Introduction
Neoadjuvant chemotherapy has emerged as the standard
of care in the treatment of inoperable and operable locally
advanced breast cancer. Neoadjuvant chemotherapy was used to afford
tumor shrinkage and render tumors treatable for inoperable and
locally advanced disease (1,2). Compared to patients with operable
primary breast cancer, neoadjuvant chemotherapy can downstage the
tumor so that breast-conserving surgery (BCS) becomes an
alternative to mastectomy (2).
Another benefit of neoadjuvant therapy is the unique opportunity
for the evaluation of treatment response with pathological complete
response (pCR) acting as a surrogate marker of survival. This
allows a more rapid assessment of the efficacy of novel
chemotherapeutic agents, also enabling early cessation of
ineffective treatments and providing an opportunity to
individualise patient treatment at an early stage. Despite the
proven benefits of neoadjuvant treatment, no neoadjuvant
chemotherapy regimens were recommended as the treatment of first
choice. Anthracycline- or taxane-based neoadjuvant chemotherapy
regimens are used widely, and other agents have been explored in
clinical studies.
Vinorelbine is a semi-synthetic third generation
vinca alkaloid with a broad spectrum of antitumor activity.
Vinorelbine acts on the dynamic equilibrium of tubulin in the
microtubulin apparatus of the cell. It inhibits tubulin
polymerisation and binds preferentially to mitotic microtubules and
blocks mitosis at G2-M, causing cell death in interphase
or at the following mitosis (3,4). The
Breast Cancer Guidelines Committee of the National Comprehensive
Cancer Network (NCCN) recommend vinorelbine as one of the first
choices for patients with recurrent or metastatic breast cancer,
but there are also other clinical studies exploring neoadjuvant
treatment (5–8). Vinorelbine-based regimens were as
effective and well-tolerated as vinorelbine-free regimens for
breast cancer patients, and called for a neoadjuvant chemotherapy
regimen as an option for primary breast cancer (5). To more clearly understand
vinorelbine-based regimens in neoadjuvant treatment for breast
cancer patients, a meta-analysis was performed of the randomized
controlled trials comparing neoadjuvant therapies with and without
the drug for patients with breast cancer.
Materials and methods
Publication search strategy
PubMed and Embase were searched until July 2013 for
randomized controlled trials regarding vinorelbine-based
neoadjuvant chemotherapy for breast cancer. No language
restrictions were used. The following search terms were used:
‘Vinorelbine’, ‘neoadjuvant’, ‘preoperative’, ‘breast neoplasm’ and
‘breast cancer’. Manual searches were performed by reviewing the
reference lists of retrieved studies, textbooks and review studies
to identify additional potentially eligible studies.
Inclusion criteria
To be considered eligible for inclusion in the
meta-analysis, the study criteria had to include: i) Patient
diagnosis of breast cancer without metastasis; ii) being a
controlled trial; iii) using vinorelbine in the neoadjuvant setting
to treat breast cancer; and iv) reporting relative risk (RR) with a
95% confidence interval (CI); if not, the reported data of pCR,
overall response rate (ORR) or BCS outcomes were sufficient to
calculate them.
Data extraction
Two investigators (Hui Gao and Qiuyun Li)
independently extracted information from the included studies.
Disagreement was resolved by discussion between the two
investigators. When multiple studies covering the same trial were
retrieved, or when studies had overlapping study publications, only
the largest number of participants with the most recent publication
was included. The following data were extracted: First author's
family name, year of publication, country of origin, regimens,
number of cases and doses of regimens. Study quality was assessed
using the Jadad score. Two investigators independently evaluated
all the included trials based on an appropriate randomization
method (0–2), an appropriate blinding method (0–2) and the study
withdrawals and dropouts (0–1). Trials were considered to be of low
quality if they reported none of the items, medium quality if they
reported on <3 and of high quality if they reported on 3–5.
Statistical methods
All the statistical tests were performed using
Stata/SE12.0 software (version 12.0; Stata Corp., College Station,
TX, USA). The strength of association between the vinorelbine-based
and vinorelbine-free regimens was assessed by calculating RR with
95% CIs based on the numbers in the controls. To test for
heterogeneity in the included studies and analyze the statistical
heterogeneity using the χ2 test, P≤0.10 was considered
to indicate a statistically significant difference. When
heterogeneity did not exist between the results, I2
heterogeneity quantitative analysis was used and the significance
level was set at 50%, so I2>50% indicated
heterogeneity in the results. A random-effects model was used to
pool the analysis when there was a genuine difference in the
result. By contrast, if the difference in the studies was due to
chance, then a fixed-effects model was used for meta-analysis.
Results
Patient characteristics
Five eligible studies (9–13) were
identified with a total of 1,492 patients with early or operable
breast cancer without distant metastasis according to the inclusion
criteria (Fig. 1). In total, 675
patients were assigned to chemotherapy combined with vinorelbine
and 817 to chemotherapy alone. The characteristics of the included
trials are summarized in Table I. The
median follow-up ranged between 2.2 and 5.1 years.
 | Table I.Characteristics of studies. |
Table I.
Characteristics of studies.
| First author
(year) | No. of patients | Agents and doses | Country | End-point | Jadad score | (Refs.) |
|---|
| Krop (2010) | 41 | N 25 mg/m2
qwk + H 2 mg/kg qwk | America | pCR, ORR, BCS | 2 | (11) |
|
| 39 | T 75 mg/m2
q3wk + C1 AUC 6 q3wk + H 2 mg/kg qwk |
|
|
|
|
| Minckwitz (2011) | 321 | T 75 mg/m2
+ A 50 mg/m2 + C 500 mg/m2 on day 1 | German | pCR, ORR, BCS | 3 | (9) |
|
| 301 | N 25 mg/m2
on days 1 and 8 + X 1,000 mg/m2 twice a day on days
1–14 |
|
|
|
|
| Ferrero (1997) | 68 | A 40 mg/m2
day 1+ V1 1.4 mg/m2 day 2 + C 300
mg/m2 days 3–5 + F 500 mg/m2 days 3–5 | France | pCR, ORR, BCS | 2 | (13) |
|
| 47 | A 40 mg/m2
day 1 + V2 3 mg/m2 day 2 + C 300
mg/m2 days 3–5 + F 500 mg/m2 days 3–5 |
|
|
|
|
|
| 77 | A 30 mg/m2
day 1+ V3 1.4 mg/m2 day 2 + C 100
mg/m2 days 1–14 + F 500 mg/m2 days 1 and
8 |
|
|
|
|
|
| 46 | A 50 mg/m2
day 1 + N 25 mg/m2 days 1 and 8 |
|
|
|
|
| Chua (2005) | 238 | N 25 mg/m2
on days 1 and 8 + E 60 mg/m2 on day 1 | UK | pCR, ORR, BCS | 2 | (12) |
|
| 210 | A 60 mg/m2
+ C 600 mg/m2 day 1 |
|
|
|
|
| Gwak (2011) | 53 | A 50 mg/m2
+ D 75 mg/m2 | Korea | pCR, ORR, BCS | 2 | (10) |
|
| 49 | A 50
mg/m2 + N 25 mg/m2 |
|
|
|
|
Krop et al (11) reported on a group receiving
vinorelbine plus trastuzumab as neoadjuvant therapy and another
receiving a standard combination of trastuzumab, docetaxel plus
carboplatin. This study was only available as an abstract, while
the full text was available for the remaining four studies. In the
study by von Minckwitz et al (9), prior data for the group with complete or
partial remission to 2 cycles TAC (docetaxel, doxorubicin and
cyclophosphamide) followed by 4 or 6 cycles of TAC were excluded
based on the inclusion criteria. All the studies included in the
meta-analysis were well-organized and had balanced populations. The
main endpoint of all the five studies was pCR, and the second
endpoint was ORR, BCS and various toxicities of the two arms.
First endpoint
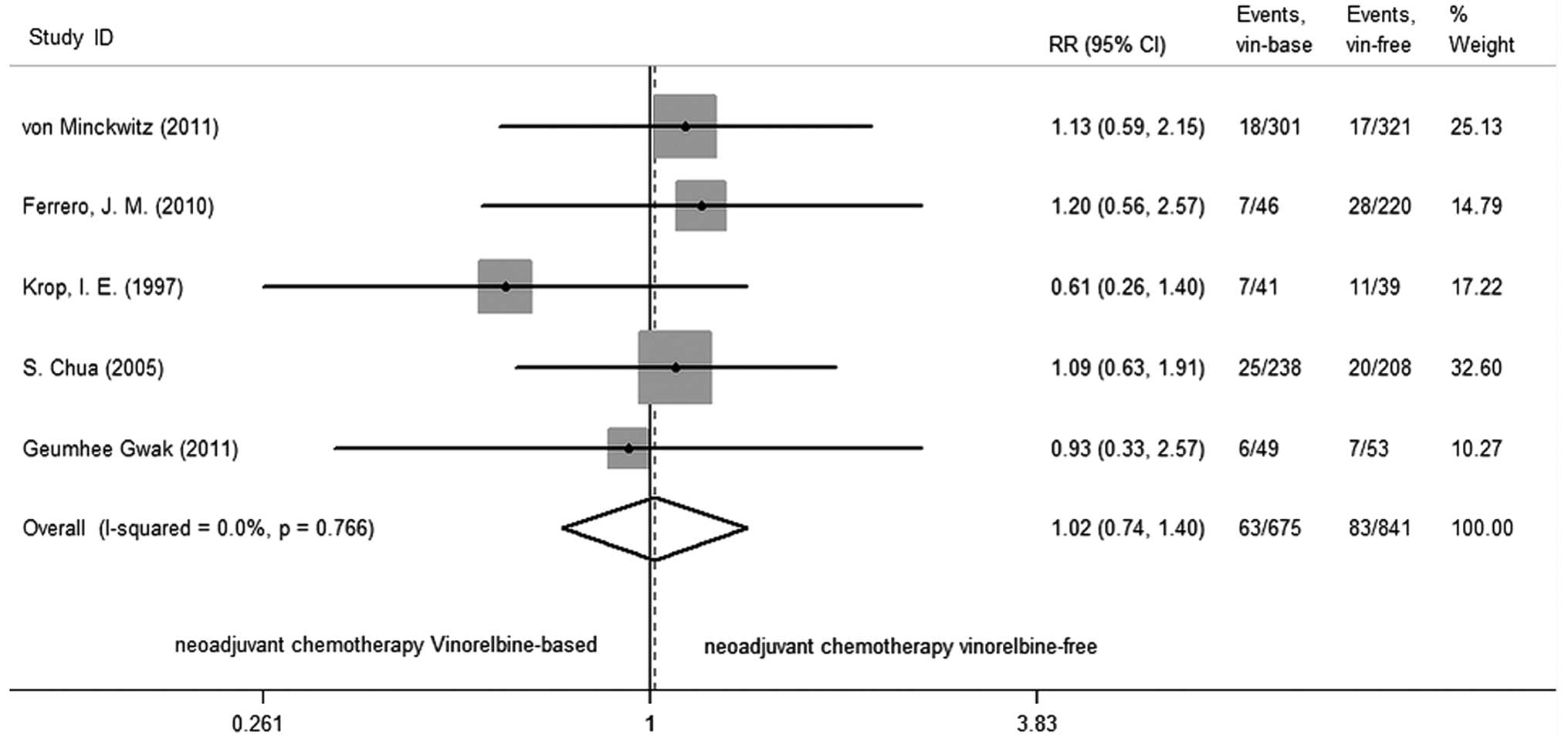
Vinorelbine-based neoadjuvant chemotherapy was not
associated with a significant improvement in pCR compared to
vinorelbine-free regimens (RR=1.016; 95% CI, 0.738–1.399; P=0.922),
There was no significant heterogeneity among studies (P=0.766,
I2=0.0%; Fig. 2).
Second endpoint
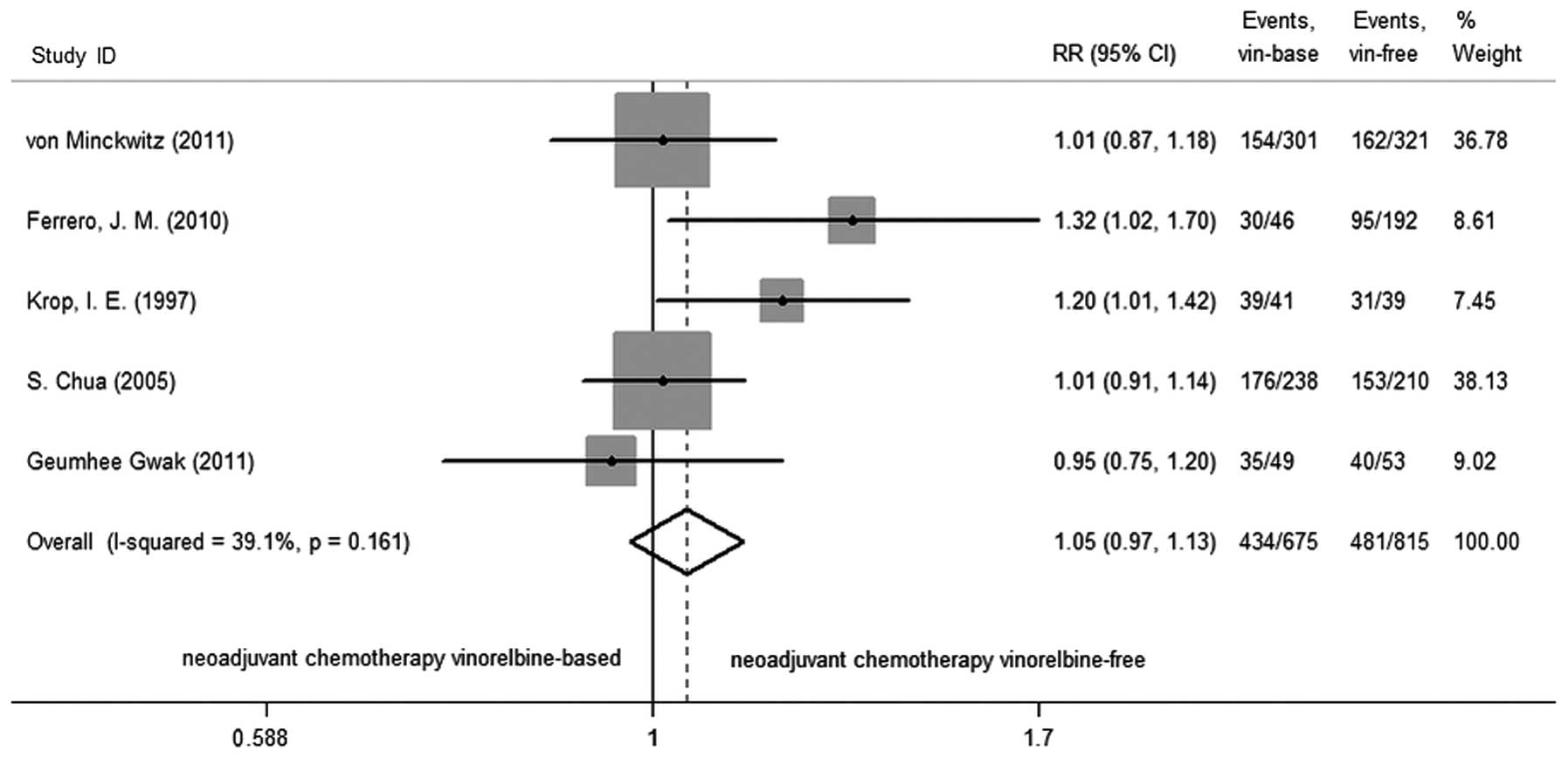
The next goal was the ORR in studies, following the
generation of a fixed-effects model. There was no change in ORR
(RR=1.048; 95% CI, 0.969–1.133; P=0.239) with vinorelbine-based
regimens compared to vinorelbine-free regimens, and the test of
heterogeneity did not exist in the studies (P=0.161,
I2=39.1%; Fig. 3).
There were three studies (9,10,13) that reported breast-conserving surgery
for 415 patients and showed that the difference in the BCS was not
statistically significant (RR=1.764; 95% CI, 0.734–4.239; P=0.205)
between vinorelbine-based and vinorelbine-free regimens with
regards to neoadjuvant therapy. The test for heterogeneity was
statistically significant (P=0.000, I2=92.6%).
Therefore, an exploratory sensitivity analysis was performed to
explore the source of the heterogeneity. Sensitivity analysis
indicated that the outcome was not robust until the study by
Ferrero et al (13) was
excluded, and the heterogeneity could be mainly due to this study.
The heterogeneity disappeared following the removal of this study,
and the result also indicated that the difference in the BCS
between the vinorelbine-based and vinorelbine-free regimens was not
statistically significant (RR=1.042; 95% CI, 0.917–1.148; P=0.526),
and the test of heterogeneity did not exist in studies (P=0.953,
I2=0.0%; Fig. 4).
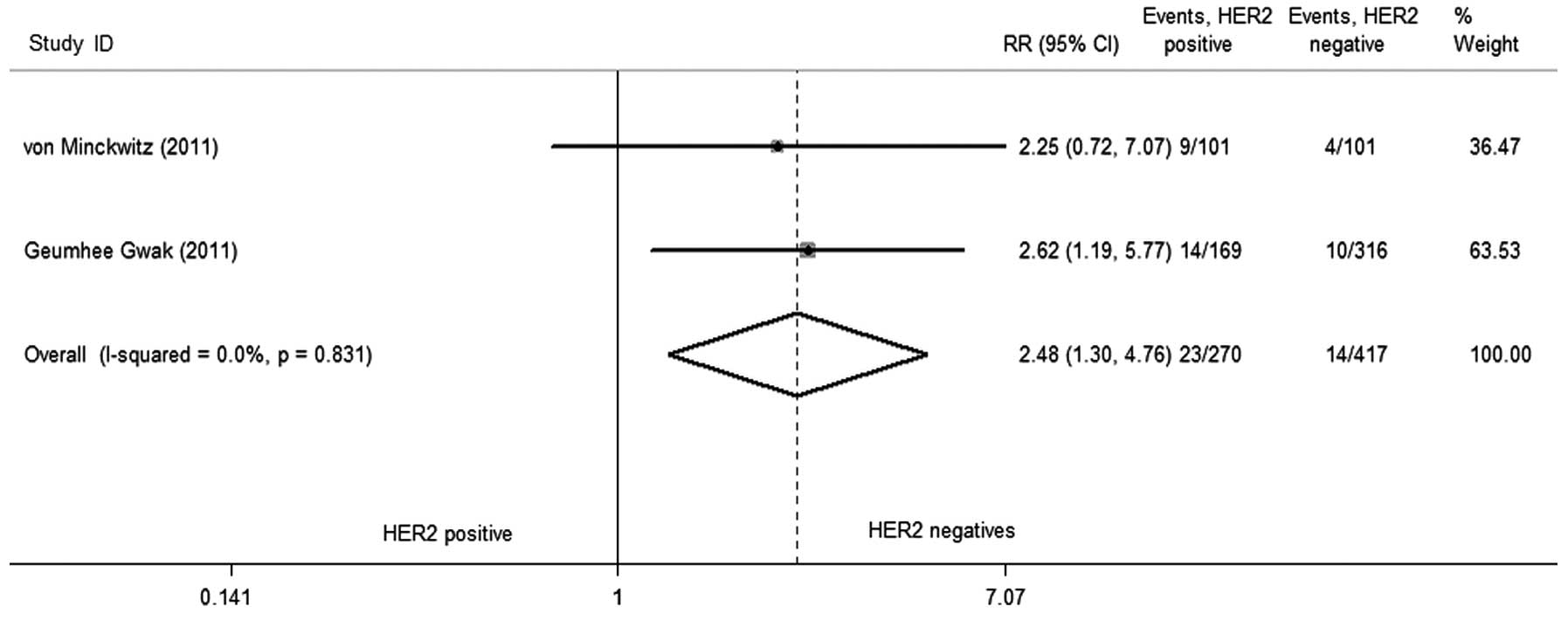
Two studies (9,10) reported
the postoperative outcomes in detail, as a hierarchical analysis,
the rate of pCR of HER2 amplified was higher compared to HER2
non-amplified (RR=2.484; 95% CI, 1.296–4.760; P=0.006; Fig. 5) in neoadjuvant chemotherapy, and the
test of heterogeneity did not exist (P=0.831, I2=0.0%).
The hormone receptor status was associated with the rate of pCR in
neoadjuvant chemotherapy. The rate of pCR of hormone
receptor-negative was significant different compared to hormone
receptor-positive (RR=0.488; 95% CI, 0.263–0.908, P=0.023; Fig. 6), and the heterogeneity was not
statistically significant (P=0.170, I2=46.9%).
Toxicity
Table II presents the
summary estimates of the vinorelbine-based and vinorelbine-free
neoadjuvant chemotherapy regimen toxicity. The results show that
treatment with vinorelbine-based regimens is associated with a
lower incidence of grade 3–4 (National Cancer Institute Common
Teminology Criteria for Adverse Events grades 3–4) alopecia (OR,
0.617; 95% CI, 0.448–0.848; P=0.003). Heterogeneity among the
studies in the analysis was not significant regarding alopecia
(P=0.378, I2=0.0%). Neutropenia (OR, 0.436; 95% CI,
0.185–1.145; P=0.058), leukopenia (OR, 0.477; 95% CI, 0.190–1.196;
P=0.114) and mucositis (OR, 0.680; 95% CI, 0.390–1.185; P=0.173)
showed no statistical significance between the two arms.
Heterogeneity among the studies in these analyses was significant,
possibly due to the use of various agents at different dosages and
the use of different control arms.
 | Table II.Summary estimate of the toxicity of
neoadjuvant chemotherapy regimens vinorelbine-based and
vinorelbine-free. |
Table II.
Summary estimate of the toxicity of
neoadjuvant chemotherapy regimens vinorelbine-based and
vinorelbine-free.
|
|
|
| Heterogeneity |
| Effect size |
|---|
|
|
|
|
|
|
|
|---|
| Adverse events | No. of studies | No. of
patients | P-value | I2
(%) | Statistical
model | OR | 95% CI | P-value |
|---|
| Mucositis | 3 | 1,172 | 0.342 |
6.9 | Fixed-effect
model | 0.680 | 0.390–1.185 | 0.173 |
| Alopecia | 2 | 1,070 | 0.378 |
0.0 | Fixed-effect
model | 0.617 | 0.448–0.848 | 0.003 |
| Leukopenia | 2 | 1,070 | 0.001 | 90.5 | Random-effect
model | 0.477 | 0.190–1.196 | 0.114 |
| Neutropenia | 3 | 1,172 | 0.000 | 92.0 | Random-effect
model | 0.436 | 0.185–1.145 | 0.058 |
Discussion
Neoadjuvant chemotherapy is one of the common
therapies for antitumor treatment, and the most common agents used
in patients with breast cancer were anthracyclines and taxane,
although uncertainty remains for which to recommend as first
choice. Vinorelbine was recommended for the treatment of recurrent
breast cancer by the NCCN guidelines, and the exploration in
neoadjuvant therapy has been in progress. Several trials reported
that vinorelbine combined with others agents caused a certain
effect (7,14–16). In
order to reassess the data that are already present in the
literature with the largest possible statistical power, we carried
out what is, to the best of our knowledge, the first meta-analysis
of the effects of including vinorelbine as part of neoadjuvant
polychemotherapy in patients with breast cancer. The findings show
no benefit from neoadjuvant therapy in patients with
vinorelbine-based compared to patients with vinorelbine-free
regimens. pCR is the most powerful predictor of neoadjuvant
chemotherapy (17,18). Patients who achieved a pCR following
neoadjuvant chemotherapy have an improved prognosis compared to
those who remain with residual disease, which shows improvements in
DFS and OS (19,20). A higher pCR rate has become one of the
indicators of neoadjuvant chemotherapy. Therefore, the first goal
of the present meta-analysis was pCR assessment. From the five
studies, we identified vinorelbine-based regimens without any
advantage in pCR (21). However, this
shows another problem, as comparing to the vinorelbine-free
regimens, vinorelbine-based regimens show no weakness in pCR in
comparison to other regimens in neoadjuvant chemotherapy.
Although the present meta-analysis did not
demonstrate an advantage of adding vinorelbine to neoadjuvant
therapy for breast cancer, certain notable results did emerge from
the included studies. von Minckwitz et al (9) and Gwak et al (10) reported four molecular outcomes in
detail following surgery. Patients with HER2-amplified or hormone
receptor-negative may have an additional benefit from neoadjuvant
therapy to achieve pCR, which suggests that patients with different
HER2 or hormone receptor status have different sensitivity for
neoadjuvant therapy, although there were poor prognostic factors
(22). The similar association
between molecularly and pCR has been reported previously. Yoo et
al (23) reported that the
triple-negative is more likely to obtain pCR when neoadjuvant
chemotherapy is administered, but there are worse survival
outcomes. Houssami et al (24)
also obtained similar results; triple-negative or
HER2+/HR− subtypes achieve higher odds of
pCR.
However, the present meta-analysis showed that the
rate of BCS was not improved following neoadjuvant therapy. Certain
strong heterogeneity was identified among the studies, and the
reasons are list as followed. First, the decision of the surgeon to
perform surgery was not only according to the result of neoadjuvant
chemotherapy, but also considering other comprehensive situations.
Second, the choice of surgery is strongly influenced by the
willingness of the patient, the level of development of the country
or region cognitive to breast cancer. Studies of different
countries illustrate different BCS. Suen et al (25) reported that 21.9% of patients with
early-stage breast cancer underwent BCS among 680 patients between
January 2001 and December 2005 in Hong Kong. Clavarezza et
al (26) reported that 34% of
patients achieved breast-conserving surgery after four 3-weekly
cycles of fluorouracil, epirubicin and cyclophosphamide followed by
12 cycles of weekly paclitaxel as neoadjuvant therapy in Italy.
Further research and feasibility studies are required to
demonstrate this.
The present analysis included five randomized
controlled trials of varying quality and had the following
limitations. First, despite the fact that no language restrictions
were applied to the literature search, only one non-English
language study was identified. It is possible that certain relevant
clinical data published in other languages may have been
overlooked. Second, one randomized controlled trial had no full
text and another one had data pooled by the author, therefore, the
heterogeneity is likely to increase. Finally, the characteristics
of the included trials were varied in patients, time and dosage.
The five trials did not use the double-blinding method. Future
studies should attempt to minimize these possible sources of
heterogeneity.
Despite the limitations of the present study, the
results strongly demonstrate that vinorelbine-based neoadjuvant
chemotherapy did not significantly improve pCR, ORR and BCS.
HER2-amplified and hormone receptor-negative patients were
significantly associated with the pathological response rate, but
not the lymph node status and tumor size.
References
|
1
|
Cleator S, Parton M and Dowsett M: The
biology of neoadjuvant chemotherapy for breast cancer. Endocr Relat
Cancer. 9:183–195. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Charfare H, Limongelli S and Purushotham
AD: Neoadjuvant chemotherapy in breast cancer. Br J Surg. 92:14–23.
2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cros S, Wright M, Morimoto M, Lataste H,
Couzinier JP and Krikorian A: Experimental antitumor activity of
Navelbine. Semin Oncol. 16 (Suppl 4):15–20. 1989.PubMed/NCBI
|
|
4
|
Goa KL and Faulds D: Vinorelbine. A review
of its pharmacological properties and clinical use in cancer
chemotherapy. Drugs Aging. 5:200–234. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang J, Xu L, Ye JM, Zhao JX, Duan XN and
Liu YH: Effects of vinorelbine plus cisplatin as second-line
neoadjuvant chemotherapy regimen in the treatment of breast cancer.
Zhonghua Yi Xue Za Zhi. 93:93–95. 2013.(In Chinese). PubMed/NCBI
|
|
6
|
Rivera-Rodriguez N, Cabanillas F,
Lawrenson L, Negron V, Pavia OA, Bruno M, Echenique MM, Carlo V,
Liboy I, et al: Results of a novel neoadjuvant chemotherapy (NAC)
regimen for breast cancer. J Clin Oncol. (abstract).
31:e116102013.
|
|
7
|
Carillio G, Aiello RA, Chiarenza M, Alì M,
Mazzola A, Marco R, Taibi E, Fallica G, Casella T, Zacchia A, et
al: Neoadjuvant trastuzumab and sequential chemotherapy with
cisplatin, vinorelbine, and docetaxel for stage II–III breast
cancer patients: Final results of a single institution phase II
study. J Clin Oncol. (abstract). 31:e115272013.
|
|
8
|
Xu L, Ye JM, Zhao JX, Duan XN and Liu YH:
Effects and toxicity of neoadjuvant chemotherapy with vinorelbine
and cisplatin in treatment of operable breast cancer previously
non-responsive to anthracyclines and taxanes-containing regimen:
Analysis of 19 cases. Zhonghua Yi Xue Za Zhi. 89:683–685. 2009.(In
Chinese). PubMed/NCBI
|
|
9
|
von Minckwitz G, Blohmer JU, Costa S,
Denkert C, Eidtmann H, Eiermann W, Gerber B, Hanusch C, Hilfrich J,
Huober J, et al: Neoadjuvant chemotherapy adapted by interim
response improves overall survival of primary breast cancer
patients - Results of the GeparTrio trial. Cancer Res. 71:S3–S2.
2011. View Article : Google Scholar
|
|
10
|
Gwak G, Kim JY, Park K, Shin YJ, Cho H,
Park SJ, Yang GH, Bae BN, Kim KW and Han S: Comparison of
doxorubicin plus docetaxel neoadjuvant chemotherapy with
doxorubicin plus vinorelbine in primary breast cancer. J Breast
Cancer. 14:129–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Krop IE, Flores L, Tuck DP, Ryan PD,
Partridge AH, Morganstern D, Najita J, Lezon-Geyda K, Winer EP and
Harris L: Phase II trial of preoperative vinorelbine/trastuzumab
(VH) or docetaxel/carboplatin/trastuzumab (TCH) in HER2-positive
breast cancer with analysis of resistance mechanisms. J Clin Oncol.
28:15S2010.
|
|
12
|
Chua S, Smith IE, Ahern RP, Coombes GA,
Hickish TF, Robinson AC, Laing RW, OBrien ME, Ebbs SR, Hong A, et
al TOPIC Trial Group: Neoadjuvant vinorelbine/epirubicin (VE)
versus standard adriamycin/cyclophosphamide (AC) in operable breast
cancer: Analysis of response and tolerability in a randomised phase
III trial (TOPIC 2). Ann Oncol. 16:1435–1441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ferrero JM, Namer M, Dufour JF, Largillier
R, Creisson A, Teissier E, Machiavello JC, Lallement M, Monticelli
J and Abbes M: Neoadjuvant chemotherapy of locally advanced breast
cancer: Historical comparison of 4 sequential combinations. Bull
Cancer. 84:10–16. 1997.PubMed/NCBI
|
|
14
|
Halim A and Wahba H: Second-line
neoadjuvant vinorelbine and gemcitabine combination in locally
advanced breast cancer showing no early response to TAC. Med Oncol.
29:454–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
O'Regan RM, Gabram S, Styblo T, Rizzo M,
Wood W, Srinivasiah J, Jonas W, Schnell F, Adams A, Nahta R, et al:
Final results of a phase 2 trial using a novel, non-anthracycline
neoadjuvant chemotherapy regimen in Her2-positive breast cancer.
Cancer Res. 72:27032012. View Article : Google Scholar
|
|
16
|
Medioni J, Huchon C, Le Frere-Belda MA,
Hofmann H, Bats AS, Eme D, Andrieu JM, Oudard S, Lecuru F and Levy
E: Neoadjuvant dose-dense gemcitabine plus docetaxel and
vinorelbine plus epirubicin for operable breast cancer: Improved
prognosis in triple-negative tumors. Drugs R D. 11:147–157. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bear HD, Anderson S, Smith RE, Geyer CE
Jr, Mamounas EP, Fisher B, Brown AM, Robidoux A, Margolese R,
Kahlenberg MS, et al: Sequential preoperative or postoperative
docetaxel added to preoperative doxorubicin plus cyclophosphamide
for operable breast cancer: National Surgical Adjuvant Breast and
Bowel Project Protocol B-27. J Clin Oncol. 24:2019–2027. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wolmark N, Wang J, Mamounas E, Bryant J
and Fisher B: Preoperative chemotherapy in patients with operable
breast cancer: nine-year results from National Surgical Adjuvant
Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 96–102.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chollet P, Amat S, Cure H, de Latour M, Le
Bouedec G, Mouret-Reynier MA, Ferriere JP, Achard JL, Dauplat J and
Penault-Llorca F: Prognostic significance of a complete
pathological response after induction chemotherapy in operable
breast cancer. Br J Cancer. 86:1041–1046. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Montagna E, Bagnardi V, Rotmensz N, Viale
G, Pruneri G, Veronesi P, Cancello G, Balduzzi A, Dellapasqua S,
Cardillo A, et al: Pathological complete response after
preoperative systemic therapy and outcome: Relevance of clinical
and biologic baseline features. Breast Cancer Res Treat.
124:689–699. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
von Minckwitz G, Kümmel S, Vogel P,
Hanusch C, Eidtmann H, Hilfrich J, Gerber B, Huober J, Costa SD,
Jackisch C, et al German Breast Group: Neoadjuvant
vinorelbine-capecitabine versus
docetaxel-doxorubicin-cyclophosphamide in early nonresponsive
breast cancer: Phase III randomized GeparTrio trial. J Natl Cancer
Inst. 100:542–551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kurebayashi J, Kanomata N, Yamashita T,
Shimo T, Mizutoh A, Moriya T and Sonoo H: Prognostic value of
phosphorylated HER2 in HER2-positive breast cancer patients treated
with adjuvant trastuzumab. Breast Cancer. Jun 8–2013.(Epub ahead of
print). PubMed/NCBI
|
|
23
|
Yoo C, Ahn JH, Jung KH, Kim SB, Kim HH,
Shin HJ, Ahn SH, Son BH and Gong G: Impact of
immunohistochemistry-based molecular subtype on chemosensitivity
and survival in patients with breast cancer following neoadjuvant
chemotherapy. J Breast Cancer. 15:203–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Houssami N, Macaskill P, von Minckwitz G,
Marinovich ML and Mamounas E: Meta-analysis of the association of
breast cancer subtype and pathologic complete response to
neoadjuvant chemotherapy. Eur J Cancer. 48:3342–3354. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suen D, Chow L and Kwong A:
Breast-conserving surgery in Hong Kong Chinese women. World J Surg.
32:2549–2553. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Clavarezza M, Turazza M, Aitini E,
Saracchini S, Garrone O, Durando A, De Placido S, Bisagni G,
Levaggi A, Bighin C, et al: Phase II open-label study of
bevacizumab combined with neoadjuvant anthracycline and taxane
therapy for locally advanced breast cancer. Breast. 22:470–475.
2013. View Article : Google Scholar : PubMed/NCBI
|