Introduction
Several cases of unexplained thromboembolic events
occurring in patients with malignant tumors have been reported.
These events were first described by Trousseau in 1865 (1) and named Trousseau's syndrome (TS). TS is
typically associated with mucin-producing tumors, such as
pancreatic, gastric or pulmonary carcinoma (2,3).
TS was considered to be infrequently associated with
ovarian cancer, representing 3.8% of all malignant diseases, until
1977 (2). Planner et al then
suggested that thromboembolic events may be more common in ovarian
cancer patients than previously reported, occurring in 44% of 59
patients with ovarian cancer and coagulation abnormalities
(4).
Recently, an increasing number of patients with
ovarian cancer have been reported to experience TS, providing an
opportunity to expand our knowledge regarding the treatment of this
syndrome. Thromboembolism in the brain is very difficult to treat
in several types of TS, and there are currently no specific
treatment guidelines.
We herein present a case series of 5 patients with
ovarian or endometrial cancer who experienced cerebral infarction
due to TS. We investigated biomarkers for early detection of TS and
evaluated treatment strategies for these patients. We may predict
acute thromboembolism by measuring D-dimer levels, which were found
to be increased in this disease, and we observed that the patients'
condition could be controlled by treatment of the primary malignant
disease. Of the 5 cases presented in this study, 1 case has been
previously published as a case report (5).
Patients and methods
Patients
From October, 2005 to September, 2014, 485 patients
suffering from endometrial, ovarian, or cervical cancer were
treated at the Shimane University Hospital (Izumo, Japan). Among
these patients, 5 patients experienced cerebral infarction
associated with TS. The treatment outcomes of patients with TS were
analyzed by reviewing their medical backgrounds, medical records
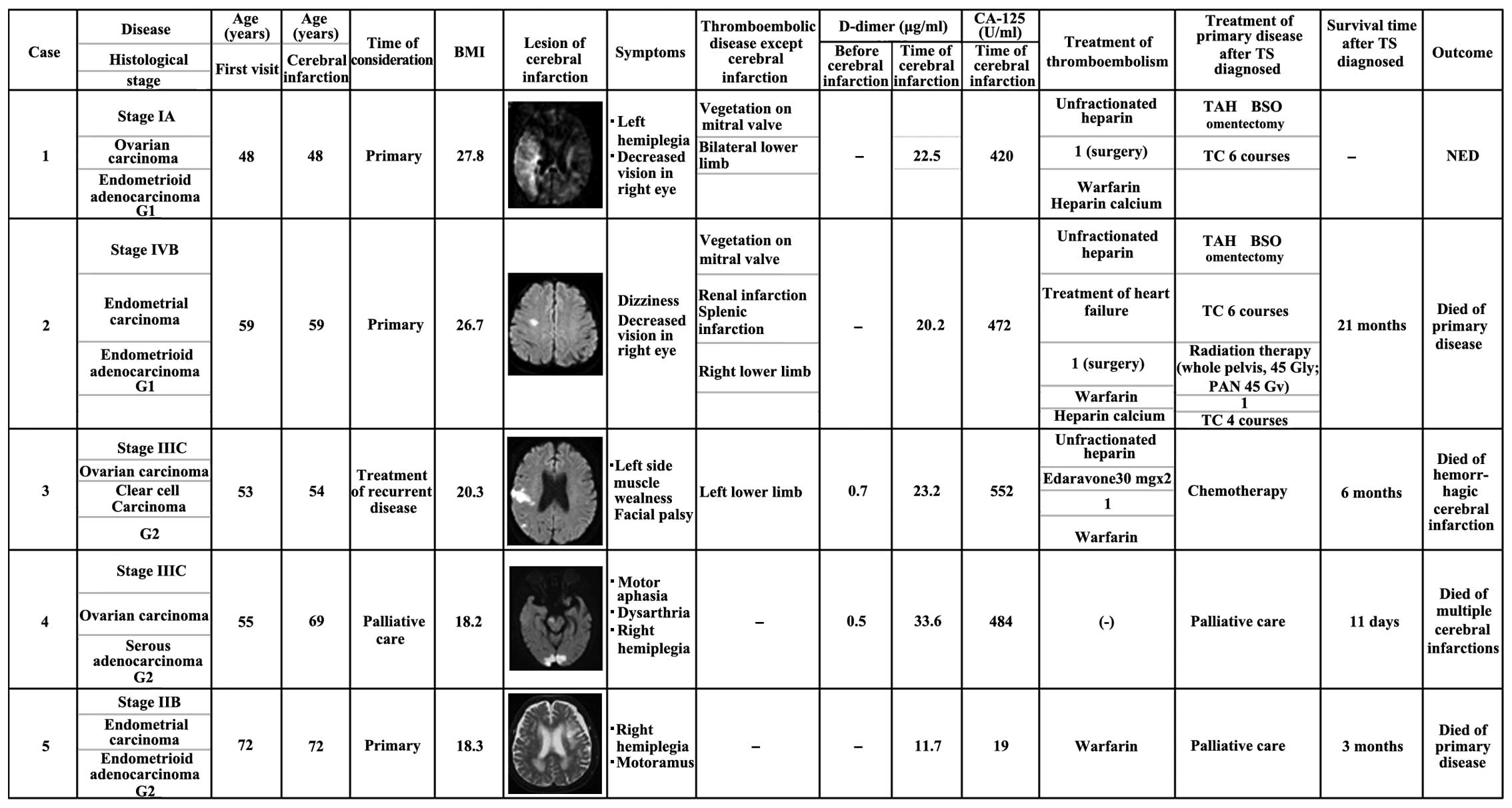
and surgical reports (Fig. 1).
Patient background
The frequency of TS among patients with
gynecological malignant disease at our hospital is shown in
Table I. Overall, 2 of 139 patients
(1.43%) with endometrial cancer and 3 of 244 patients (2.08%) with
ovarian cancer experienced TS. No cervical cancer patients at our
hospital experienced TS. The patient backgrounds are summarized in
Table I. The average age of the
patients was 57.4 years (range, 48–72 years). The average body mass
index was 22.2 kg/m2 (range, 18.2–27.8
kg/m2). Tumor histology included endometrial cancer
(n=2; both endometrioid) and ovarian cancer (n=3; 1 endometrioid, 1
serous and 1 clear cell). There was no history of any condition
commonly associated with thrombosis, such as uncontrollable
diabetes mellitus or hyperlipidemia.
 | Table I.Patients with Trousseau's
syndrome. |
Table I.
Patients with Trousseau's
syndrome.
| Primary disease | Cases, n/total
(%) |
|---|
| Cervical cancer | 0/202 |
| Endometrial
cancer | 2/139 (1.43) |
| Ovarian cancer | 3/144 (2.08) |
Findings at diagnosis
The findings at diagnosis are summarized in Table II. All 5 patients (100.0%) had
neurological symptoms, including hemiplegia (n=3), facial palsy
(n=2), decreased vision (n=2), dizziness (n=1), muscle weakness
(n=1) and dysarthria (n=1). Several cerebral infarction lesions
were observed in the right hemicerebrum, right parietal lobe, left
frontal lobe, right postcentral gyrus, bilateral posterior lobe,
cerebellum and brain stem. Of the 5 patients, 3 (60%) had other
types of thromboembolism; among those cases, the thrombosis
involved a lower limb in all patients (3/3, 100.0%). Other
thrombosis sites included the pulmonary artery (n=1) and the renal
and splenic arteries (n=1). Mitral valve vegetations were observed
in 2 cases.
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
| Characteristics | No. |
|---|
| Age at diagnosis,
years [median (range)] | 57.4 (48–72) |
| Disease |
|
| Ovarian
cancer | 3 |
|
Endometrioid cancer | 2 |
| Tumor histology |
|
|
Endometrial cancer,
endometrioid | 2 |
| Ovarian
cancer, endometrioid | 1 |
| Ovarian
cancer, clear cell | 1 |
| Ovarian
cancer, serous | 1 |
| Body mass index,
kg/m2 [median (range)] | 22.2 (18.2–27.8) |
Patients with TS exhibited high serum carbohydrate
antigen (CA)-125 levels on laboratory examination. The average
serum CA-125 level when the infarctions occurred was 390 U/ml
(range, 19–552 U/ml). Moreover, all patients (5/5, 100.0%)
presented with significantly elevated D-dimer levels. The average
D-dimer level was 41 µg/ml (range, 11.4–108.3 µg/ml). Of note,
these two markers fluctuated in parallel with tumor activity and
infarctions occurred when the two markers were elevated
simultaneously.
Results
Thromboembolism treatment results
The treatment results are summarized in Table II. The majority of the patients had
received anticoagulation treatment with unfractionated heparin by
continuous intravenous drip, and warfarin. This resulted in a good
course for 1 patient with recurrent ovarian cancer (case 3), who
recovered and was able to resume chemotherapy. However, 3 months
later, the patient developed hemorrhagic cerebral infarction,
despite an international normalized ratio (INR) of 2.8 (Table II). The patient was unable to receive
further active treatment for her recurrent disease and she
succumbed 9 days after the second hemorrhagic cerebral infarction
(6 months after the first acute cerebral infarction).
The remaining 2 patients (cases 1 and 2) initially
received this treatment, but 1 patient (case 2) developed recurrent
aseptic vegetations on the mitral valve, whereas cerebral, renal
and splenic embolisms recurred during anticoagulation treatment.
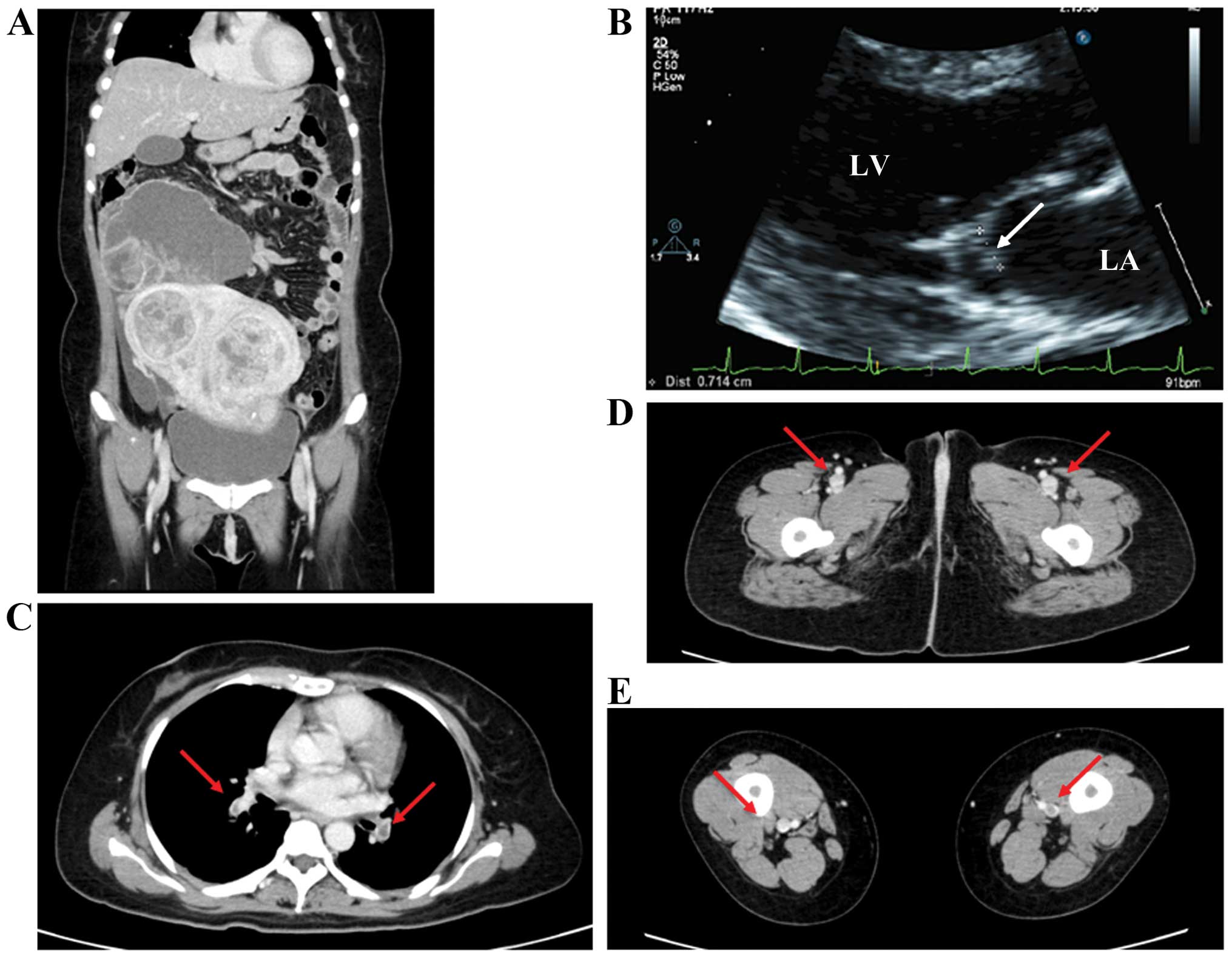
One patient (case 1) also experienced worsening of a pulmonary
embolism (Fig. 2). We therefore
decided to treat their primary disease through tumor reduction to
provide relief and improve the quality of life. The patients
underwent tumor resection, which resulted in disappearance of the
thromboembolism. After surgery, anticoagulation therapy was resumed
with low-molecular-weight heparin by continuous intravenous drip
and subcutaneous injection. The mitral vegetations disappeared and
the other emboli almost disappeared.
Another patient with recurrent ovarian cancer (case
4) was unable to receive anticoagulation therapy, as she was
already diagnosed with therapy-related myelodysplastic syndrome
(WHO; refractory cytopenia with multilineage dysplasia). The
patient was at high risk of bleeding due to anticoagulation
therapy, so we were only able to monitor her condition and provide
best supportive care; her first stroke was widely spread to the
brainstem, and she succumbed to acute cerebral infarction 11 days
after the stroke.
Primary disease treatment results
One patient (case 1) underwent total hysterectomy +
bilateral salpingo-oophorectomy + omentectomy. Optimal debulking
surgery without pelvic or para-aortic lymph node dissection was
performed, and the patient received adjuvant chemotherapy
consisting of carboplatin (area under the curve = 5) and paclitaxel
(175 mg/m2). The other patient (case 2) underwent total
hysterectomy + bilateral salpingo-oophorectomy + pelvic and
para-aortic lymph node dissection, and she received the same
chemotherapy as case 1. In addition, that patient also received
radiotherapy (whole pelvic irradiation, 45 Gy; para-aortic lymph
nodes, 45 Gy). Both patients had a good postoperative course,
without recurrence of thromboembolism. One patient (case 2)
survived for 21 months after the diagnosis of TS, but eventually
succumbed to recurrent malignant disease. The other patient (case
1) remains alive 14 months after treatment, with no evidence of
recurrence.
Discussion
We have treated 5 patients with cerebral infarction
due to TS and made two observations regarding this syndrome: First,
when the cerebral infarctions occurred, the D-dimer level was
suddenly and significantly elevated (Fig.
1; Table III). To the best of
our knowledge, this observation was not made by previous studies.
As each of these 5 patients experienced sudden elevation of D-dimer
levels, we must consider that TS occurs while these levels are
elevated. The present results also suggest that, even if patients
with progressive malignant disease are treated by anticoagulation
therapy, thromboembolism may still occur, despite an INR within the
therapeutic range. We may predict the risk of acute thromboembolism
by measuring the D-dimer level, which is almost always elevated in
this disease. Moreover, we observed an association between D-dimer
level and serum CA-125 level. A significant increase in CA-125
levels has been reported in mucin-producing tumors, and TS is known
to occur in association with such tumors (2,6). We
observed that tumors with mucin-producing characteristics exhibited
an association between the levels of D-dimer and serum CA-125
(parallel change) in TS. This suggests a role for potential
biomarkers for early development of TS.
 | Table III.Clinical and laboratory data. |
Table III.
Clinical and laboratory data.
| Data | No. (%) |
|---|
| Symptoms |
|
|
Hemiplegia | 3 |
| Facial
palsy | 2 |
|
Dizziness | 1 |
| Decreased
vision | 2 |
| Muscle
weakness | 1 |
|
Dysarthria | 1 |
| Thromboembolic
disease (except cerebral infarction) |
|
| Lower
limb | 3 (60%) |
|
Vegetations on the mitral
valve | 2 (40%) |
| Pulmonary
artery infarction | 1 |
| Renal and
splenic infarction | 1 |
| Laboratory data |
|
| CA-125
level at the time of cerebral infarction, U/ml (range) | 390 (19–552) |
| D-dimer
concentration, µg/ml (range) |
|
|
Under stable
conditions | 0.6 (0.5–0.7) |
|
At the time of
cerebral infarction | 41 (11.4–105.3) |
Second, we may control thromboembolism by
effectively controlling the primary disease. Two patients with
recurrence (cases 3 and 4) and 1 patient in whom the primary
disease could not be controlled (case 5) succumbed to
cerebrovascular disease. By contrast, in the remaining 2 patients
(cases 1 and 2), who were diagnosed with primary disease and
cerebrovascular disease simultaneously, thromboembolism was cured
with anticoagulation therapy and tumor resection. The primary
approach for treating malignancy-related TS is the elimination of
the causative tumor, regardless of the underlying disease
mechanism. Treatment directed against the underlying malignancy may
also significantly improve the hypercoagulable state.
Levitan et al suggested that the rate of deep
venous thrombosis/pulmonary embolism was 16/10,000 for patients
with head and neck cancers and 22/10,000 for those with bladder or
breast cancer, but it steadily increased to 85, 96, 110, 117 and
120/10,000 for patients with stomach cancer, lymphoma, and tumors
of the pancreas, brain and ovaries, respectively (7). Planner et al also suggested that
thromboembolic events may be more common in ovarian carcinoma than
previously considered, occurring in 44% of 59 patients with ovarian
carcinoma and coagulation abnormalities (4). The definition of TS includes
neurological symptoms which occur at distant sites from the
malignant tumor and is associated with malignant disease. In a
narrow sense, TS was considered to induce strokes due to
hypercoagulability, and to have a poor prognosis (8). It has been reported that patients with
lung, prostate, breast or ovarian carcinoma, or malignant
hematological diseases, tend to suffer from cerebral infarction
more frequently compared with patients with other malignant
diseases (9).
The primary approach to treating TS is to eliminate
the causative tumor; however, this is often not possible. It is
commonly mentioned in the literature that heparin is the preferred
treatment (2,10–15). In
recent years, low-molecular-weight heparins have become popular, in
part due to their improved pharmacokinetics, the ability to
administer single daily dosages, and the reduced incidence of
heparin-induced thrombocytopenia (16–18). In 2
of our patients, we initially used low-molecular-weight heparins,
but eventually switched to warfarin, as it was difficult for the
patients to continue using heparin at home, although it is well
known that heparins are better for the treatent of TS.
In this report, we described cerebral infarction
with TS due to malignant gynecological disease. Thromboembolisms in
the brain may be difficult to treat in several types of TS. We
experienced cases in which both TS and the primary disease could be
effectively controlled with conventional heparin treatment.
We treated 5 TS patients whose condition could be
controlled through treatment of the primary malignant disease
(Table IV). Patients with TS with
recurrent and difficult-to-control malignant disease are almost
impossible to cure. It is well known that if the primary disease is
difficult to control, the patients have a poor prognosis.
 | Table IV.Treatments and outcomes. |
Table IV.
Treatments and outcomes.
| Treatment of
thromboembolism |
|
|
Unfractionated heparin →
calcium heparin → warfarin | 3 (60%) |
|
Warfarin | 1 |
| No
treatment | 1 |
| Treatment of primary
disease after diagnosis of TS |
|
| Primary
treatment for primary disease | 2 |
|
Surgery,
chemotherapy (PTX+CBDCA) | 1 |
|
Surgery,
chemotherapy (PTX+CBDCA), RT | 1 |
|
Chemotherapy for reccurent
disease | 1 |
|
Palliative care | 2 |
| Treatment
outcomes |
|
| NED | 1 |
| Died from
primary disease | 2 |
| Died from
multiple cerebral infarctions | 2 |
To the best of our knowledge, this is the first
report to describe the association between D-dimer and serum CA-125
level as potential biomarkers for TS. In addition, if patients have
cerebral infarction and pulmonary embolism, cancer treatment need
not be discontinued, as it is possible to treat patients with TS
via tumor resection.
References
|
1
|
Trousseau A: Phlegmasia alba dolens.
Lectures on Clinical Medicine, delivered at the Hotel-Dieu, Paris.
5:(London, England). New Sydenham Society. 281–332. 1865.
|
|
2
|
Sack GH Jr, Levin J and Bell WR:
Trousseau's syndrome and other manifestations of chronic
disseminated coagulopathy in patients with neoplasms: Clinical,
pathophysiologic, and therapeutic features. Medicine (Baltiinore).
56:1–37. 1977. View Article : Google Scholar
|
|
3
|
Viselli AL, Feuer GA and Granai CO: Lower
limb ischemic venous thrombosis in patients with advanced ovarian
carcinoma. Gynecol Oncol. 49:262–265. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Planner RS, O'Sullivan EF, Campbell JJ and
Ball DL: The hypercoagulable state and pulmonary embolism in
patients with ovarian carcinoma. Aust N Z J Obstet Gynaecol.
18:209–212. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ito S, Yoshitomi H, Pak M, Kawahara H,
Oshima T, Ito S, Watanabe N, Sato H, Adachi T, Takeda M, et al:
Trousseau syndrome with nonbacterial thrombotic endocarditis in a
patient with uterine cancer. Intern Med. 52:1353–1358. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shao B, Wahrenbrock MG, Yao L, David T,
Coughlin SR, Xia L, Varki A and McEver RP: Carcinoma mucins trigger
reciprocal activation of platelets and neutrophils in a murine
model of Trousseau syndrome. Blood. 118:4015–4023. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Levitan N, Dowlati A, Remick SC, Tahsildar
HI, Sivinski LD, Beyth R and Rimm AA: Rates of initial and
recurrent thromboembolic disease among patients with malignancy
versus those without malignancy. Risk analysis using Medicare
claims date. Medicine (Baltimore). 78:285–291. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vernon S: Trousseau's syndrome:
Thrombophlebitis with carcinoma. J Abdom Surg. 3:137–138.
1961.PubMed/NCBI
|
|
9
|
Cestari DM, Weine DM, Panageas KS, Segal
AZ and DeAngelis LM: Stroke in patients with cancer: Incidents and
etiology. Neurology. 62:2025–2030. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang YY, Chan DK, Cordato D, Shen Q and
Sheng AZ: Stroke risk factor, pattern and outcome in patients with
cancer. Acta Neurol Scand. 114:378–383. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bell WR, Starksen NF, Tong S and
Porterfield JK: Trousseau's syndrome. Devastating coagulopathy in
the absence of heparin. Am J Med. 79:423–430. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Krauth D, Holden A, Knapic N, Liepman M
and Ansell J: Safety and efficacy of long-term oral anticoagulation
in cancer patients. Cancer. 59:983–985. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Walsh-McMonagle D and Green D:
Low-molecular-weight heparin in the management of Trousseau's
syndrome. Cancer. 80:649–655. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meyer G, Marjanovic Z, Valcke J, Lorcerie
B, Gruel Y, Solal-Celigny P, Le Maignan C, Extra JM, Cottu P and
Farge D: Comparison of low-molecular-weight heparin and warfarin
for the secondary prevention of venous thromboembolism in patients
with cancer: A randomized controlled study. Arch Intern Med.
162:1729–1735. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Levine MN: Managing thromboembolic disease
in the cancer patient: Efficacy and safety of antithrombotic
treatment options in patients with cancer. Cancer Treat Rev.
28:145–149. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Castelli R, Porro F and Tarsia P: The
heparins and cancer: Review of clinical trials and biological
properties. Vasc Med. 9:205–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zacharski LR and Loynes JT:
Low-molecular-weight heparin in oncology. Anticancer Res.
23:2789–2793. 2003.PubMed/NCBI
|
|
18
|
McCart GM and Kayser SR: Therapeutic
equivalency of low-molecular-weight heparins. Ann Pharmacother.
36:1042–1057. 2002. View Article : Google Scholar : PubMed/NCBI
|