Introduction
Prostate cancer is currently one of the most common
malignant tumors in men aged >50 years. The global
age-standardized incidence rate of prostate cancer in 2008 was
~30/100,000 individuals, which is second only to that of lung
cancer (1). Screening for prostate
cancer mostly relies on digital rectal examination, measurement of
prostate-specific antigen (PSA) level, magnetic resonance imaging
(MRI) and ultrasound (US). The current standard for diagnosing
prostate cancer in men at risk relies on a transrectal US
(TRUS)-guided biopsy test, which is blind to cancer location. This
method has the advantages of speed, ease, cost-effectiveness,
availability and portability, and it is more suitable for wide-area
sampling of the prostate, including the far-lateral peripheral
zones (2). However, despite an
increasing number of biopsy cores being included in TRUS-guided
biopsy protocols, the current standard of including 10–14
randomized cores lacks sensitivity and frequently detects
clinically insignificant disease (3–5).
It was recently suggested that targeted biopsies of
suspicious lesions detected by multi-parametric (mp)-MRI may
increase the diagnostic accuracy of TRUS-guided biopsy (6). mp-MRI combines T2-weighted images with
diffusion-weighted images and dynamic contrast enhancement
(7,8).
This method exhibits increased sensitivity and specificity and has
become the standard imaging technique for biopsy guidance (9,10). As
mp-MRI and biopsy are performed on different days, with the latter
commonly performed using a TRUS probe, a number of devices that use
image-fusion software have been developed to overlay the suspicious
area on mp-MRI onto the US images at the time of biopsy (11).
In the present study, a meta-analysis of data
extracted from published studies using MRI-US image fusion targeted
prostate biopsy was performed to assess the accuracy of prostate
cancer detection compared with that of systematic biopsy.
Materials and methods
Literature search strategy
A literature search was conducted through the
PubMed, EMBASE and China National Knowledge Infrastructure
databases for studies published prior to July 21st, 2015, using the
key words (‘prostate cancer’, ‘prostate neoplasm’ or ‘prostate’) in
combination with (‘magnetic resonance imaging’, ‘MRI’ or ‘MR’) and
(‘transrectal ultrasound’ or ‘TRUS’) and (‘fusion’, ‘registration’,
‘targeted’, ‘target’, ‘computer’ or ‘software’). Only articles
written in English or Chinese and studies on human subjects were
included. In addition, references of relevant articles were
manually searched to identify potentially eligible studies.
Eligibility criteria
Two authors assessed each identified study
independently. The inclusion of individual studies required that
software-based MRI-US fusion targeted prostate biopsies and
systematic biopsies had been performed within the same study. In
addition, each study was required to contain overall or significant
cancer detection results for the two modalities. To allow for a
valid comparison, only studies directly comparing the two
techniques were included. When multiple studies contained
overlapping data, only the most informative study was included.
Meeting abstracts, editorials, case reports, letters and reviews
were excluded.
Data extraction
Two investigators blinded to each others' results
independently reviewed the full manuscripts of the eligible
studies. The information extracted from each study included first
author, year of publication, study design, population (sample size,
age, PSA, prostate volume and prior biopsy), type of anaesthesia,
systematic biopsy (number of cores and sampling route), MRI-US
image fusion targeted biopsy procedure (software used, sampling
route, time flow and number of cores per lesion) and separate
histological outcomes for systematic vs. targeted biopsy (overall
detection rate of cancer and detection rate of clinically
significant and insignificant cancer). Disagreements between the
two reviewers were resolved through discussion.
Statistical analysis
All the analyses were performed using the
statistical Stata software, version SE/12 (StataCorp LP, College
Station, TX, USA). The main outcome was the detection rate of
overall prostate cancer and the secondary outcomes were the
detection rates of clinically significant and insignificant disease
by MRI-TRUS image fusion targeted biopsy compared with the
systematic biopsy technique. The definition used to determine
clinical significance was that used by each individual study.
Fixed-effects or random-effects meta-analysis was performed to pool
the original studies on the basis of their relative risk (RR),
depending on the result of the heterogeneity analysis. Forest plots
were created to summarize all studies, the pooled estimate and
corresponding 95% confidence intervals (95% CIs) in a single
overview.
Heterogeneity was assessed using the I2
statistical method, with I2>50% indicating
significant heterogeneity. When heterogeneity was confirmed, a
sensitivity analysis was performed by successively excluding each
individual study. Subgroup analysis was performed according to
several characteristics. Publication bias was assessed using Begg's
funnel plot and Egger's test. All the P-values were two-tailed and
P<0.05 was considered to indicate statistically significant
differences.
Results
Study selection and
characteristics
Of the 800 articles retrieved during the initial
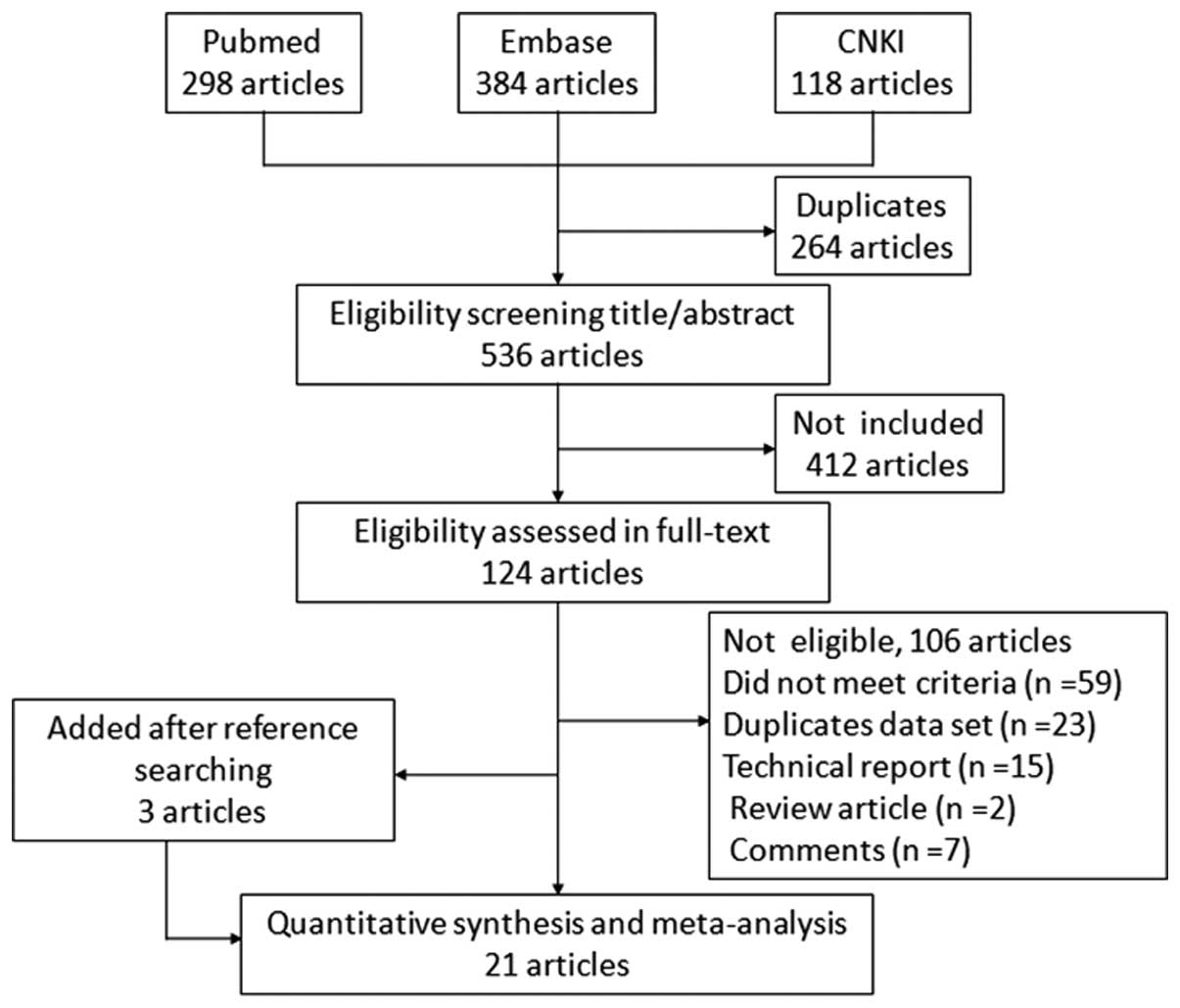
search, 21 (2,12–31) met
the inclusion criteria. A flow chart of the study selection process
is presented in Fig. 1.
A total of 3,415 patients were included, with a
sample size ranging from 20 to 1,003 patients. The majority of
studies originated from 6 countries, with studies from the USA
comprising the largest proportion (n=10). A total of 6 studies had
been conducted on biopsy-naive patients, 4 on patients with a
previous negative prostate biopsy, and 11 studies reported on a
mixed cohort (either biopsy-naive patients, or those having
undergone previous prostate biopsy). All mp-MRI scans had been
performed on either a 1.5- or a 3-T scanner and 9 different image
fusion platforms currently used in the clinical setting to perform
MRI-TRUS targeted biopsies were identified in this meta-analysis.
The standard comparator was a 8- to 12-core TRUS biopsy in 18
studies, whereas 3 other studies used transperineal template
biopsies, or a combination of the two. The characteristics of the
included studies are summarized in Table
I.
 | Table I.Main characteristics of the studies
included in the meta-analysis. |
Table I.
Main characteristics of the studies
included in the meta-analysis.
|
|
| Population
characteristics |
|
| MRI-TRUS fusion
targeted biopsy |
|---|
|
|
|
|
|
|
|
|---|
| Author, year
(Refs.) | Study design | Country | Sample size (n) | Age (years) | PSA (ng/ml) | Prostate volume
(ml) | Prior biopsy | Anaesthesia | System biopsy | Magnetic field
(T) | Software used | Sampling
method | Targeted biopsy
first | No. of cores per
lesion |
|---|
| Baco et al
2015 (29) | RCT | USA | 175 | Mean (range), 65
(59–69) | Mean (range), 7.3
(5.5–9.9) | Mean (range), 42
(30–59) | Biopsy naive | Local | TRUS 12 cores | 1.5 | UroStation | Transrectal | Yes | Med (range), 2
(1–4) |
| Siddiqui et
al 2015 (30) | Cohort | USA | 1003 | Mean ± SD,
62.1±7.5 | Med (IQR), 6.7
(4.4–10.7) | Med (IQR), 49
(36–71) | Mixed | NR | TRUS 12 cores | 3 | UroNav | Transrectal | Yes | 2 |
| Borkowetz et
al 2015 (31) | Cohort | Germany | 263 | Med (range), 66
(47–83) | Med (range), 8.3
(0.39–86.57) | Med (range), 50
(12–220) | Mixed | General or
spinal | TRUS 12 cores | 3 | Biojet | Transperineal | Yes | ≥2 |
| Sankineni et
al 2015 (12) | Cohort | USA | 33 | Mean (range), 63
(52–76) | Mean (range), 8.4
(1.22–65.20) | Mean (range), 53
(12–125) | Mixed | NR | TRUS 12 cores | 3 | UroNav | Transrectal | NR | NR |
| Zhang et al
2015 (13) | Cohort | China | 62 | Mean ± SD,
68.38±6.57 | Mean ± SD,
10.21±5.57 | Mean ± SD,
34.05±9.86 | Biopsy naive | General | TRUS 12 cores | 3 | RVS | Transrectal | Yes | ≥2 |
| de Gorski et
al 2015 (14) | Cohort | France | 232 | Mean ± SD,
64±6.4 | Mean ± SD,
6.65±1.8 | Mean ± SD,
40±24.3 | Biopsy naive | NR | TRUS 12 cores | 1.5 | UroStation | Transrectal | No | NR |
| Ukimura et
al 2015 (15) | Cohort | USA | 127 | Med, 69 | Med, 5.8 | NR | Mixed | Local | TRUS 10–12
cores | 3 | UroStation | Transrectal | No | ≥1 |
| Junker et al
2015 (16) | Cohort | Australia | 50 | Mean ± SD,
63.7±7.9 | Mean ± SD,
7.6±4.2 | Mean ± SD,
49.2±21.9 | Mixed | NR | TRUS 10 cores | 3 | Logiq | Transrectal | Yes | Mean, 3.9 |
| Shoji et al
2015 (17) | Cohort | Japan | 20 | Med (range), 70
(52–83) | Med (range), 7.4
(3.54–19.9) | Med (range), 38
(24–68) | Biopsy naive | Spinal | Transperineal 12
cores | 1.5 | Biojet | Transrectal | Yes | NR |
| Salami et al
2015 (18) | Cohort | USA | 140 | NR | NR | NR | Negative | NR | TRUS 12 cores | 3 | UroNav | Transrectal | Yes | 2 |
| Mozer et al
2015 (19) | Cohort | France | 152 | Med (IQR), 63.7
(59.3–67.5) | Med (IQR), 6
(5–7.9) | Med (IQR), 38.5
(30–55) | Biopsy naive | NR | TRUS 12 cores | 1.5 | UroStation | Transrectal | No | 2 or 3 |
| Volkin et al
2015 (25) | Cohort | USA | 162 | Med (range), 63
(44–80) | Med (range), 8.4
(0.3–95.8) | Med (range), 48
(19–187) | Mixed | Local | TRUS 12 cores | 3 | NR | Transrectal | No | ≥2 |
| Rastinehad et
al 2014 (20) | Cohort | USA | 105 | Mean (range), 65.8
(42–87) | Mean (range), 9.2
(0.6–62) | NR | Mixed | NR | TRUS 12 cores | 3 | UroNav | Transrectal | Yes | NR |
| Sonn et al
2014 (26) | Cohort | USA | 105 | Med (IQR), 65
(59–70) | Med (IQR), 7.5
(5–11.2) | Med (IQR), 58
(39–82) | Negative | Local | TRUS 12 cores | 3 | Artemis | Transrectal | Yes | Mean (range), 4.2
(1–9) |
| Wysock et al
2014 (27) | Cohort | USA | 125 | Med (range), 65
(56.3–71) | Med (range), 5.1
(3.5–7.3) | Med (range), 46
(31–62.5) | Mixed | Local | TRUS 12 cores | 3 | Artemis | Transrectal | Yes | 2 |
| Fiard et al
2013 (21) | Cohort | France | 20 | Med (range), 65
(62–68) | Med (range), 6.3
(5.3–10) | Med (range), 39
(29–49) | Mixed | Local or
general | TRUS 12 cores | 3 | UroStation | Transrectal | No | 2 |
| Delongchamps et
al 2013 (23) | Cohort | France | 133 | Mean ± SD,
64.5±7.9 | Mean ± SD,
9±3.9 | Mean ± SD,
58.3±28.6 | Biopsy naive | NR | TRUS 10–12
cores | 1.5 | Koelis | Transrectal | No | ≥2 |
| Puech et al
2013 (24) | Cohort | France | 95 | Med (range), 65
(49–76) | Mean ± SD,
10.05±8.8 | Mean ± SD,
52±24 | Mixed | Local | TRUS 12 cores | 1.5 | Virtual
Navigator | Transrectal | No | 2 |
| Kuru et al
2013 (28) | Cohort | Germany | 347 | Med (range), 65.3
(42–82) | Mean (range), 9.85
(0.5–104) | Mean (range), 48.7
(9–108) | Mixed | General | Transperineal 24
cores | 3 | BiopSee | Transperineal | Yes | Med (range), 4
(2–6) |
| Vourganti et
al 2012 (22) | Cohort | USA | 195 | Med (range), 62
(37–80) | Med (range), 9.13
(0.3–103) | Med (range), 56
(16–187) | Negative | Local | TRUS 12 cores | 3 | NR | Transrectal | No | Med (range), 5
(2–14) |
| Miyagawa et
al 2010 (2) | Cohort | Japan | 85 | Med (range), 69
(56–84) | Med (range), 9.9
(4.0–34.2) | Med (range), 37.2
(18–141) | Negative | Spinal | TRUS/transperineal
10–11 cores | 1.5 | RVS | Transperineal | Yes | Mean, 1.9 |
Overall prostate cancer detection
Details regarding diagnostic criteria and detection
ratios in the individual studies are presented in Table II. Across the 21 studies, the
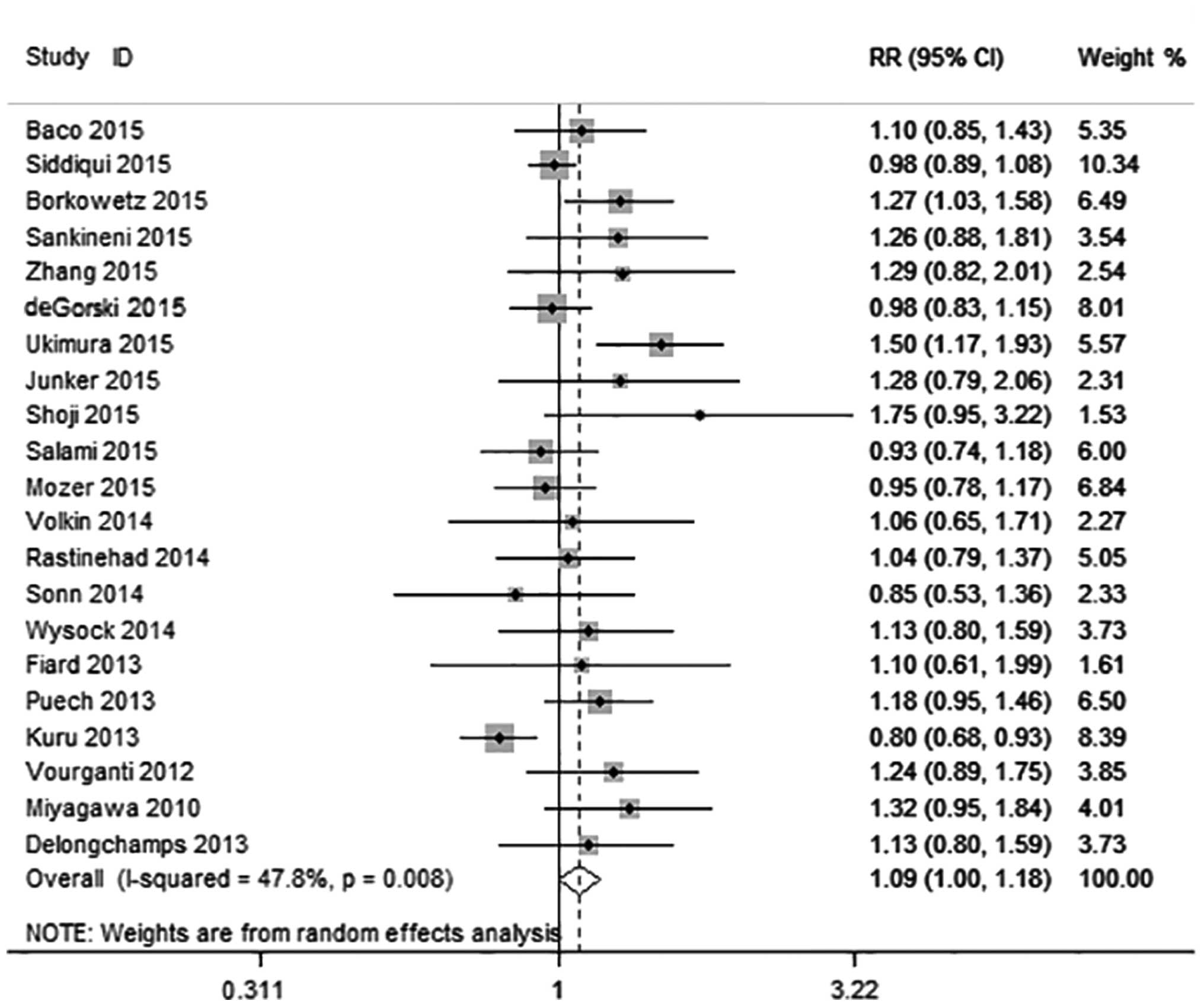
prevalence of prostate cancer was 63.0% (2,153/3,415). MRI-US
fusion biopsy detected overall prostate cancer in 1,562 of 3,315
patients and systematic biopsy in 1,496 of 3,313 patients,
resulting in an RR of 1.09 (95% CI: 1.00–1.18; P=0.047) (Fig. 2). The heterogeneity among these
studies was moderate (I2=47.8%; χ2=38.34;
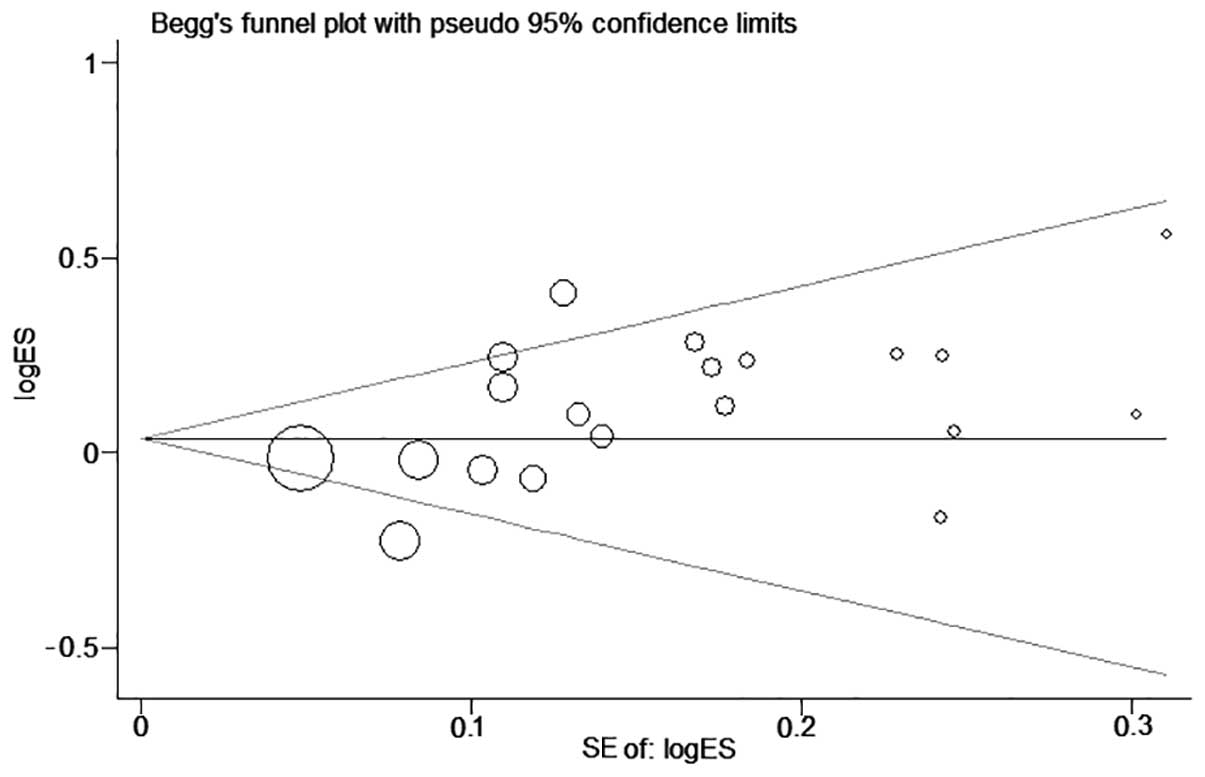
P=0.047). Publication bias in this overall analysis was revealed by
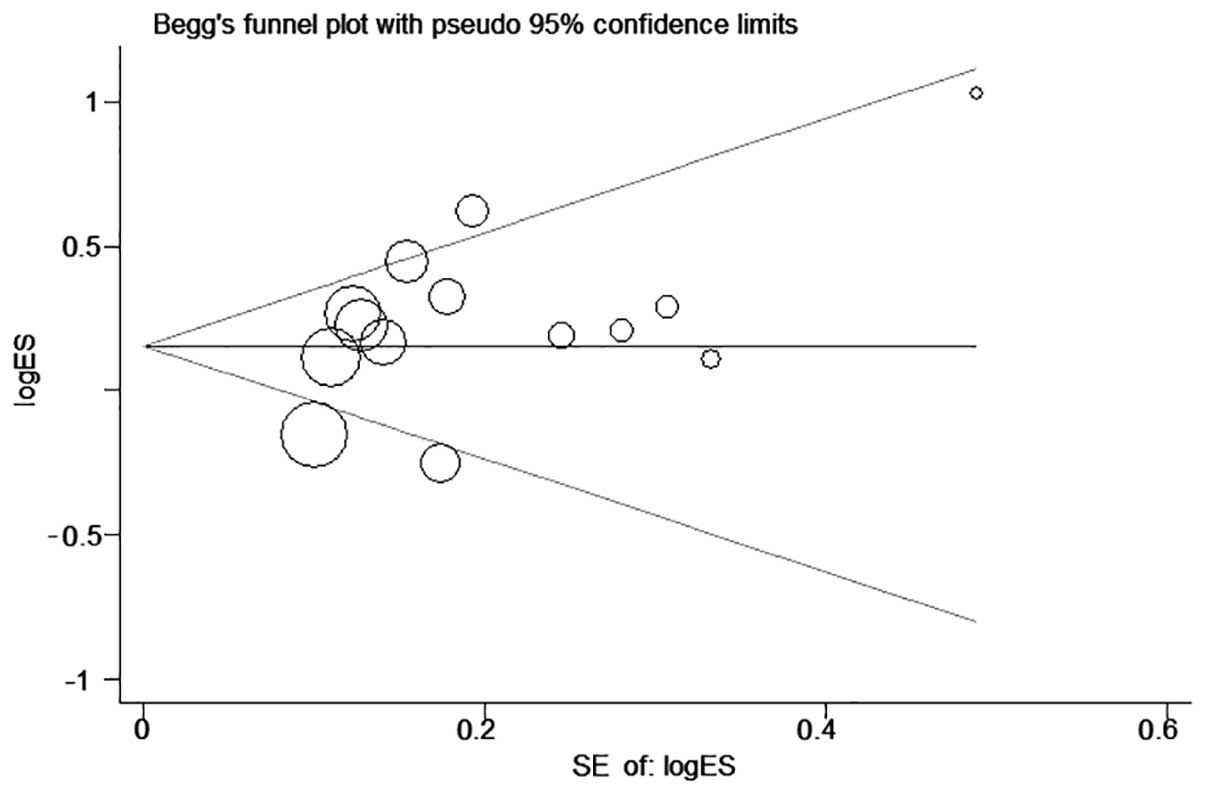
the Begg's funnel plot (P=0.205, Begg's test; P=0.017, Egger's
test) (Fig. 3).
 | Table II.Quality assessment of the studies
included in the meta-analysis. |
Table II.
Quality assessment of the studies
included in the meta-analysis.
|
|
|
| Overall cancer
detection (n/total) | Clinically
significant cancer detection (n/total) |
|---|
|
|
|
|
|
|
|---|
| Author, year
(Refs.) | Main race | Definition of
clinically significant disease | Fusion biopsy | System biopsy | Fusion biopsy | System biopsy |
|---|
| Baco et al,
2015 (29) | Caucasian | Gleason ≥7 or
maximum cancer core length ≥5 mm | 51/86 | 48/89 | 33/86 | 44/89 |
| Siddiqui et
al, 2015 (30) | Caucasian | NR | 461/1,003 | 469/1,003 | – | – |
| Borkowetz et
al, 2015 (31) | Caucasian | Gleason >6, or
>2 cores, or >50% of any core | 116/263 | 91/263 | 94/263 | 75/263 |
| Sankineni et
al, 2015 (12) | Caucasian | Gleason >3+4
with 25% biopsy core involvement | 24/33 | 19/33 | 16/33 | 13/33 |
| Zhang et al,
2015 (13) | Asian | Gleason ≥3+4 or
cancer core length ≥4 mm | 27/62 | 21/62 | 14/62 | 5/62 |
| de Gorski et
al, 2015 (14) | Caucasian | Gleason ≥3+4 or
cancer core length ≥4 mm | 126/232 | 129/232 | 102/232 | 91/232 |
| Ukimura et
al, 2015 (15) | Caucasian | Gleason ≥3+4 or
cancer core length ≥5 mm | 78/127 | 52/127 | 54/127 | 29/127 |
| Junker et
al, 2015 (16) | Caucasian | NR | 23/50 | 18/50 | – | – |
| Shoji et al,
2015 (17) | Asian | NR | 14/20 | 8/20 | – | – |
| Salami et
al, 2015 (18) | Caucasian | Gleason >6, or
>2 cores, or >50% of any core | 68/140 | 73/140 | 67/140 | 43/140 |
| Mozer et al,
2015 (19) | Caucasian | Gleason ≥3+4 or
cancer core length ≥4 mm | 82/152 | 86/152 | 66/152 | 56/152 |
| Volkin et
al, 2014 (25) | Caucasian | NR | 19/42 | 18/42 | – | – |
| Rastinehad et
al, 2014 (20) | Caucasian | Gleason >6, or
>2 cores, or >50% of any core | 53/105 | 51/105 | 47/105 | 34/105 |
| Sonn et al,
2014 (26) | Caucasian | Gleason ≥3+4 or
cancer core length ≥4 mm | 24/102 | 27/97 | 21/102 | 15/97 |
| Wysock et
al, 2014 (27) | Caucasian | Gleason ≥3+4 | 45/125 | 40/125 | 29/125 | 24/125 |
| Fiard et al,
2013 (21) | Caucasian | Gleason ≥3+4 or
total cancer length ≥10 mm | 11/20 | 10/20 | 10/20 | 9/20 |
| Delongchamps et
al, 2013 (23) | Caucasian | NR | 45/125 | 40/125 | – | – |
| Puech et al,
2013 (24) | Caucasian | Gleason ≥3+4 or
cancer core length ≥3 mm | 66/95 | 56/95 | 64/95 | 49/95 |
| Kuru et al,
2013 (28) | Caucasian | NCCN criteria | 128/253 | 161/253 | 104/253 | 121/253 |
| Vourganti et
al, 2012 (22) | Caucasian | NR | 56/195 | 45/195 | – | – |
| Miyagawa et
al, 2010 (2) | Asian | NR | 45/85 | 34/85 | – | – |
| Totala |
|
| 1,562/3,315 | 1,496/3,313 | 721/1,795 | 608/1,793 |
Clinically significant prostate cancer
detection
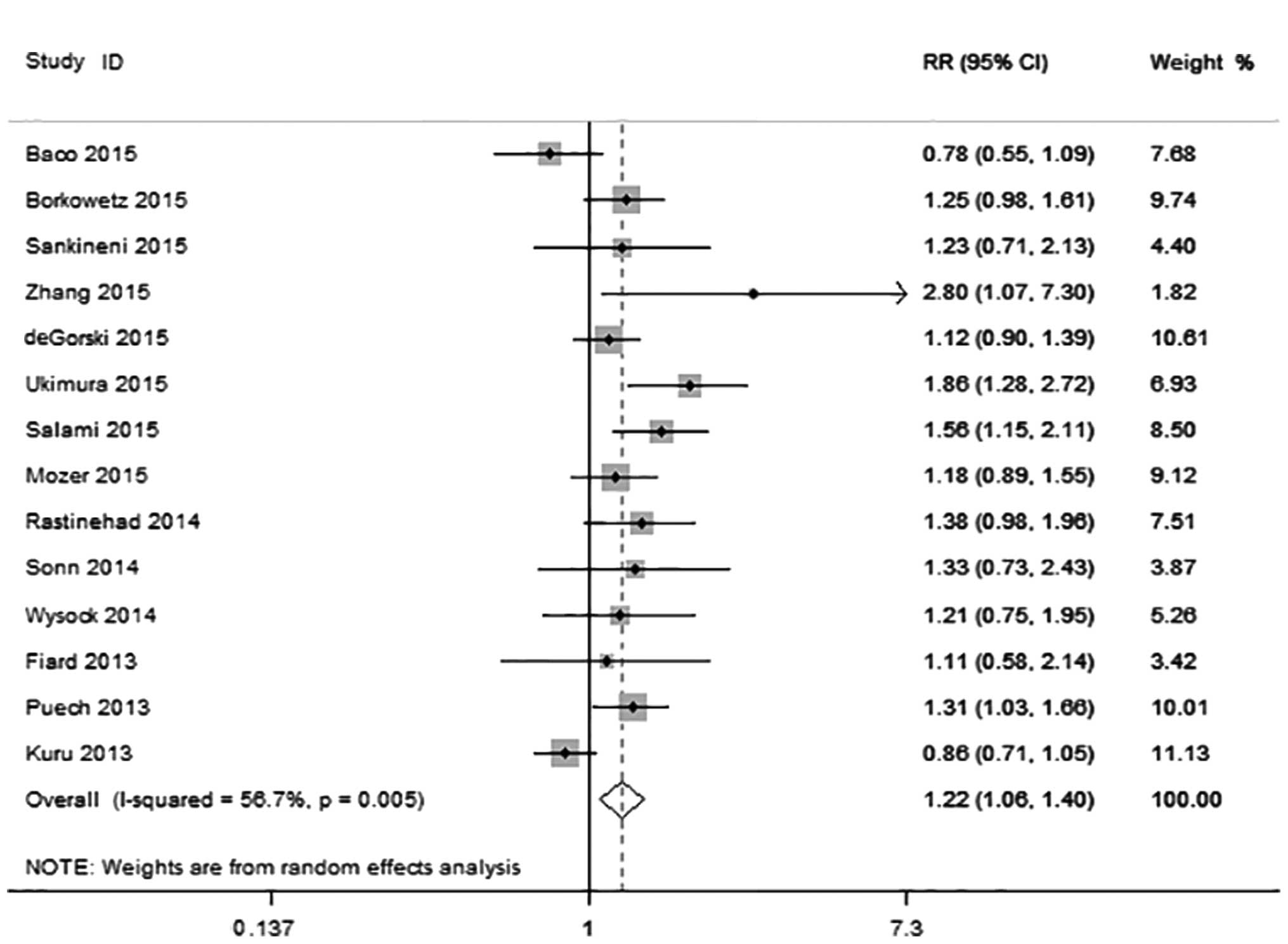
A total of 14 studies including 1,884 patients were
eligible for inclusion in the analysis. The prevalence of
clinically significant and insignificant prostate cancer was 47.2%
(890/1,884) and 16.6% (313/1,884), respectively. Clinically
significant prostate cancer was diagnosed in 721 of the 1,795
patients with MRI-US fusion biopsy compared with 608 of 1,793
patients with systematic biopsy, with an RR of 1.22 (95% CI:
1.06–1.40; P=0.005) (Fig. 4).
However, heterogeneity was observed among these studies
(I2=56.7%; χ2=30.04; P=0.005). Begg's funnel
plots revealed little publication bias in this analysis (Fig. 5), whereas the Egger's and Begg's tests
indicated there was no publication bias.
The results of the subgroup analysis for clinically
significant prostate cancer detection are presented in Table III. MRI-US fusion biopsy exhibited a
significantly higher detection rate of clinically significant
prostate cancer compared with systematic biopsy in 7 subgroups, but
there was heterogeneity in all subgroups apart from that of
patients with a previous negative biopsy.
 | Table III.Results of subgroup analysis of
significant prostate cancer detection. |
Table III.
Results of subgroup analysis of
significant prostate cancer detection.
|
|
| Heterogeneity |
| Meta-analysis |
|---|
|
|
|
|
|
|
|---|
| Subgroups | No. of studies | I2
(%) | P-value | Effects model | RR (95% CI) | P-value |
|---|
| Study design |
|
| Paired
cohort | 13 | 50.3 | 0.02 | Random | 1.258
(1.100–1.438) | 0.001 |
|
Comparative series | 1 | – | – | – | 0.776
(0.552–1.091) | 0.145 |
| Main race |
|
|
Caucasian | 13 | 55.2 | 0.008 | Random | 1.198
(1.047–1.370) | 0.009 |
|
Asian | 1 | – | – | – | 2.800
(1.074–7.302) | 0.035 |
| Prior biopsy |
|
|
Mixed | 8 | 59.8 | 0.015 | Random | 1.235
(1.023–1.491) | 0.028 |
| Biopsy
naive | 4 | 62.4 | 0.047 | Random | 1.101
(0.833–1.457) | 0.498 |
|
Previous negative | 2 |
0.0 | 0.645 | Fixed | 1.498
(1.141–1.967) | 0.004 |
| Strength of
magnetic field |
|
| 3T | 10 | 61.5 | 0.005 | Random | 1.311
(1.073–1.601) | 0.008 |
|
1.5T | 4 | 51.6 | 0.102 | Random | 1.104
(0.914–1.333) | 0.307 |
| Sampling
method |
|
|
Transrectal | 12 | 40.3 | 0.072 | Random | 1.269
(1.104–1.459) | 0.001 |
|
Transperineal | 2 | 81.7 | 0.019 | Random | 1.029
(0.710–1.492) | 0.879 |
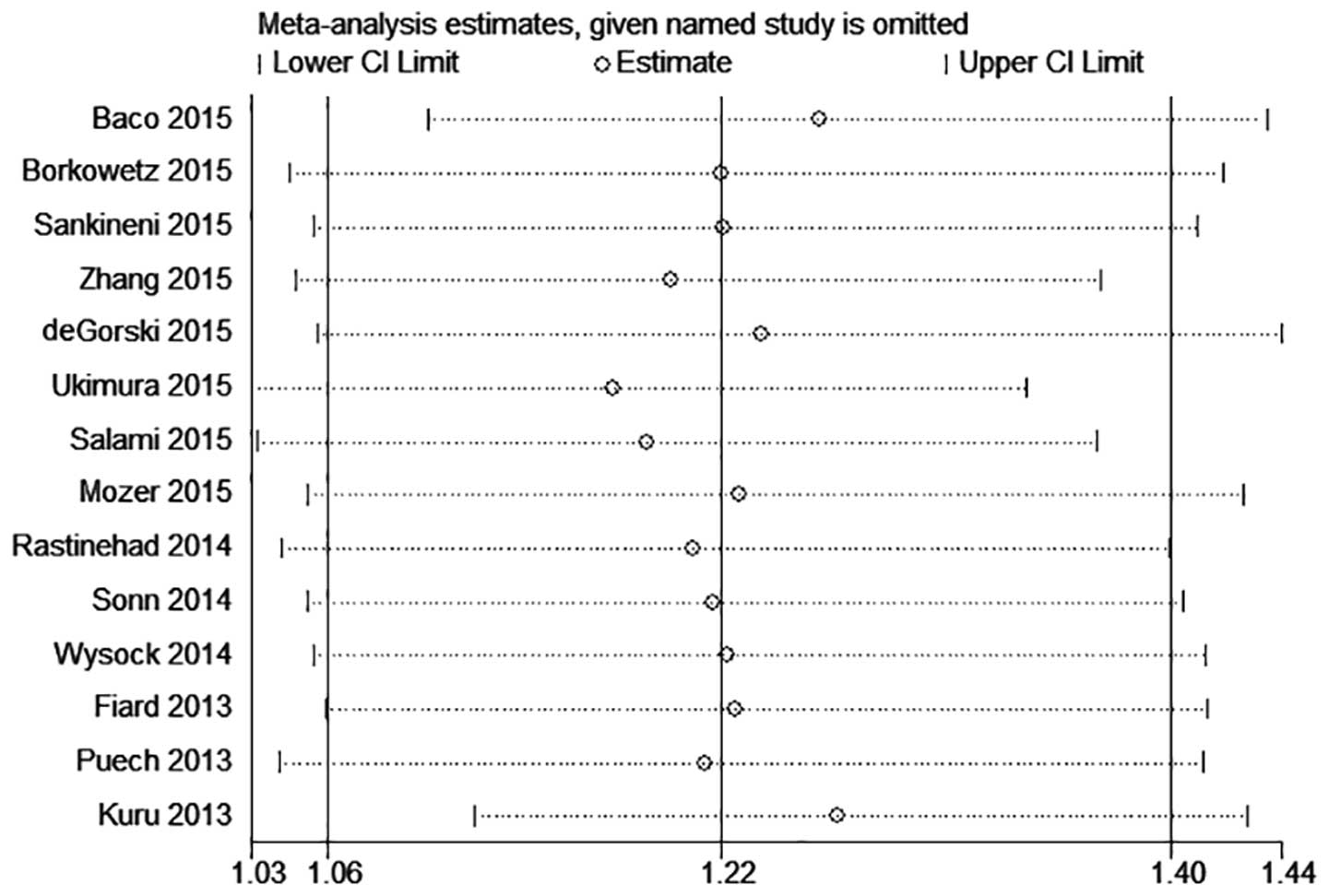
Sensitivity analysis of the 14 studies demonstrated
that the results of Kuru et al (28) diverged from those of most other trials
(Fig. 6). Following exclusion of the
Kuru et al trial, there was no significant variation in the
RR value, but the heterogeneity decreased (Table IV).
 | Table IV.Results of sensitivity analysis of
significant prostate cancer detection. |
Table IV.
Results of sensitivity analysis of
significant prostate cancer detection.
|
| Heterogeneity
test | Pooled
estimate |
|---|
|
|
|
|
|---|
| Sensitivity
analysis | I2
(%) |
tau2 | RR (95% CI) | P-value |
|---|
| Kuru et al
(28) incorporated | 56.7 | 0.0350 | 1.218
(1.060,1.399) | 0.005 |
| Kuru et al
(28) excluded | 34.8 | 0.0162 | 1.265
(1.119,1.429) | <0.001 |
Clinically insignificant prostate cancer was
diagnosed in 178 of 1,795 patients by MRI-US fusion biopsy and in
256 of 1,793 patients by systematic biopsy, resulting in a RR of
0.73 (95% CI: 0.51–1.05; P=0.089); there was high heterogeneity
among these studies (I2=67.5%; χ2=39.97;
P<0.01).
Discussion
The current gold standard technique for diagnosing
prostate cancer in men at risk is systematic prostate biopsy using
TRUS. However, there are discrepancies between the results of
systematic prostate biopsy and radical prostatectomy specimens
(32), as only 24–40% of TRUS-guided
biopsy results are consistent with the pathological findings
following prostatectomy (33).
Furthermore, systematic biopsy may not be able to detect all cases
of clinically significant prostate cancer, which may delay the
treatment of a tumor with a high Gleason score (3–5). The
optimal biopsy strategy should selectively detect clinically
significant prostate cancer and minimize clinically insignificant
prostate cancer detection to avoid consequent overtreatment. The
present meta-analysis demonstrated that MRI-US fusion targeted
biopsy may be a promising strategy with certain advantages over
systematic biopsy.
The results of the present study demonstrated that
the overall prostate cancer detection rate of MRI-US fusion biopsy
is higher compared with that of systematic biopsy, with an RR of
1.09. The difference between the results of the present study and
those of a prior systematic review (34) may be due to the larger sample size and
updated data included herein.
It was reported that mp-MRI exhibits a high
diagnosis rate of clinically significant prostate cancer when
compared to the histological findings following radical
prostatectomy (35), which is in line
with the results of the present study. In our study, MRI-US fusion
biopsy had an RR of 1.22 for detecting significant prostate cancer,
which means that MRI-US fusion biopsy has a 22% increased detection
rate for clinically significant prostate cancer compared with
systematic biopsy.
MRI-US fusion biopsy and systematic biopsy did not
significantly differ in the detection of clinically insignificant
prostate cancer; however, an RR of 0.73 indicated that MRI-US had a
better performance in terms of avoiding detection of insignificant
prostate cancer compared with systematic biopsy in most studies.
Thus, the application of MRI-US fusion biopsy may help reduce
oversampling of potentially insignificant prostate cancers.
Several studies also compared MRI-US fusion with the
systematic approach on a per-core basis (13,21,30). The
results demonstrated that MRI-US-guided biopsy required fewer cores
for successful tumor detection, thereby reducing patient discomfort
compared with systematic biopsy.
The present meta-analysis had several limitations
that may reduce the strength of the conclusions. Studies with
negative results are less likely to be published, which may result
in the overstatement of beneficial effects in meta-analyses. In the
analysis of the overall prostate cancer detection rate, Begg's test
yielded a P-value of 0.205, while Egger's test yielded a P-value of
0.017, indicating the presence of publication bias, as Egger's test
has a higher sensitivity.
In the analysis of the detection of clinically
significant prostate cancer, Begg's test and Egger's test indicated
no publication bias. However, significant heterogeneity was found
in this analysis (I2=56.7%). Subgroup analysis revealed
the presence of heterogeneity in all subgroups apart from that
including patients with a previous negative biopsy, indicating that
the heterogeneity originated in the category of prior biopsy, but
not study design, main race, strength of magnetic field or sampling
method. The sensitivity analysis revealed that, after excluding the
trial by Kuru et al (28),
heterogeneity was markedly decreased, while the RR value was not
significantly affected. Kuru et al (28) obtained a higher RR compared with that
of the other studies, possibly due to the BiopSee system used in
their study, in which US, TRUS/MRI fusion, biopsy planning,
perineal targeting, 3D mapping and automated documentation are
integrated into a single system. The definition of significant
prostate cancer, which included intermediate or high-risk tumors
according to the National Comprehensive Cancer Network criteria
(36), may also explain their higher
RR. In addition, significant heterogeneity may be attributed to the
variability across the studies in terms of criteria for defining
clinically significant tumors, the methodology of targeted biopsy
and the number of cores per target.
On the basis of biopsy data alone, it may be
methodologically incorrect to conclude that MRI-US fusion biopsy
detects more significant prostate cancers compared with systematic
biopsy. This conclusion may be a statistical or methodological
effect rather than a true clinical fact. All patients would have to
undergo radical prostatectomy and assessment of the final pathology
to draw clinically relevant conclusions. Such studies are warranted
in the future.
In summary, a meta-analysis of the currently
available high-level clinical studies was performed to evaluate the
efficacy of MRI-US fusion prostate biopsy. It was revealed that
MRI-US fusion prostate biopsy has a higher detection rate of
prostate cancer compared with systematic biopsy. MRI-US fusion
biopsy also detects more clinically significant and fewer
insignificant prostate cancers compared with systematic protocols.
It is therefore recommended that mp-MRI is performed in patients
suspected of having prostate cancer in order to optimize the
detection of clinically significant prostate cancer, while reducing
the burden of biopsies.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81370781).
References
|
1
|
Soerjomataram I, Lortet-Tieulent J, Parkin
DM, Ferlay J, Mathers C, Forman D and Bray F: Global burden of
cancer in 2008: A systematic analysis of disability-adjusted
life-years in 12 world regions. Lancet. 380:1840–1850. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miyagawa T, Ishikawa S, Kimura T, Suetomi
T, Tsutsumi M, Irie T, Kondoh M and Mitake T: Real-time virtual
sonography for navigation during targeted prostate biopsy using
magnetic resonance imaging data. Int J Urol. 17:855–860. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Babaian RJ, Toi A, Kamoi K, Troncoso P,
Sweet J, Evans R, Johnston D and Chen M: A comparative analysis of
sextant and an extended 11-core multisite directed biopsy strategy.
J Urol. 163:152–157. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Presti JC Jr, O'Dowd GJ, Miller MC, Mattu
R and Veltri RW: Extended peripheral zone biopsy schemes increase
cancer detection rates and minimize variance in prostate specific
antigen and age related cancer rates: Results of a community
multi-practice study. J Urol. 169:125–129. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campos-Fernandes JL, Bastien L, Nicolaiew
N, Robert G, Terry S, Vacherot F, Salomon L, Allory Y, Vordos D,
Hoznek A, et al: Prostate cancer detection rate in patients with
repeated extended 21-sample needle biopsy. Eur Urol. 55:600–606.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sonn GA, Margolis DJ and Marks LS: Target
detection: Magnetic resonance imaging-ultrasound fusion-guided
prostate biopsy. Urol Oncol. 32:903–911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dickinson L, Ahmed HU, Allen C, Barentsz
JO, Carey B, Futterer JJ, Salomon L, Allory Y, Vordos D, Hoznek A,
et al: Magnetic resonance imaging for the detection, localisation,
and characterisation of prostate cancer: Recommendations from a
European consensus meeting. Eur Urol. 59:477–494. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barentsz JO, Richenberg J, Clements R,
Choyke P, Verma S, Villeirs G, Rouviere O, Logager V and Fütterer
JJ: European Society of Urogenital Radiology: ESUR prostate MR
guidelines 2012. Eur Radiol. 22:746–757. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haffner J, Lemaitre L, Puech P, Haber GP,
Leroy X, Jones JS and Villers A: Role of magnetic resonance imaging
before initial biopsy: Comparison of magnetic resonance
imaging-targeted and systematic biopsy for significant prostate
cancer detection. BJU Int. 108:E171–E178. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hambrock T, Somford DM, Hoeks C, Bouwense
SA, Huisman H, Yakar D, van Oort IM, Witjes JA, Fütterer JJ and
Barentsz JO: Magnetic resonance imaging guided prostate biopsy in
men with repeat negative biopsies and increased prostate specific
antigen. J Urol. 183:520–527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moore CM, Robertson NL, Arsanious N,
Middleton T, Villers A, Klotz L, Taneja SS and Emberton M:
Image-guided prostate biopsy using magnetic resonance
imaging-derived targets: A systematic review. Eur Urol. 63:125–140.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sankineni S, George AK, Brown AM,
Rais-Bahrami S, Wood BJ, Merino MJ, Pinto PA, Choyke PL and Turkbey
B: Posterior subcapsular prostate cancer: Identification with mpMRI
and MRI/TRUS fusion-guided biopsy. Abdom Imaging. 40:2557–2565.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Q, Wang W, Yang R, Zhang G, Zhang B,
Li W, Huang H and Guo H: Free-hand transperineal targeted prostate
biopsy with real-time fusion imaging of multiparametric magnetic
resonance imaging and transrectal ultrasound: Single-center
experience in China. Int Urol Nephrol. 47:727–733. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Gorski A, Rouprêt M, Peyronnet B, Le
Cossec C, Granger B, Comperat E and Cussenot O: Accuracy of
magnetic resonance imaging/ultrasound fusion targeted biopsies to
diagnose clinical significant prostate cancer in enlarged compared
to smaller prostates. J Urol. 194:669–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ukimura O, Marien A, Palmer S, Villers A,
Aron M, de Castro AA, Leslie S, Shoji S, Matsugasumi T, Gross M, et
al: Trans-rectal ultrasound visibility of prostate lesions
identified by magnetic resonance imaging increases accuracy of
image-fusion targeted biopsies. World J Urol. 33:1669–1676. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Junker D, Schäfer G, Heidegger I, Bektic
J, Ladurner M, Jaschke W and Aigner F: Multiparametric magnetic
resonance imaging/transrectal ultrasound fusion targeted biopsy of
the prostate: Preliminary results of a prospective single-centre
study. Urol Int. 94:313–318. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shoji S, Hiraiwa S, Endo J, Hashida K,
Tomonaga T, Nakano M, Sugiyama T, Tajiri T, Terachi T and Uchida T:
Manually controlled targeted prostate biopsy with real-time fusion
imaging of multiparametric magnetic resonance imaging and
transrectal ultrasound: An early experience. Int J Urol.
22:173–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Salami SS, Ben-Levi E, Yaskiv O, Ryniker
L, Turkbey B, Kavoussi LR, Villani R and Rastinehad AR: In patients
with a previous negative prostate biopsy and a suspicious lesion on
magnetic resonance imaging, is a 12-core biopsy still necessary in
addition to a targeted biopsy? BJU Int. 115:562–570. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mozer P, Rouprêt M, Le Cossec C, Granger
B, Comperat E, de Gorski A, Cussenot O and Renard-Penna R: First
round of targeted biopsies using magnetic resonance
imaging/ultrasonography fusion compared with conventional
transrectal ultrasonography-guided biopsies for the diagnosis of
localised prostate cancer. BJU Int. 115:50–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rastinehad AR, Turkbey B, Salami SS,
Yaskiv O, George AK, Fakhoury M, Beecher K, Vira MA, Kavoussi LR,
Siegel DN, et al: Improving detection of clinically significant
prostate cancer: Magnetic resonance imaging/transrectal ultrasound
fusion guided prostate biopsy. J Urol. 191:1749–1754. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fiard G, Hohn N, Descotes JL, Rambeaud JJ,
Troccaz J and Long JA: Targeted MRI-guided prostate biopsies for
the detection of prostate cancer: Initial clinical experience with
real-time 3-dimensional transrectal ultrasound guidance and
magnetic resonance/transrectal ultrasound image fusion. Urology.
81:1372–1378. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vourganti S, Rastinehad A, Yerram NK, Nix
J, Volkin D, Hoang A, Turkbey B, Gupta GN, Kruecker J, Linehan WM,
et al: Multiparametric magnetic resonance imaging and ultrasound
fusion biopsy detect prostate cancer in patients with prior
negative transrectal ultrasound biopsies. J Urol. 188:2152–2157.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Delongchamps NB, Peyromaure M, Schull A,
Beuvon F, Bouazza N, Flam T, Zerbib M, Muradyan N, Legman P and
Cornud F: Prebiopsy magnetic resonance imaging and prostate cancer
detection: Comparison of random and targeted biopsies. J Urol.
189:493–499. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Puech P, Rouvière O, Renard-Penna R,
Villers A, Devos P, Colombel M, Bitker MO, Leroy X,
Mège-Lechevallier F, Comperat E, et al: Prostate cancer diagnosis:
Multiparametric MR-targeted biopsy with cognitive and transrectal
US-MR fusion guidance versus systematic biopsy-prospective
multicenter study. Radiology. 268:461–469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Volkin D, Turkbey B, Hoang AN,
Rais-Bahrami S, Yerram N, Walton-Diaz A, Nix JW, Wood BJ, Choyke PL
and Pinto PA: Multiparametric magnetic resonance imaging (MRI) and
subsequent MRI/ultrasonography fusion-guided biopsy increase the
detection of anteriorly located prostate cancers. BJU Int.
114:E43–E49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sonn GA, Chang E, Natarajan S, Margolis
DJ, Macairan M, Lieu P, Nix JW, Wood BJ, Choyke PL and Pinto PA:
Value of targeted prostate biopsy using magnetic
resonance-ultrasound fusion in men with prior negative biopsy and
elevated prostate-specific antigen. Eur Urol. 65:809–815. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wysock JS, Rosenkrantz AB, Huang WC,
Stifelman MD, Lepor H, Deng FM, Melamed J and Taneja SS: A
prospective, blinded comparison of magnetic resonance (MR)
imaging-ultrasound fusion and visual estimation in the performance
of MR-targeted prostate biopsy: The PROFUS trial. Eur Urol.
66:343–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuru TH, Roethke MC, Seidenader J,
Simpfendörfer T, Boxler S, Alammar K, Rieker P, Popeneciu VI, Roth
W, Pahernik S, et al: Critical evaluation of magnetic resonance
imaging targeted, transrectal ultrasound guided transperineal
fusion biopsy for detection of prostate cancer. J Urol.
190:1380–1386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baco E, Rud E, Eri LM, Moen G, Vlatkovic
L, Svindland A, Eggesbø HB and Ukimura O: A randomized controlled
trial to assess and compare the outcomes of two-core prostate
biopsy guided by fused magnetic resonance and transrectal
ultrasound images and traditional 12-core systematic biopsy. Eur
Urol. 69:149–156. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Siddiqui MM, Rais-Bahrami S, Turkbey B,
George AK, Rothwax J, Shakir N, Okoro C, Raskolnikov D, Parnes HL,
Linehan WM, et al: Comparison of MR/ultrasound fusion-guided biopsy
with ultrasound-guided biopsy for the diagnosis of prostate cancer.
JAMA. 313:390–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Borkowetz A, Platzek I, Toma M, Laniado M,
Baretton G, Froehner M, Koch R, Wirth M and Zastrow S: Comparison
of systematic transrectal biopsy to transperineal magnetic
resonance imaging/ultrasound-fusion biopsy for the diagnosis of
prostate cancer. BJU Int. 116:873–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ploussard G, Salomon L, Xylinas E, Allory
Y, Vordos D, Hoznek A, Abbou CC and de la Taille A: Pathological
findings and prostate specific antigen outcomes after radical
prostatectomy in men eligible for active surveillance - does the
risk of misclassification vary according to biopsy criteria? J
Urol. 183:539–544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dominguez-Escrig JL, McCracken SR and
Greene D: Beyond diagnosis: Evolving prostate biopsy in the era of
focal therapy. Prostate Cancer. 2011:3862072011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Valerio M, Donaldson I, Emberton M, Ehdaie
B, Hadaschik BA, Marks LS, Mozer P, Rastinehad AR and Ahmed HU:
Detection of clinically significant prostate cancer using magnetic
resonance imaging-ultrasound fusion targeted biopsy: A systematic
review. Eur Urol. 68:8–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Puech P, Potiron E, Lemaitre L, Leroy X,
Haber GP, Crouzet S, Kamoi K and Villers A: Dynamic
contrast-enhanced-magnetic resonance imaging evaluation of
intraprostatic prostate cancer: Correlation with radical
prostatectomy specimens. Urology. 74:1094–1099. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Carroll PR, Parsons JK, Andriole G,
Bahnson RR, Castle EP, Catalona WJ, Dahl DM, Davis JW, Epstein JI,
Etzioni RB, et al: NCCN Guidelines Insights: Prostate Cancer Early
Detection, Version 2.2016. J Natl Compr Canc Netw. 14:509–519.
2016.PubMed/NCBI
|