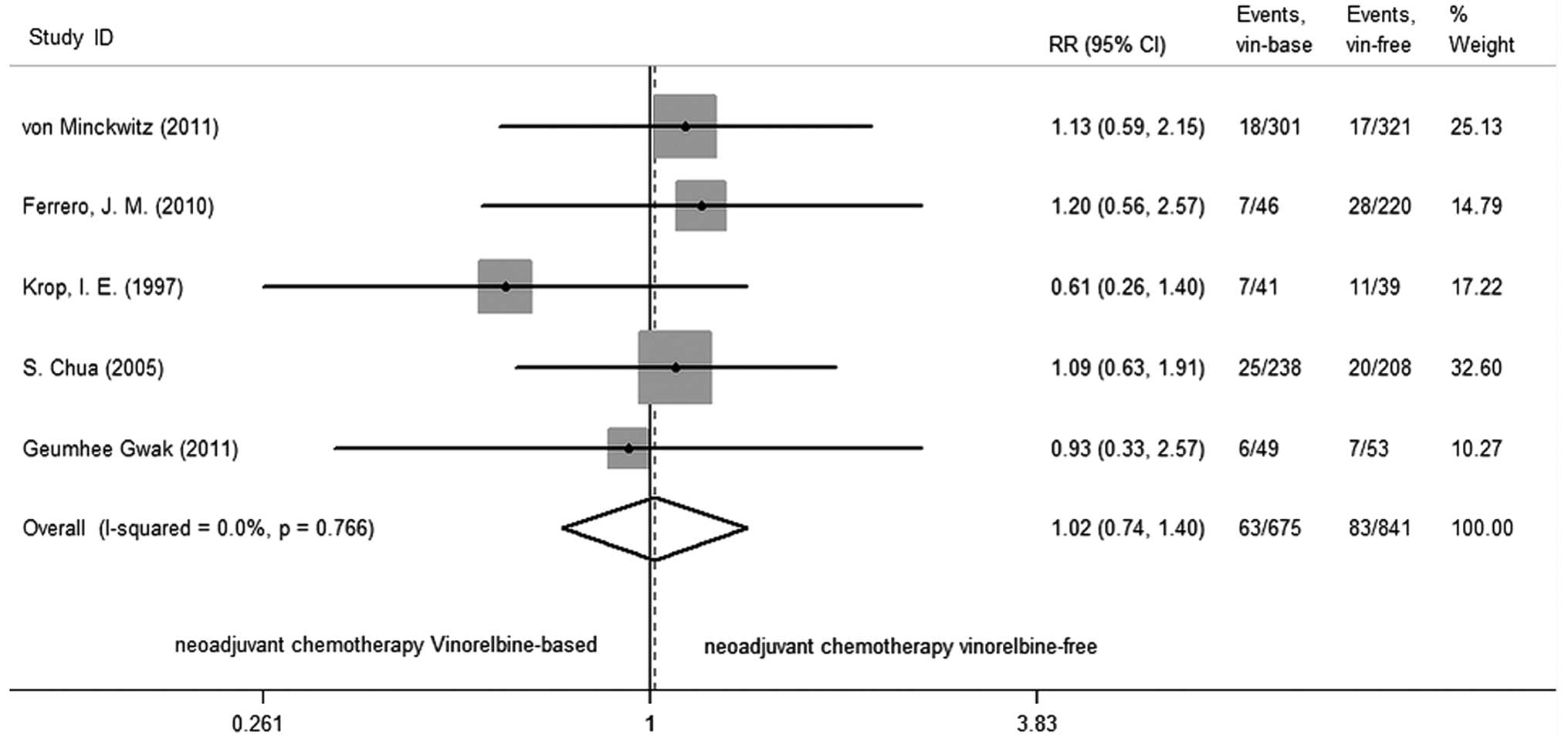
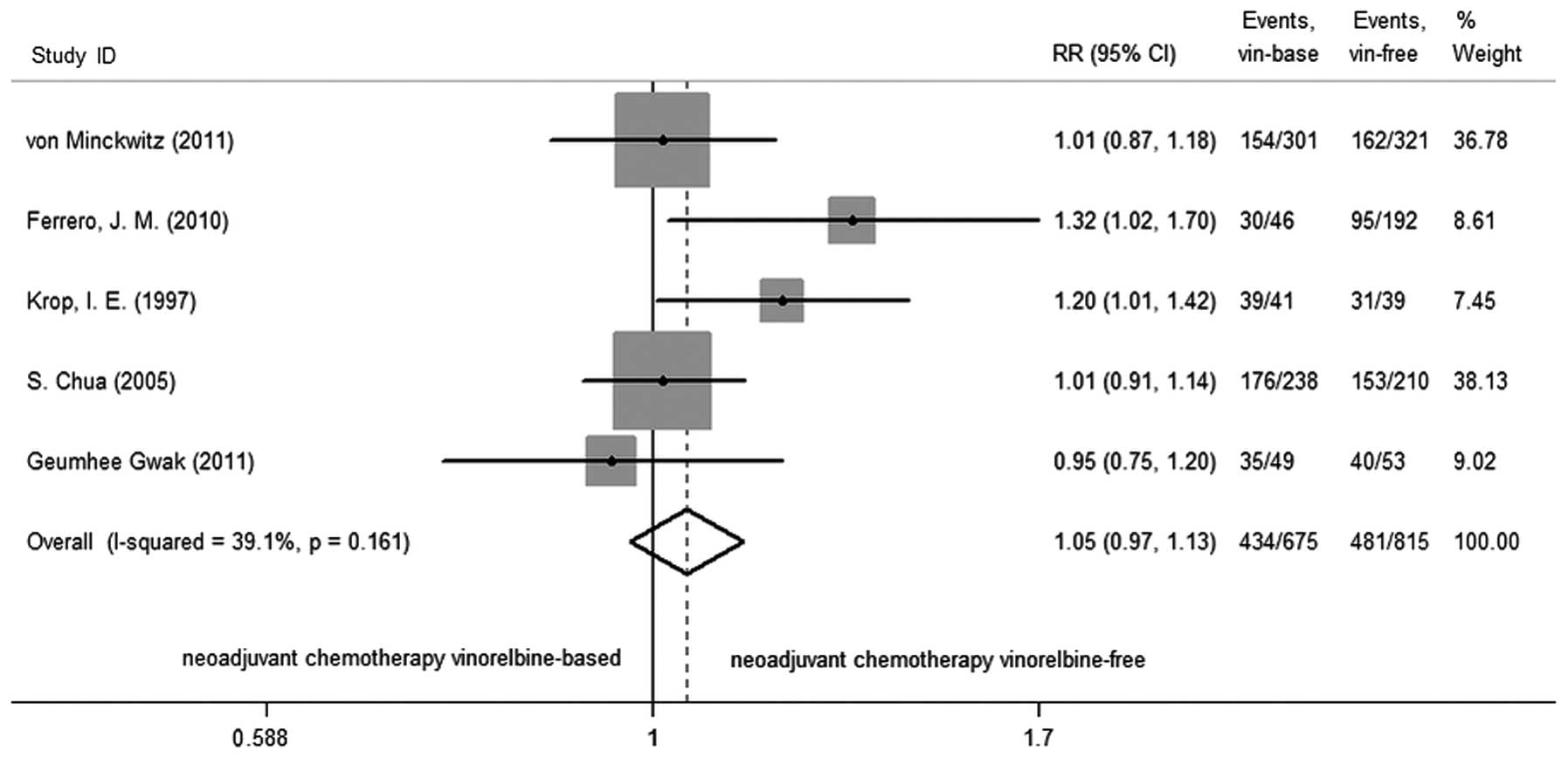
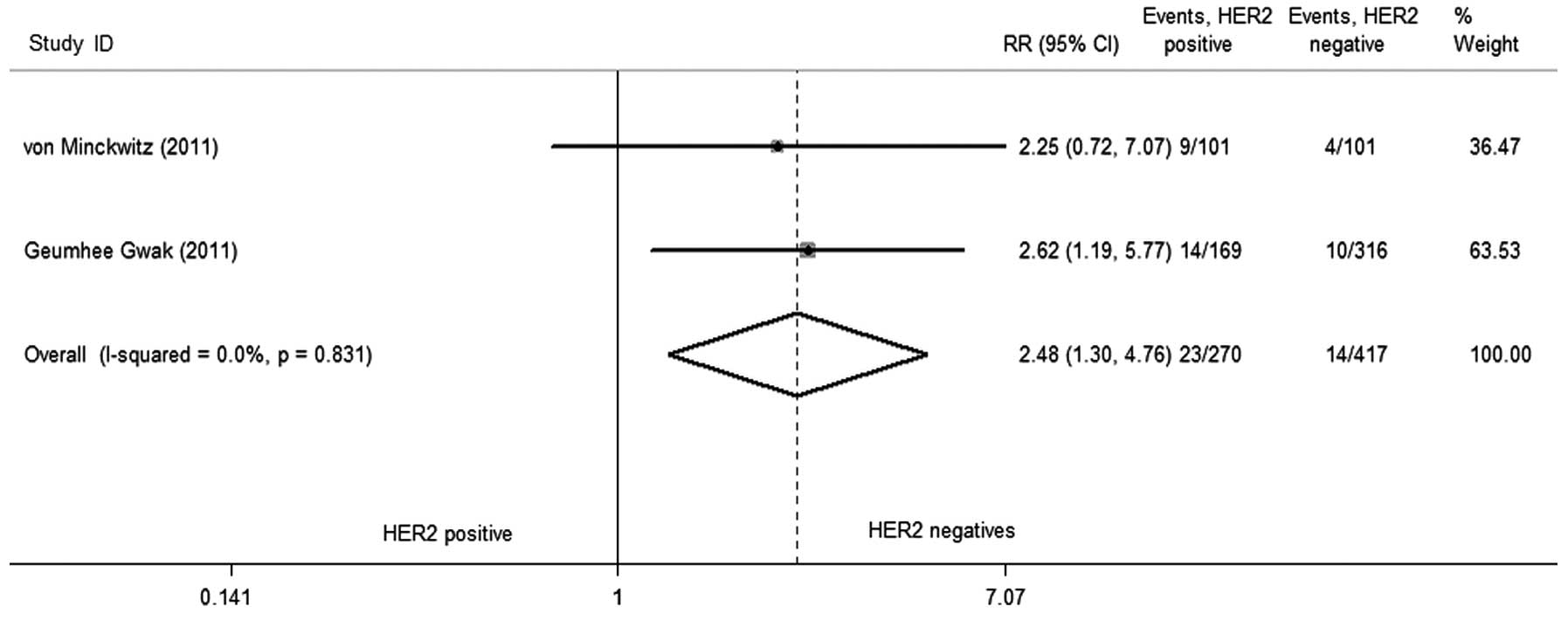
|
1
|
Cleator S, Parton M and Dowsett M: The
biology of neoadjuvant chemotherapy for breast cancer. Endocr Relat
Cancer. 9:183–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Charfare H, Limongelli S and Purushotham
AD: Neoadjuvant chemotherapy in breast cancer. Br J Surg. 92:14–23.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cros S, Wright M, Morimoto M, Lataste H,
Couzinier JP and Krikorian A: Experimental antitumor activity of
Navelbine. Semin Oncol. 16 (Suppl 4):15–20. 1989.PubMed/NCBI
|
|
4
|
Goa KL and Faulds D: Vinorelbine. A review
of its pharmacological properties and clinical use in cancer
chemotherapy. Drugs Aging. 5:200–234. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Xu L, Ye JM, Zhao JX, Duan XN and
Liu YH: Effects of vinorelbine plus cisplatin as second-line
neoadjuvant chemotherapy regimen in the treatment of breast cancer.
Zhonghua Yi Xue Za Zhi. 93:93–95. 2013.(In Chinese). PubMed/NCBI
|
|
6
|
Rivera-Rodriguez N, Cabanillas F,
Lawrenson L, Negron V, Pavia OA, Bruno M, Echenique MM, Carlo V,
Liboy I, et al: Results of a novel neoadjuvant chemotherapy (NAC)
regimen for breast cancer. J Clin Oncol. (abstract).
31:e116102013.
|
|
7
|
Carillio G, Aiello RA, Chiarenza M, Alì M,
Mazzola A, Marco R, Taibi E, Fallica G, Casella T, Zacchia A, et
al: Neoadjuvant trastuzumab and sequential chemotherapy with
cisplatin, vinorelbine, and docetaxel for stage II–III breast
cancer patients: Final results of a single institution phase II
study. J Clin Oncol. (abstract). 31:e115272013.
|
|
8
|
Xu L, Ye JM, Zhao JX, Duan XN and Liu YH:
Effects and toxicity of neoadjuvant chemotherapy with vinorelbine
and cisplatin in treatment of operable breast cancer previously
non-responsive to anthracyclines and taxanes-containing regimen:
Analysis of 19 cases. Zhonghua Yi Xue Za Zhi. 89:683–685. 2009.(In
Chinese). PubMed/NCBI
|
|
9
|
von Minckwitz G, Blohmer JU, Costa S,
Denkert C, Eidtmann H, Eiermann W, Gerber B, Hanusch C, Hilfrich J,
Huober J, et al: Neoadjuvant chemotherapy adapted by interim
response improves overall survival of primary breast cancer
patients - Results of the GeparTrio trial. Cancer Res. 71:S3–S2.
2011. View Article : Google Scholar
|
|
10
|
Gwak G, Kim JY, Park K, Shin YJ, Cho H,
Park SJ, Yang GH, Bae BN, Kim KW and Han S: Comparison of
doxorubicin plus docetaxel neoadjuvant chemotherapy with
doxorubicin plus vinorelbine in primary breast cancer. J Breast
Cancer. 14:129–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krop IE, Flores L, Tuck DP, Ryan PD,
Partridge AH, Morganstern D, Najita J, Lezon-Geyda K, Winer EP and
Harris L: Phase II trial of preoperative vinorelbine/trastuzumab
(VH) or docetaxel/carboplatin/trastuzumab (TCH) in HER2-positive
breast cancer with analysis of resistance mechanisms. J Clin Oncol.
28:15S2010.
|
|
12
|
Chua S, Smith IE, Ahern RP, Coombes GA,
Hickish TF, Robinson AC, Laing RW, OBrien ME, Ebbs SR, Hong A, et
al TOPIC Trial Group: Neoadjuvant vinorelbine/epirubicin (VE)
versus standard adriamycin/cyclophosphamide (AC) in operable breast
cancer: Analysis of response and tolerability in a randomised phase
III trial (TOPIC 2). Ann Oncol. 16:1435–1441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferrero JM, Namer M, Dufour JF, Largillier
R, Creisson A, Teissier E, Machiavello JC, Lallement M, Monticelli
J and Abbes M: Neoadjuvant chemotherapy of locally advanced breast
cancer: Historical comparison of 4 sequential combinations. Bull
Cancer. 84:10–16. 1997.PubMed/NCBI
|
|
14
|
Halim A and Wahba H: Second-line
neoadjuvant vinorelbine and gemcitabine combination in locally
advanced breast cancer showing no early response to TAC. Med Oncol.
29:454–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O'Regan RM, Gabram S, Styblo T, Rizzo M,
Wood W, Srinivasiah J, Jonas W, Schnell F, Adams A, Nahta R, et al:
Final results of a phase 2 trial using a novel, non-anthracycline
neoadjuvant chemotherapy regimen in Her2-positive breast cancer.
Cancer Res. 72:27032012. View Article : Google Scholar
|
|
16
|
Medioni J, Huchon C, Le Frere-Belda MA,
Hofmann H, Bats AS, Eme D, Andrieu JM, Oudard S, Lecuru F and Levy
E: Neoadjuvant dose-dense gemcitabine plus docetaxel and
vinorelbine plus epirubicin for operable breast cancer: Improved
prognosis in triple-negative tumors. Drugs R D. 11:147–157. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bear HD, Anderson S, Smith RE, Geyer CE
Jr, Mamounas EP, Fisher B, Brown AM, Robidoux A, Margolese R,
Kahlenberg MS, et al: Sequential preoperative or postoperative
docetaxel added to preoperative doxorubicin plus cyclophosphamide
for operable breast cancer: National Surgical Adjuvant Breast and
Bowel Project Protocol B-27. J Clin Oncol. 24:2019–2027. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wolmark N, Wang J, Mamounas E, Bryant J
and Fisher B: Preoperative chemotherapy in patients with operable
breast cancer: nine-year results from National Surgical Adjuvant
Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 96–102.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chollet P, Amat S, Cure H, de Latour M, Le
Bouedec G, Mouret-Reynier MA, Ferriere JP, Achard JL, Dauplat J and
Penault-Llorca F: Prognostic significance of a complete
pathological response after induction chemotherapy in operable
breast cancer. Br J Cancer. 86:1041–1046. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Montagna E, Bagnardi V, Rotmensz N, Viale
G, Pruneri G, Veronesi P, Cancello G, Balduzzi A, Dellapasqua S,
Cardillo A, et al: Pathological complete response after
preoperative systemic therapy and outcome: Relevance of clinical
and biologic baseline features. Breast Cancer Res Treat.
124:689–699. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
von Minckwitz G, Kümmel S, Vogel P,
Hanusch C, Eidtmann H, Hilfrich J, Gerber B, Huober J, Costa SD,
Jackisch C, et al German Breast Group: Neoadjuvant
vinorelbine-capecitabine versus
docetaxel-doxorubicin-cyclophosphamide in early nonresponsive
breast cancer: Phase III randomized GeparTrio trial. J Natl Cancer
Inst. 100:542–551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kurebayashi J, Kanomata N, Yamashita T,
Shimo T, Mizutoh A, Moriya T and Sonoo H: Prognostic value of
phosphorylated HER2 in HER2-positive breast cancer patients treated
with adjuvant trastuzumab. Breast Cancer. Jun 8–2013.(Epub ahead of
print). PubMed/NCBI
|
|
23
|
Yoo C, Ahn JH, Jung KH, Kim SB, Kim HH,
Shin HJ, Ahn SH, Son BH and Gong G: Impact of
immunohistochemistry-based molecular subtype on chemosensitivity
and survival in patients with breast cancer following neoadjuvant
chemotherapy. J Breast Cancer. 15:203–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Houssami N, Macaskill P, von Minckwitz G,
Marinovich ML and Mamounas E: Meta-analysis of the association of
breast cancer subtype and pathologic complete response to
neoadjuvant chemotherapy. Eur J Cancer. 48:3342–3354. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suen D, Chow L and Kwong A:
Breast-conserving surgery in Hong Kong Chinese women. World J Surg.
32:2549–2553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clavarezza M, Turazza M, Aitini E,
Saracchini S, Garrone O, Durando A, De Placido S, Bisagni G,
Levaggi A, Bighin C, et al: Phase II open-label study of
bevacizumab combined with neoadjuvant anthracycline and taxane
therapy for locally advanced breast cancer. Breast. 22:470–475.
2013. View Article : Google Scholar : PubMed/NCBI
|