of NSCLC patients (HR, 0.539; 95% CI,
0.451–0.643; P<0.001). Table III
showed that the MST of the patients with 2–3, 4–5 and ≥6
chemotherapy cycles in the DDP group and in the NDP group was 10,
18 and 24 months vs. 12, 20 and 26 months, respectively. However,
no statistical difference was identified between the DDP and NDP
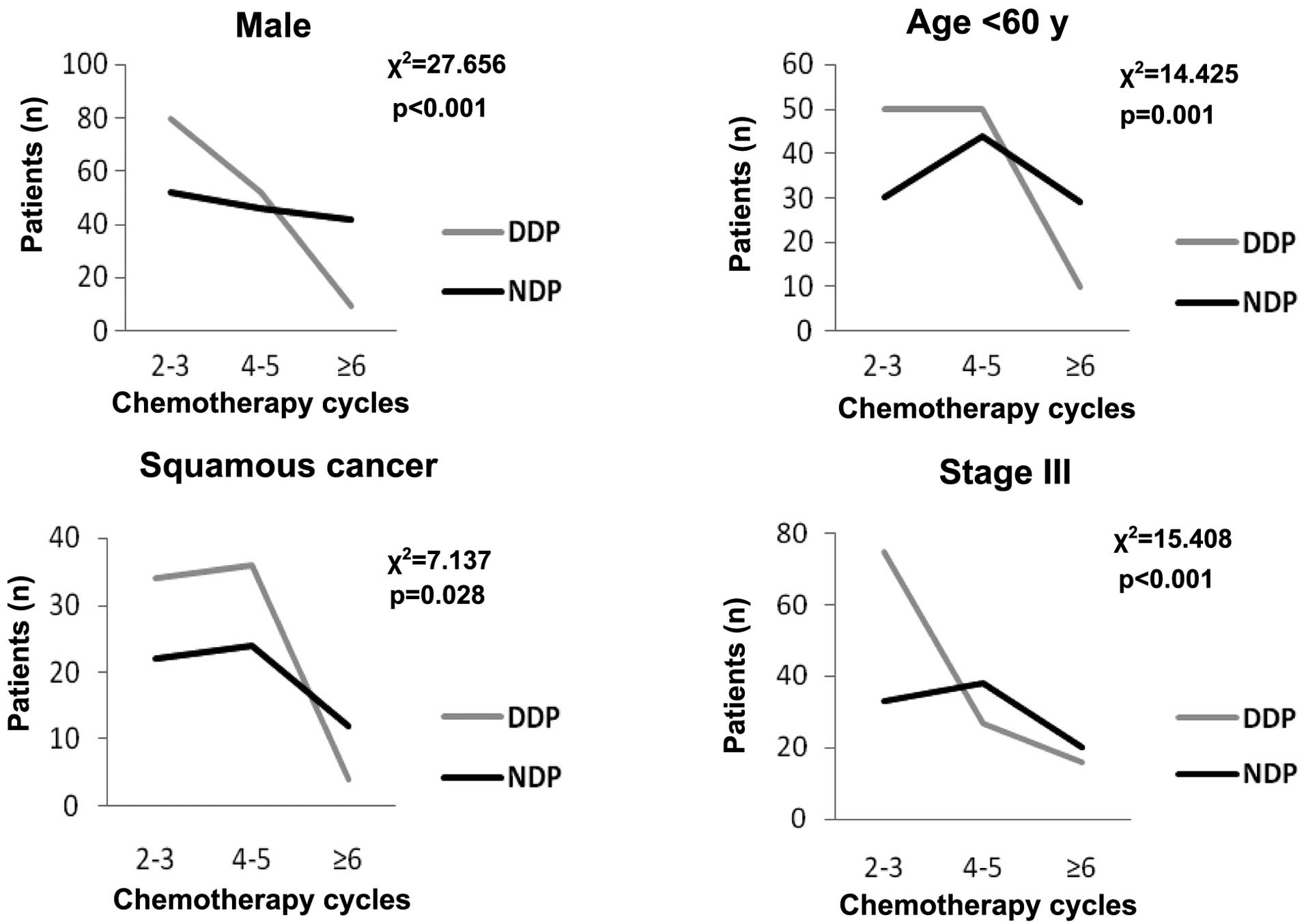
groups (χ2=0.040, P=0.980). Table III also showed that NDP-based
chemotherapy was beneficial regardless of smoking status. No
statistical differences were observed between the two groups for
female patients, patients aged >60 years and patients with
non-squamous cancer. However, younger patients (<60 years), male
patients, patients with squamous cancer and stage III in the NDP
group had a longer survival time compared to patients with the same
characteristics in the DDP group (21 vs. 16 months, P<0.001; 20
vs. 14 months, P<0.001; 24 vs. 16 months, P=0.021; and 20 vs. 15
months, P<0.001, respectively). For further study in these
subgroups, the distribution of the chemotherapy cycles was
significantly different in the different platinum agent groups
(Fig. 2). Thus, the chemotherapy
cycles were the main reason that caused the different survival
time.
 | Table III.Log-rank test for comparing overall
survival time in the subgroups. |
Table III.
Log-rank test for comparing overall
survival time in the subgroups.
|
|
| Median survival
time (95% CI) |
|
|
|---|
|
|
|
|
|
|
|---|
| Variables | n | DDP (n=202) | NDP (n=190) | χ2 | P-value |
|---|
| Gender |
|
|
|
|
|
Female | 111 | 16 (15.1–16.9) | 16 (9.9–22.1) | 0.582 | 0.445 |
|
Male | 281 | 14 (11.7–16.3) | 20 (16.8–23.2) | 14.225 | <0.001 |
| Age, years |
|
<60 | 212 | 16 (14.5–17.5) | 21 (12.4–29.6) | 21.121 | <0.001 |
|
≥60 | 180 | 14 (11.6–17.3) | 16 (13.2–17.8) | 0.068 | 0.795 |
| Smoking status |
|
|
|
|
|
|
Non-smoker | 194 | 16 (14.4–17.6) | 20 (14.6–25.4) | 7.029 | 0.008 |
| Current
smoker | 198 | 15 (10.2–19.8) | 19 (16.2–21.2) | 6.217 | 0.013 |
| Histology type |
|
|
|
|
|
| Sq | 132 | 16 (10.4–19.6) | 24 (20.4–31.6) | 10.305 | <0.001 |
|
Non-Sq | 260 | 15 (14.4–17.6) | 17 (14.8–21.2) | 0.345 | 0.557 |
| Stage |
|
|
|
|
|
|
I–II | 40 | 34 (20.6–40.4) | 38 (30.1–45.9) | 0.669 | 0.413 |
|
III | 209 | 15 (12.7–17.3) | 20 (18.1–21.9) | 5.360 | 0.021 |
| IV | 143 | 10 (9.0–11.0) | 14 (12.5–15.5) | 2.508 | 0.113 |
| Cycles |
|
|
|
|
|
|
2–3 | 184 | 10 (7.8–12.2) | 12 (10.3–13.7) | 5.106 | 0.204 |
|
4–5 | 148 | 18 (14.5–22.5) | 20 (15.9–28.1) | 0.053 | 0.818 |
| ≥6 | 60 | 22 (20.9–30.1) | 24 (14.0–36.0) | 1.121 | 0.290 |
Table II also showed
that stage was an independent predictive factor for the overall
survival time of NSCLC patients (HR=2.099; 95% CI, 1.756–2.510;
P<0.001). As the characteristic baseline regarding stage between
DDP and NDP groups was balanced, further analysis was not
performed.
Toxicity
The hematological and non-hematological toxicities
are summarized in Table IV. No grade
3 or 4 renal toxicity or neurotoxicity was observed in either of
the two groups. A significant difference was observed in
thrombocytopenia, nausea/vomiting, anorexia and weight loss between
the two groups. The rates of thrombocytopenia were higher in the
NDP compared to the DDP group (12.1 vs. 5.4%, P=0.019). However,
the rates of nausea/vomiting, anorexia and weight loss were higher
in the DDP compared to the NDP group (36.1 vs. 8.4%, P<0.001;
17.3 vs. 5.8%, P<0.001; and 9.9 vs. 1.0%, P<0.001,
respectively).
 | Table IV.Toxicity of grades 3–4 of different
platinum agents. |
Table IV.
Toxicity of grades 3–4 of different
platinum agents.
| Variables | DDP, n (%) | NDP, n (%) | χ2 | P-value |
|---|
| Hematologic |
|
|
|
|
|
Anemia | 8 (4.0) | 3 (1.6) | 2.036 | 0.154 |
|
Neutropenia | 42 (20.8) | 32 (16.8) | 0.998 | 0.192 |
|
Thrombocytopenia | 11 (5.4) | 23 (12.1) | 5.482 | 0.019 |
|
Non-hematologic |
|
|
|
|
|
Nausea/vomiting | 73 (36.1) | 16 (8.4) | 48.862 | <0.001 |
|
Anorexia | 35 (17.3) | 11 (5.8) | 12.582 | <0.001 |
| Renal
toxicity | 0 (0.0) | 0 (0.0) | N.A. | N.A. |
|
Neurotoxicity | 0 (0.0) | 0 (0.0) | N.A. | N.A. |
| Weight
loss | 20 (9.9) | 2 (1.0) | 13.642 | <0.001 |
Discussion
Chemotherapy is the major method for treatment of
lung cancer, owing to its high mortality and morbidity rates;
recently, the use of platinum-based chemotherapeutic agents have
allowed for significant advances in the survival of patients with
NSCLC. For a number of years, DDP has been the major agent in these
regimens. However, its relatively high rates of renal and
gastrointestinal toxicities lead numerous patients in China to give
up chemotherapy. Carboplatin and NDP are DDP analogs, with a
relatively lower toxicity profile. As NDP has the same
administration method and dosage as DDP, it has become the most
popular platinum-based agent for NSCLC patients in China. Although
numerous trials have compared the effect and survival benefit of
DDP and carboplatin in NSCLC (18–21), only
few trials have compared NDP with DDP. Cao et al (22) reported that NDP had similar response
rates to DDP in the treatment of nasopharyngeal carcinoma.
Yamashita et al (23) reported
that the overall survival rates of NDP at 1, 2 and 3 years were
lower than those of DDP (40, 13 and 13% vs. 56, 42 and 8%,
respectively) in the treatment of esophageal cancer, but no
significant difference was found between the two groups. However,
in the study, the two groups had an unequal number of patients (12
on the NDP regimen vs. 29 on the DDP regimen). Therefore, the
survival benefit of NDP has remained an unsolved issue thus
far.
In the present study, the patients receiving
NDP-based chemotherapy had higher survival rates than those treated
with DDP. The MST was greater by 5 months, whereas the 1- and
2-year overall survival rates were also higher in the NDP group.
The observed survival benefits of NDP can be explained as follows:
Firstly, the two groups have similar baseline characteristics,
except for the chemotherapy cycles. As patients receiving NDP-based
treatment experience less toxicity and show good compliance with
the chemotherapy regimen, these patients can complete more cycles
of the chemotherapy. The chemotherapy cycle number was an
independent predictive factor. More chemotherapy cycles can reduce
the mortality risk for 46%. Scotti et al (24) also came to the same conclusion that
the number of chemotherapy courses persisted as a significant
mortality predictor at multivariate regression analysis, with a
reduced mortality risk for 5–6 chemotherapy cycles in comparison to
3–4 cycles (HR, 0.44). Secondly, the differential weight loss
effect of NDP could also account for the observed survival benefit.
More specifically, the rate of weight loss was much higher in DDP-
compared to NDP-treated patients. Yang et al (25) reported that lung cancer patients
undergoing weight loss had shorter MST than those not losing weight
(6.4 vs. 9.2 months, P<0.001). Finally, the present study also
showed that the type of platinum agent used was an independent
predictive factor for the overall survival time and HR is 0.764.
Preclinical and in vitro studies have found that the plasma
concentration profile of unbound platinum following NDP infusion is
similar to that of total platinum, and that the protein-binding
affinity of NDP is lower than that of DDP (26). Thus, NDP has been demonstrated to have
higher antitumor activity than DDP (2).
In the present study, male patients, patients <60
years of age, and patients with squamous cancer and stage III in
the NDP group had a much longer survival time than patients with
similar characteristics in the DDP group. In the further study, the
distribution of chemotherapy cycles was significantly different in
different platinum agent groups, which suggested that the
chemotherapy cycles were the main reason that caused the different
survival time. However, Yamamoto et al (27) observed that when NDP was used in
advanced NSCLC patients, partial responses were observed in 13
(33%) of the 39 patients, while 12 of the 13 patients who responded
had squamous cell carcinoma. Teramoto et al (28) also reported that NDP responded better
in squamous cell carcinoma of the lung. Thus, further clinical
trials are required to confirm these observations.
In conclusion, NDP-based chemotherapy prolongs the
median survival time of NSCLC patients, compared to DDP-based
chemotherapy. The observed survival benefit is due to the reduced
toxicity of NDP, which allows patients to tolerate more cycles of
chemotherapy. A slow toxicity and high life quality were the
tendencies of the advanced cancer treatment currently. Thus, NDP
may be a more reasonable choice than DDP in clinical practice. In
addition, noteworthy information is also provided regarding the
impact of gender, age and histological type, which may improve
treatments by targeting specific patient populations.
Acknowledgements
The authors thank the Chinese National Natural
Science Foundation (grant nos. 30801367 and 81272599). They also
thank the colleagues of the Cancer Centre (Daping Hospital) for
collecting the patient information, including Mrs. Lei Xia, Mrs.
Juan Li, Mrs. Wei Luo, Mrs. Shuai Wang, Mrs. Yan Feng, Mrs. Qian
Zhou, Mrs. Hong Peng and Miss Shu Chen. Thank you to Editage for
providing editorial and publication support.
References
|
1
|
Pastorino U: Lung cancer screening. Br J
Cancer. 102:1681–1686. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanzawa F, Matsushima Y, Nakano H, et al:
Antitumor activity of a new platinum compound (glycolate-o,o′)
diammineplatinum (II) (254-S), against non-small cell lung
carcinoma grown in a human tumor clonogenic assay system.
Anticancer Res. 8:323–327. 1998.
|
|
3
|
Suzumura Y, Kato T, Ueda R and Ota K:
Effect of treatment schedule on antitumor activity of glycolate-0,
0′-diammineplatinum (II), a new platinum derivative: comparison
with cis-diamminedichloroplatinum (II). Anticancer Res.
9:1083–1088. 1989.PubMed/NCBI
|
|
4
|
Hida S, Okada K and Yoshida O: Advantages
in combination chemotherapy using cisplatin and its analogues for
human testicular tumor xenografts. Jpn J Cancer Res. 81:425–430.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koshiyama M, Kinezaki M, Uchida T and
Sumitomo M: Chemosensitivity testing of a novel platinum analog,
nedaplatin (254-S), in human gynecological carcinomas: a comparison
with cisplatin. Anticancer Res. 25:4499–4502. 2005.PubMed/NCBI
|
|
6
|
Kuruse K, Fukuoka M, et al: A phase II
clinical study of cis-diammine glycolato platinum, 254-S, for
primary lung cancer. Gan To Kagaku Ryoho. 19:879–884.
1992.PubMed/NCBI
|
|
7
|
Zheng J, Wang G, Yang GY, et al: Induction
chemotherapy with nedaplatin with 5-FU followed by
intensity-modulated radiotherapy concurrent with chemotherapy for
locoregionally advanced nasopharyngeal carcinoma. Jpn J Clin Oncol.
40:425–431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Naito Y, Kubota K, Ohmatsu H, et al: Phase
II study of nedaplatin and docetaxel in patients with advanced
squamous cell carcinoma of the lung. Ann Oncol. 22:2471–2475. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oshita F, Ohe M, Honda T, et al: Phase II
study of nedaplatin and irinotecan with concurrent thoracic
radiotherapy in patients with locally advanced non-small-cell lung
cancer. Br J Cancer. 103:1325–1330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Osawa S, Furuta T, Sugimoto K, et al:
Prospective study of daily low-dose nedaplatin and continuous
5-fluorouracil infusion combined with radiation for the treatment
of esophageal squamous cell carcinoma. BMC Cancer. 9:4082009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jin J, Xu X, Wang F, et al: Second-line
combination chemotherapy with docetaxel and nedaplatin for
Cisplatin-pretreated refractory metastatic/recurrent esophageal
squamous cell carcinoma. J Thorac Oncol. 4:1017–1021. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kitamura H, Taguchi K, Kunishima Y, et al:
Paclitaxel, ifosfamide and nedaplatin as second-line treatment for
patients with metastatic urothelial carcinoma: a phase II study of
the SUOC group. Cancer Sci. 102:1171–1175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yin M, Zhang H, Li H, et al: The toxicity
and long-term efficacy of nedaplatin and paclitaxel treatment as
neoadjuvant chemotherapy for locally advanced cervical cancer. J
Surg Oncol. 105:206–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sasaki Y, Saijo N and Tamura T: Comparison
of the antitumor activity of cisplatin and its derivatives with
special stress on the pharmacokinetics of active form of drugs in
the plasma determined by colony assay. Proc Am Soc Clin Oncol.
6:341987.
|
|
15
|
Furuse K, Fukuoka M, Asamoto H, et al: A
randomized comparative study of 254-S plus vindesine (VDS) vs.
cisplatin (CDDP) plus VDS in patients with advanced non-small cell
lung cancer (NSCLC). Gan To Kagaku Ryoho. 19:1019–1026.
1992.PubMed/NCBI
|
|
16
|
Guo JF, Zhang B, Wu F, et al: A phase Ⅱ
trial of docetaxel plus nedaplatin and 5-fluorouracil in treating
advanced esophageal carcinoma. Chin J Cancer. 29:348–352. 2010.
View Article : Google Scholar
|
|
17
|
Cancer Therapy Evaluation Program, .
Common Terminology Criteria for Adverse Events. Version 3.0. DCTD,
NCI, NIH, DHHS. 2006.
|
|
18
|
Ohe Y, Ohashi Y, Kubota K, et al:
Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
four-arm cooperative study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hotta K, Matsuo K, Ueoka H, Kiura K,
Tabata M and Tanimoto M: Meta-analysis of randomized clinical
trials comparing Cisplatin to Carboplatin in patients with advanced
non-small-cell lung cancer. J Clin Oncol. 22:3852–3859. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang J, Liang X, Zhou X, Huang R and Chu
Z: A meta-analysis of randomized controlled trials comparing
carboplatin-based to cisplatin-based chemotherapy in advanced
non-small cell lung cancer. Lung Cancer. 57:348–358. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ardizzoni A, Boni L, Tiseo M, et al:
Cisplatin- versus carboplatin-based chemotherapy in first-line
treatment of advanced non-small-cell lung cancer: an individual
patient data meta-analysis. J Natl Cancer Inst. 99:847–857. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cao KJ, Zhang AL, Ma WJ, Huang PY, Luo DH
and Xia WX: Nedaplatin or cisplatin combined with 5-fluorouracil
for treatment of stage III–IVa nasopharyngeal carcinoma: a
randomized controlled study. Zhonghua Zhong Liu Za Zhi. 33:50–52.
2011.PubMed/NCBI
|
|
23
|
Yamashita H, Nakagawa K, Tago M, et al:
Radiation therapy combined with cis-diammine-glycolatoplatinum
(nedaplatin) and 5-fluorouracil for Japanese stage II–IV esophageal
cancer compared with cisplatin plus 5-fluorouracil regimen: a
retrospective study. Dis Esophagus. 19:15–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Scotti V, Meattini I, Saieva C, et al:
Limited-stage small-cell lung cancer treated with early
chemo-radiotherapy: the impact of effective chemotherapy. Tumori.
98:53–59. 2012.PubMed/NCBI
|
|
25
|
Yang R, Cheung MC, Pedroso FE, Byrne MM,
Koniaris LG and Zimmers TA: Obesity and weight loss at presentation
of lung cancer are associated with opposite effects on survival. J
Surg Res. 170:e75–e83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ota K, Oguma T and Shimamura K:
Pharmacokinetics of platinum in cancer patients following
intravenous infusion of cis-diammine (glycolato) platinum, 254-S.
Anticancer Res. 14:1383–1387. 1994.PubMed/NCBI
|
|
27
|
Yamamoto N, Tamura T, Kurata T, et al:
Dose-finding and pharmacokinetic study of nedaplatin in elderly
patients with advanced non-small cell lung cancer. Cancer Chemother
Pharmacol. 65:79–88. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Teramoto K, Asada Y, Ozaki Y, et al: phase
II study of docetaxel plus nedaplatin in patients with metastatic
non-small-cell lung cancer. Cancer Chemother Pharmacol. 70:531–537.
2012. View Article : Google Scholar : PubMed/NCBI
|