Introduction
Chemotherapy with molecular-targeted drugs improves
the survival rate of patients with unresectable advanced colorectal
cancer (CRC). Although chemotherapy is effective for a subset of
patients with CRC, the incidence of adverse events (AEs) often
affects treatment continuity. Regorafenib (1), which is an oral multikinase inhibitor,
is commonly used as third- or fourth-line chemotherapy in
refractory CRC patients (2). Several
clinical trials (2–4) have demonstrated the efficacy of
regorafenib for refractory CRC patients. The most frequent
regorafenib-related AEs of grade ≥3 in the CORRECT trial were
hand-foot skin reaction, fatigue, diarrhea, hypertension and rash
or desquamation (2). Regarding the
incidence of regorafenib-related hepatotoxicity, including
hyperbilirubinaemia and elevation of aspartate aminotransferase
(AST) and alanine aminotransferase (ALT) levels, it was relatively
low in a non-Japanese population, while it was high in a Japanese
population (5). These results were
supported with the CONCUR trial (3),
which was performed by institutions in Asia.
The present study reports the case of a CRC patient
with multiple liver metastases, who developed liver atrophy and
massive ascites following regorafenib treatment. To the best of our
knowledge, this is the first report of a rare AE induced by
regorafenib treatment.
Case report
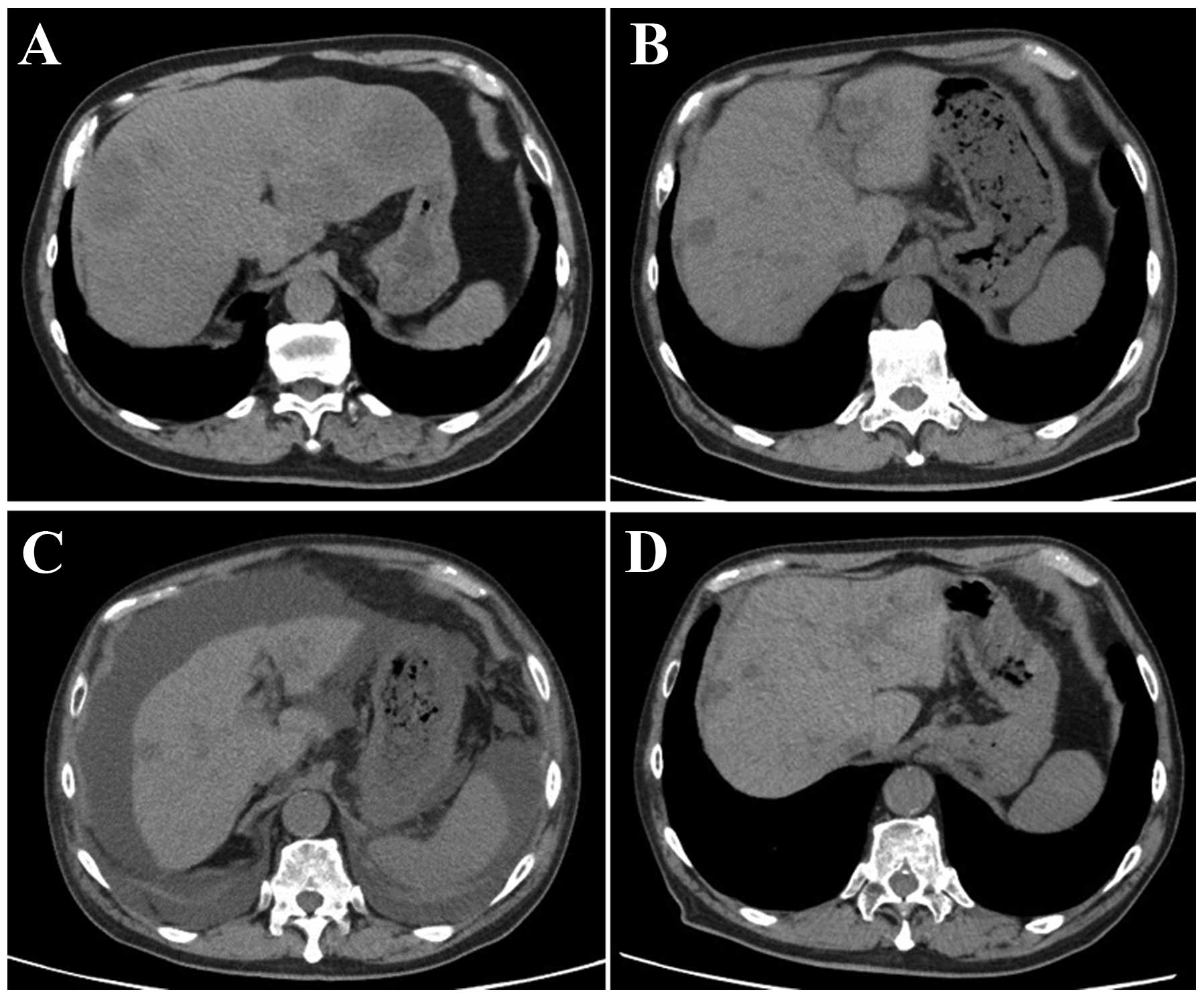
A 70-year-old man, who was diagnosed with advanced
rectal cancer with multiple liver metastases (Fig. 1A), received chemotherapy without
surgical treatment from November, 2013 onwards. First, the patient
was treated with XELOX + bevacizumab (2,000 mg/day capecitabine on
days 1–14 and 150 mg oxaliplatin on day 1+150 mg bevacizumab on day
1) for three cycles. The regimen was changed to SOX + bevacizumab
(100 mg/day S-1 on days 1–14, 150 mg oxaliplatin on day 1+150 mg
bevacizumab on day 1) due to severe AEs, including hand-foot
syndrome. Ten cycles of the SOX treatment were administered over 10
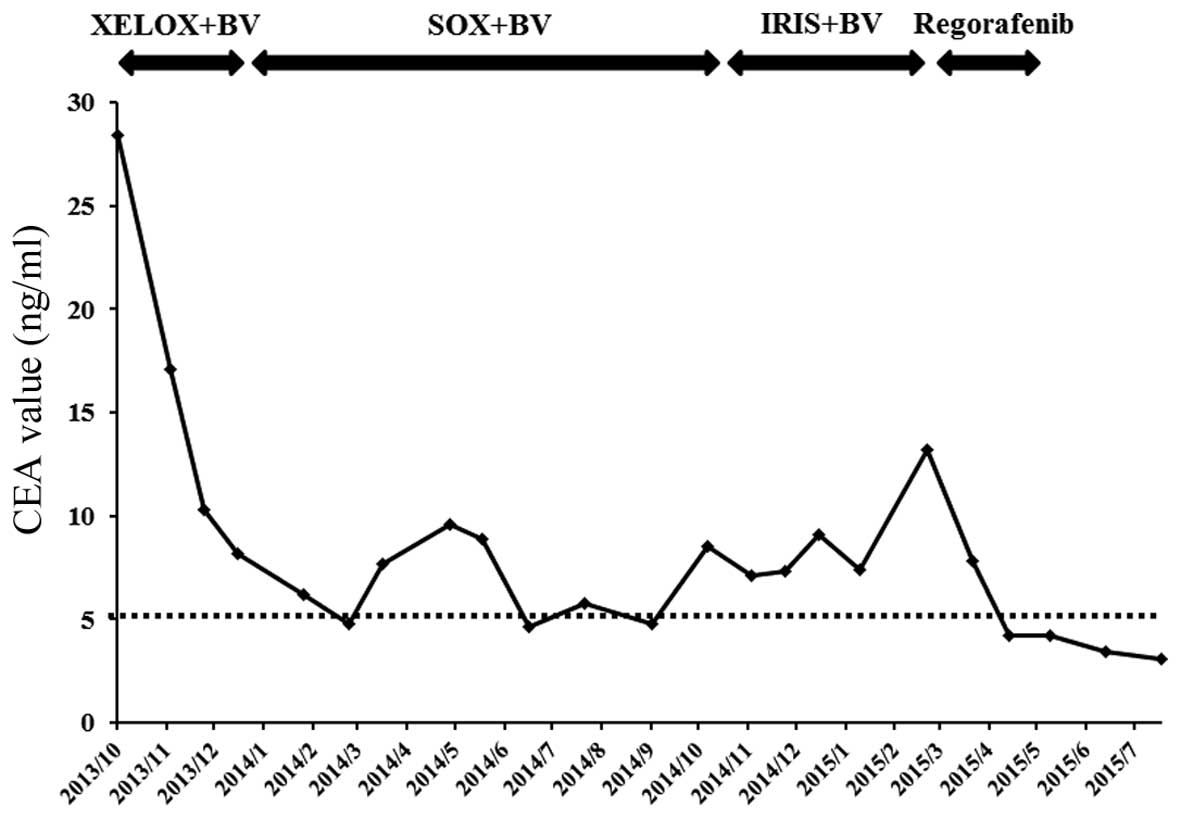
months, achieving stable disease, until the carcinoembryonic
antigen (CEA) level was elevated to 8.5 ng/ml (normal range,
<5.0 ng/ml) (Fig. 2). IRIS +
bevacizumab (100 mg/day S-1 on days 1–14, 150 mg irinotecan on day
1+150 mg bevacizumab on day 1) treatment was subsequently
administered. However, the IRIS + bevacizumab treatment was not
effective for the patient, reflected by the increase in the CEA
level to 13.2 ng/ml (Fig. 2). The
primary rectal cancer almost disappeared during chemotherapy. The
RAS status could not be examined. Regorafenib (160 mg/day for 3
weeks) was then administered as third-line chemotherapy. Following
completion of one course, the patient complained of abdominal
distension. Although no abnormal findings were observed on computed
tomography (CT) examination prior to the regorafenib treatment
(Fig. 1B), liver atrophy and massive
ascites were identified (Fig. 1C).
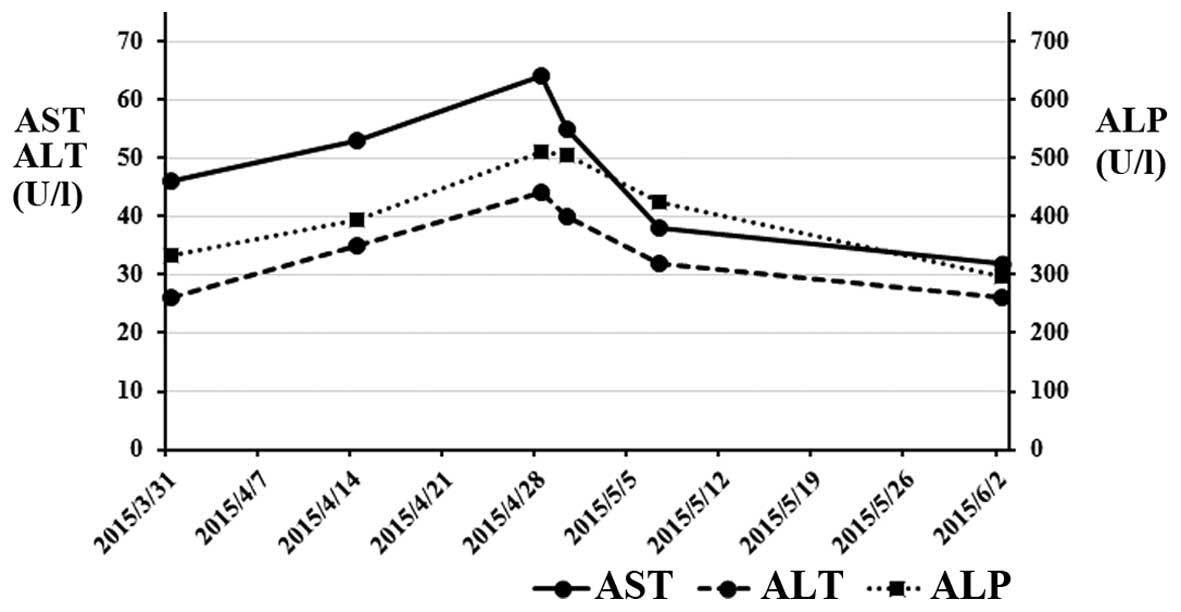
The blood tests after regorafenib treatment revealed elevated
levels of AST, ALT and alkaline phosphatase (ALP) to 55 IU/l
(normal range, 12–32 IU/l), 40 IU/l (normal range, 6–30 IU/l) and
505 IU/l (normal range, 109–335 IU/l), respectively (Table I). For the evaluation of liver
function, 99mTc-galactosyl human serum albumin
scintigraphy, which reflects the number and function of
hepatocytes, was performed. The uptake ratio of the liver to the
liver plus heart at 15 min was 0.77. The estimated value of
indocyanine green clearance was 21%. Endoscopic examination of the
esophagus revealed mild esophageal varices without red color sign.
The patient was admitted to the Aizu Medical Center (Aizuwakamatsu,
Japan) and ~2,000 ml of ascitic fluid were aspirated daily for 1
week by abdominal puncture. The patient was administered oral
diuretics, including 20 mg/day of furosemide and 25 mg/day of
spironolactone. Albumin was administered to correct the albumin
deficit. The levels of AST, ALT and ALP decreased from the peak
values observed on admission (Fig.
3). The patient was discharged from the hospital 16 days after
the treatment. The CT examination after 1 month revealed that the
volume of the liver had recovered and the ascites had disappeared
(Fig. 1D). Furthermore, almost all
the liver metastases were reduced in size. The CEA level, which was
elevated prior to regorafenib treatment, was reduced to normal
(Fig. 2).
 | Table I.Blood test results following
regorafenib treatment. |
Table I.
Blood test results following
regorafenib treatment.
| Parameters | Values |
|---|
| WBC count | 4,320/µl |
| RBC count |
4.01×104/µl |
| Hemoglobin | 12.4 g/dl |
| Hematocrit | 39.4% |
| PLT count |
6.2×103/ml |
| Total protein | 5.0 g/dl |
| Albumin | 1.8 g/dl |
| AST | 55 IU/l |
| ALT | 40 IU/l |
| LDH | 328 IU/l |
| ALP | 505 IU/l |
| γ-GTP | 80 IU/l |
| Total bilirubin | 0.6 mg/dl |
| BUN | 27.4 mg/dl |
| Creatinine | 1.29 mg/dl |
| Na | 134 mEq/l |
| K | 4.7 mEq/l |
| Cl | 106 mEq/l |
| HPT | 91.6% |
| PT | 100 % |
| PTINR | 1.0 |
| APTT | 31.5 sec |
| Fibrinogen | 209.3 mg/dl |
| ATIII | 69% |
| FDP | 10.9 µg/ml |
| D-dimer | 5.8 µg/ml |
| Ammonia | 65 µg/dl |
Discussion
The present study reported the case of a CRC patient
with liver atrophy and massive ascites induced by regorafenib
treatment. The patient had received oxaliplatin-based and
irinotecan-based chemotherapy prior to regorafenib. Although
accumulation of these chemotherapeutic agents may be associated
with liver dysfunction, liver atrophy was observed following
administration of regorafenib alone and the size of the liver was
restored following discontinuation of regorafenib treatment.
Therefore, regorafenib was considered to be the cause of liver
atrophy and massive ascites in the present case. To the best of our
knowledge, this is the first report of atrophic changes of the
liver and massive ascites caused by regorafenib treatment in a CRC
patient with multiple liver metastases. Recently, chemotherapeutic
agents, including molecular-targeted drugs, antivascular
endothelial growth factor antibodies, anti-epidermal growth factor
receptor antibodies and multikinase inhibitors, have been
administered to refractory CRC patients and have improved their
survival rate. However, careful observation for AEs is required
during chemotherapeutic treatment. The most frequent
regorafenib-related AEs of grade ≥3 in the CORRECT trial were
hand-foot skin reaction, fatigue, diarrhea, hypertension and rash
or desquamation (2). Drug-induced
hepatotoxicity is a common AE, although the frequency and the type
of drug-induced liver damage differ depending on the drugs
administered. Regarding hepatotoxicity caused by regorafenib, the
incidence of all-grade hepatotoxicity, including
hyperbilirubinaemia (8%), AST elevation (2%) and ALT elevation
(<1%), was relatively low in a non-Japanese population, while
the incidence of hyperbilirubinaemia (15%), AST elevation (19%) and
ALT elevation (12%) was high in a Japanese population (5). These results were supported by the
CONCUR trial (3), a clinical trial
on non-Japanese Asian patients. The incidence of
hyperbilirubinemia, AST elevation and ALT elevation was 48.5, 31.6
and 31.6%, respectively. In the present case, the blood tests
associated with liver function revealed an increase and a peak at 4
weeks after the initiation of regorafenib treatment, which
corresponded to grade 1 hepatotoxicity, according to the Common
Terminology Criteria for Adverse Events, version 3.0 (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf).
Other AEs, including hand-foot skin reaction, fatigue, diarrhea,
hypertension and rash or desquamation, were not observed at that
time. Therefore, it would be difficult to detect liver damage only
from the results of the blood tests if the patient had not
complained of abdominal distension.
In general, drug-induced liver injury (DILI) is
classified into intrinsic and idiosyncratic liver injury. The
former is induced by drug toxicity in a dose-dependent manner,
whereas the latter is further divided into allergic and metabolic
idiosyncratic liver injuries, which are associated with host
factors, including immune response and metabolism associated with
genetic polymorphisms. The majority of DILIs are idiosyncratic. The
area under the curve of regorafenib treatment varied for intake of
the same dose, depending on the individual. Regorafenib was
metabolized to M-2 and M-5, which act through CYP3A4 (6). UGT1A9 is associated with the
inactivation of these products through glucuronate conjugation
(6). Therefore, the UGT1A9 gene
polymorphism may be associated with the toxicity of regorafenib.
Sorafenib is also a multikinase inhibitor, which has a similar
structure to regorafenib (7).
Sorafenib is also metabolized by CYP3A4-mediated oxidation and
UGT1A9-mediated glucuronidation. Ye et al (8) reported that sorafenib metabolism was
significantly altered in the liver tumor tissue of a hepatocellular
carcinoma patient, due to an evident decrease of the expression
level of CYP3A4 and UGT1A9. Boudou-Rouquette et al (9) reported that the UGT1A9 polymorphism
(rs17868320) was significantly associated with grade >2 diarrhea
in patients treated with sorafenib. Although there were no data
confirming that the patient had the UGT1A9 polymorphism, it was
hypothesized that these genetic polymorphisms confer a clinical
benefit but induce severe toxicity in the patient.
Similar changes on imaging were previously reported
in breast cancer with multiple liver metastases (10,11) and
other cancers with multiple liver metastases, including pancreatic
(12), esophageal (13) and thyroid cancer (14). The morphological changes of the liver
in these cases are referred to as ‘pseudocirrhosis’.
Pseudocirrhosis is a radiological term that indicates a shape
similar to that of macronodular cirrhosis in the absence of the
typical histopathological findings of cirrhosis, and it occurs in
cancer patients with multiple liver metastases during chemotherapy.
The progression and regression of liver metastases may cause
pseudocirrhosis. Although the precise mechanism underlying the
development of pseudocirrhosis remains unknown, the response to
systemic chemotherapeutic agents may induce cirrhotic changes with
tumor shrinkage following chemotherapy. Previous reports (10–12) have
indicated that pseudocirrhosis may be associated with nodular
regenerative hyperplasia (NRH) caused by chemotherapy-induced
hepatic injury. NRH is characterized by widespread transformation
of normal liver parenchyma into hyperplastic regenerative nodules
without bridging fibrosis, a characteristic that distinguishes this
entity from liver cirrhosis (15).
This injury is characteristically asymptomatic in its early phases,
with only mild elevations in transaminase levels (16). Oxaliplatin is a well-known causative
drug for the development of NRH (17). Previous studies on breast cancer
reported that multiple liver metastases worsened when atrophic
changes occurred in the liver (10,11).
Kang et al (12) reported a
reversible change in a pancreatic cancer patient with multiple
liver metastases, who received 8 cycles of chemotherapy with
gemcitabine and 5-fluorouracil. When liver atrophy was observed
during treatment, the values of the liver enzymes were elevated,
while the level of carbohydrate antigen 19-9 was markedly
decreased. This was also the case in our patient, although the
chemotherapeutic agents were different. In the present case, the
volume of the liver was markedly decreased, with a mild elevation
of the aminotransferase levels. Following withdrawal of
regorafenib, the volume of the liver was almost completely restored
to that prior to the initiation of regorafenib treatment.
Furthermore, the CEA level returned to normal following treatment
with regorafenib. The majority of cancer patients with
pseudocirrhosis have a poor prognosis due to progression of liver
metastases. However, effective treatment may also induce cirrhotic
changes due to the potent antitumor effect.
Symptomatic treatment was administered for the liver
dysfunction, in addition to withdrawal of regorafenib, according to
the treatment for cirrhosis with ascites. In the present case, the
patient survived after severe regorafenib-induced hepatic injury.
The course of treatment in the present as well as another case
(12) suggests that pseudocirrhosis
with a mild elevation of aminotransferase levels during
chemotherapy may be reversed by discontinuation of the
chemotherapeutic agents. In such cases, where the chemotherapeutic
agents administered are potent enough to induce liver injury and
reversible pseudocirrhosis, potent antitumor effects may also be
expected. This study presents an extremely rare case of recovery
from pseudocirrhosis; however, the majority of cancer patients with
pseudocirrhosis induced by chemotherapeutic agents eventually
succumb due to cancer progression. Clinicians should be aware that
regorafenib may induce atrophic changes of the liver and generate
massive ascites in CRC patients with multiple liver metastases.
References
|
1
|
Wilhelm SM, Dumas J, Adnane L, Lynch M,
Carter CA, Schütz G, Thierauch KH and Zopf D: Regorafenib (BAY
73–4506): A new oral multikinase inhibitor of angiogenic, stromal
and oncogenic receptor tyrosine kinases with potent preclinical
antitumor activity. Int J Cancer. 129:245–255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grothey A, Van Cutsem E, Sobrero A, Siena
S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, et
al: CORRECT Study Group: Regorafenib monotherapy for previously
treated metastatic colorectal cancer (CORRECT): An international,
multicentre, randomised, placebo-controlled, phase 3 trial. Lancet.
381:303–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li J, Qin S, Xu R, Yau TC, Ma B, Pan H, Xu
J, Bai Y, Chi Y, Wang L, et al: CONCUR Investigators: Regorafenib
plus best supportive care versus placebo plus best supportive care
in Asian patients with previously treated metastatic colorectal
cancer (CONCUR): A randomised, double-blind, placebo-controlled,
phase 3 trial. Lancet Oncol. 16:619–629. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim ST, Kim TW, Kim KP, Kim TY, Han SW,
Lee JY, Lim SH, Lee MY, Kim H and Park YS: Regorafenib as Salvage
Treatment in Korean Patients with Refractory Metastatic Colorectal
Cancer. Cancer Res Treat. 47:790–795. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoshino T, Komatsu Y, Yamada Y, Yamazaki
K, Tsuji A, Ura T, Grothey A, Van Cutsem E, Wagner A, Cihon F, et
al: Randomized phase III trial of regorafenib in metastatic
colorectal cancer: Analysis of the CORRECT Japanese and
non-Japanese subpopulations. Invest New Drugs. 33:740–750. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rey JB, Launay-Vacher V and Tournigand C:
Regorafenib as a single-agent in the treatment of patients with
gastrointestinal tumors: An overview for pharmacists. Target Oncol.
10:199–213. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Carr BI, Cavallini A, Lippolis C,
D'Alessandro R, Messa C, Refolo MG and Tafaro A: Fluoro-Sorafenib
(Regorafenib) effects on hepatoma cells: Growth inhibition,
quiescence, and recovery. J Cell Physiol. 228:292–297. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye L, Yang X, Guo E, Chen W, Lu L, Wang Y,
Peng X, Yan T, Zhou F and Liu Z: Sorafenib metabolism is
significantly altered in the liver tumor tissue of hepatocellular
carcinoma patient. PLoS One. 9:e966642014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boudou-Rouquette P, Narjoz C, Golmard JL,
Thomas-Schoemann A, Mir O, Taieb F, Durand JP, Coriat R, Dauphin A,
Vidal M, et al: Early sorafenib-induced toxicity is associated with
drug exposure and UGTIA9 genetic polymorphism in patients with
solid tumors: A preliminary study. PLoS One. 7:e428752012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee SL, Chang ED, Na SJ, Kim JS, An HJ, Ko
YH and Won HS: Pseudocirrhosis of breast cancer metastases to the
liver treated by chemotherapy. Cancer Res Treat. 46:98–103. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeong WK, Choi SY and Kim J:
Pseudocirrhosis as a complication after chemotherapy for hepatic
metastasis from breast cancer. Clin Mol Hepatol. 19:190–194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang SP, Taddei T, McLennan B and Lacy J:
Pseudocirrhosis in a pancreatic cancer patient with liver
metastases: A case report of complete resolution of pseudocirrhosis
with an early recognition and management. World J Gastroenterol.
14:1622–1624. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobashigawa C, Nakamoto M, Hokama A,
Hirata T, Kinjo F and Fujita J: Pseudocirrhosis in metastatic
esophageal cancer. South Med J. 103:488–489. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harry BL, Smith ML, Burton JR Jr, Dasari
A, Eckhardt SG and Diamond JR: Medullary thyroid cancer and
pseudocirrhosis: Case report and literature review. Curr Oncol.
19:e36–e41. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wanless IR: Micronodular transformation
(nodular regenerative hyperplasia) of the liver: A report of 64
cases among 2,500 autopsies and a new classification of benign
hepatocellular nodules. Hepatology. 11:787–797. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ghabril M and Vuppalanchi R: Drug-induced
nodular regenerative hyperplasia. Semin Liver Dis. 34:240–245.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Viganò L, Rubbia-Brandt L, De Rosa G,
Majno P, Langella S, Toso C, Mentha G and Capussotti L: Nodular
Regenerative Hyperplasia in Patients Undergoing Liver Resection for
Colorectal Metastases After Chemotherapy: Risk Factors,
Preoperative Assessment and Clinical Impact. Ann Surg Oncol.
22:4149–4157. 2015. View Article : Google Scholar : PubMed/NCBI
|