Introduction
Lung cancer remains the leading cause of
cancer-related mortality worldwide, with ~1.5 million new cases
diagnosed annually (1). Approximately
87% of lung cancer patients have non-small cell lung cancer
(NSCLC), and approximately one-third of NSCLC patients have locally
advanced stage III disease (LA-NSCLC) at the time of diagnosis
(2,3).
For the treatment of LA-NSCLC, clinical trials have demonstrated
that radiation therapy alone is associated with a 5-year survival
rate of only ~5% (4,5). Concurrent chemoradiotherapy (CCRT) was
found to result in survival improvement compared with radiation
alone (6–8) and sequential CRT (9–15) and is
currently the standard treatment for LA-NSCLC. A NSCLC
Collaborative Group meta-analysis also demonstrated that CCRT, as
compared with sequential CRT, improved the survival of patients
with LA-NSCLC (16).
However, for LA-NSCLC patients, the prognosis
following CCRT is still poor, with a median survival time of 15–18
months (17). Recently, close
attention has been paid to the addition of consolidation
chemotherapy (CCT) after CCRT for LA-NSCLC. Previous phase II
studies of CCRT followed by CCT have reported promising response
rates and survival results (18–20). In
addition, 5 randomized phase III studies were recently reported to
evaluate the survival benefit of CCT after CCRT compared with that
of CCRT alone (21–25). However, the efficacy of CCT after CCRT
in improving survival in LA-NSCLC patients remains controversial.
We therefore conducted a meta-analysis of published phase III
randomized controlled trials (RCTs) to quantitatively evaluate the
survival benefit of patients who received the two regimens.
Materials and methods
Eligibility criteria
CCRT was defined as chemotherapy administered during
radiotherapy. Radiation should be similar in both arms of the
trial. CCRT followed by CCT was defined as chemotherapy
administered after CCRT. RCTs comparing CCT after CCRT with CCRT
alone were conducted, using the Preferred Reporting Items for
Systematic Reviews and Meta-Analyses standards (26) as the basis for reporting the materials
and methods of this study. The following eligibility criteria for
this meta-analysis were set prior to collecting the articles: i)
Phase III RCTs; ii) studies involving patients with stage III
locally advanced NSCLC based upon international staging criteria
(27); iii) hazard ratios (HRs) and
confidence intervals (CIs) of the patients who received CCRT and
CCRT followed by CCT should be calculated at specific time
intervals after therapy from the survival rates in the article; iv)
the median follow-up time of the study should be ≥3 years.
Data collection
Published and unpublished trials were sought by
searching electronic databases (PubMed, Embase, Cochrane Library
and Chinese Biology Medicine) without language restriction, using
the Cochrane collaboration optimal search strategy for identifying
RCTs. This was supplemented by manual searches. Two investigators
independently searched eligible trials and discrepancies were
resolved through discussion. Non-English publications were
evaluated upon their English abstract and the translation of their
main text. Using the keywords ‘concurrent chemoradiotherapy +
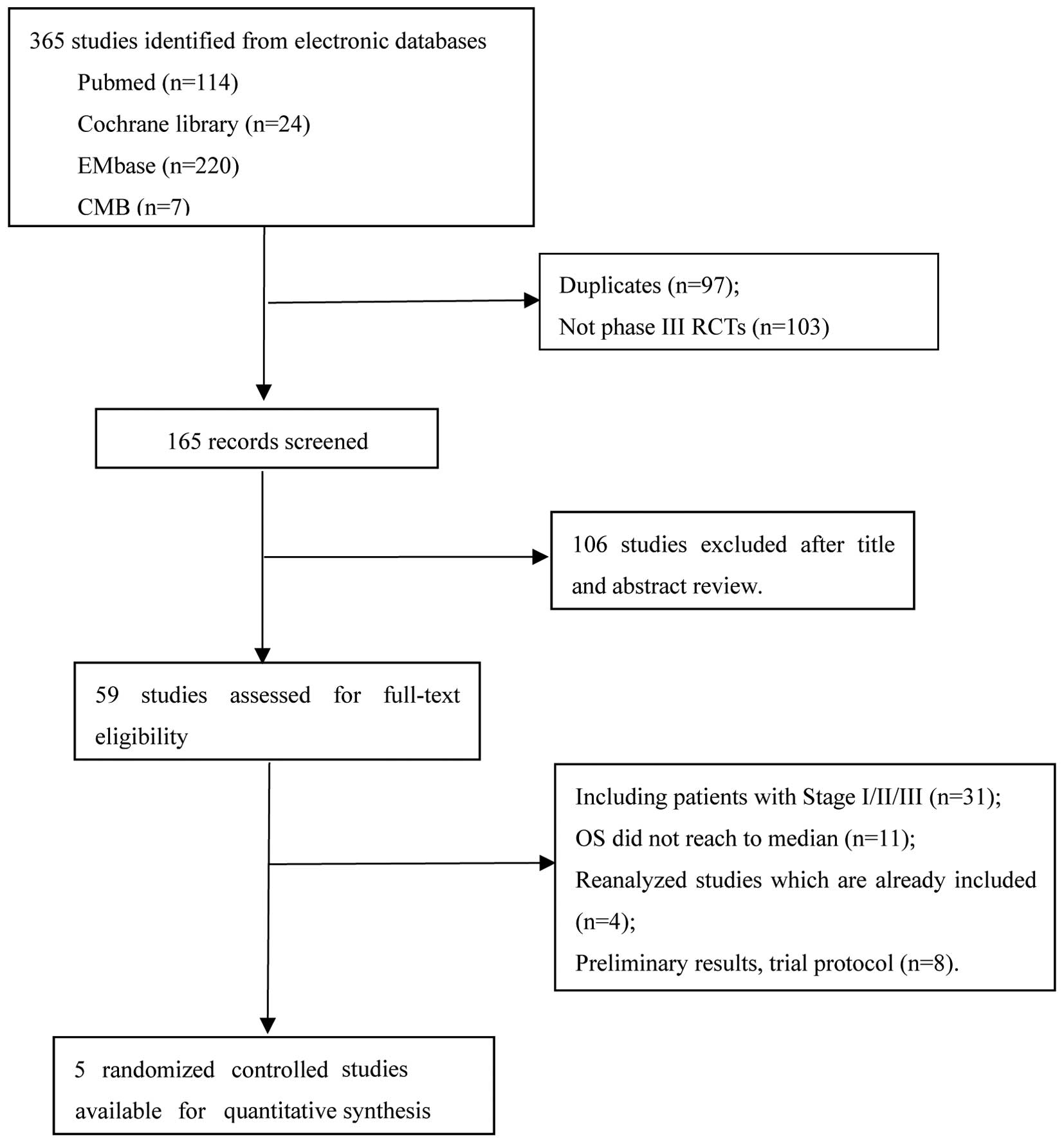
consolidation chemotherapy + non-small cell lung cancer’, 365
citations were identified in total. Unrelated articles were
excluded and, finally, only 5 studies (21–25)
fulfilled all our eligibility criteria. Study characteristics were
also recorded (period during which the study was conducted,
chemoradiotherapy regimen and median follow-up) and patient
characteristics [age, gender, cancer stage, performance status
(PS), forced expiratory volume in 1 second (FEV1) and
toxicity].
Validity assessment
Two reviewers independently evaluated the quality of
the studies, with disagreements resolved by consensus. Using the
Cochrane approach to allocation concealment, the trials were
described as having adequate, unclear, or inadequate concealment
(28). The reviewers assessed whether
there was blinding of outcome assessment and adequate description
of withdrawals (29). The adequacy of
the method of randomization was assessed as described by Jadad
et al (29). Finally, an
assessment was made as to whether the trial results used
intention-to-treat analysis (30,31). The
authors of the included studies were asked to verify the
assessments of study methodology where possible.
Statistical analysis
The primary endpoint was overall survival (OS),
which was defined as the time from randomisation until death from
any cause. The secondary endpoints were acute toxicity rates and
progression-free survival (PFS), which was defined as the time from
random assignment until first event (local or distant progression
or death from any cause). Surviving patients were censored at the
date of the last follow-up. The survival rates were derived from
the published survival curves when not provided explicitly in the
text or tables. Data extraction from the survival curves was
independently performed by two researchers, and the mean measured
values were used for the meta-analysis.
Statistical analyses for the meta-analysis were
performed with Review Manager software for Windows, version 5.3
(Cochrane Collaboration, Oxford, UK, 2014) and a pooled relative
risk was calculated with 95% CIs. Analyses were stratified by
trials. The log-rank test was used to estimate the observed and
expected number of events and associated variances were used to
calculate individual trial and overall combined odds ratios (ORs)
and their 95% CIs by the fixed-effects model. To undertake a
random-effects meta-analysis, the standard errors of the
study-specific estimates are adjusted to incorporate a measure of
the extent of variation, or heterogeneity, among the treatment
effects observed in different studies. Chi-quare (χ2)
heterogeneity tests were used to test for statistical heterogeneity
among trials. The I2 statistics were also used to assess
the proportion of variability in the results attributable to
heterogeneity across studies; I2<25%, I2
of ≥25% but <50%, and I2 ≥50% were interpreted as
indicating low-level, intermediate-level and high-level
heterogeneity, respectively (28).
Analyses by patient characteristics were performed to study the
interaction between the treatment effect and the following
characteristics: Gender, age, PS, FEV1, stage and toxicities. All
P-values were two-sided. P<0.05 was considered to indicate
statistically significant differences.
Results
Study characteristics
We identified 5 randomized phase III studies
(21–25) including 958 patients, which
investigated the survival of LA-NSCLC patients treated with CCRT
followed by CCT (Fig. 1). All 5
studies reported mature data on survival benefit and toxicity,
whereas 3 studies were reported as meeting abstracts (21,23,24). In 2
of these 3 trials (21,23), patients lacked specific OS and PFS;
thus, their survival rates were not included in our meta-analysis,
but the patient characteristics in those 2 trials are available.
Our meta-analysis on OS and PFS was only based on 3 trials with 768
patients who were randomly assigned. The analyses of patient
characteristics were based on all 5 trials and 958 patients.
Treatment regimens
Two trials (21,23) used
the same chemotherapy regimen in both arms. In 1 trial (21), induction chemotherapy with paclitaxel
200 mg/cm2 was used prior to CCRT followed by CCT.
Paclitaxel (45 mg/m2) and carboplatin (area under the
curve = 2) were used as CCRT in another study (23). Another 3 trials (22,24,25) used
cisplatin combined with one other drug (etoposide, docetaxel or
vinorelbine). All the trials used a two-dimensional radiation
technique as CCRT; the total dose was 66 Gy in 4 trials and 59.4 Gy
in 1 trial (22). In the 3 trials
(22,23,25), 3
cycles of CCT were scheduled, using the same chemotherapy regimen
as CCRT. In another 2 trials (21,24) 7 and
2 cycles of CCT were delivered accordingly. The trial
characteristics are summarized in Table
I. There was no significant difference between the two
treatments according to particular patient characteristics, such as
age, gender, PS, histology, or clinical stage, in terms of benefit
(Table II).
 | Table I.Characteristics of phase III
randomized clinical trials of concurrent chemoradiotherapy (CCRT)
with or without consolidation chemotherapy (CCT). |
Table I.
Characteristics of phase III
randomized clinical trials of concurrent chemoradiotherapy (CCRT)
with or without consolidation chemotherapy (CCT).
|
|
|
|
| CCRT with CCT |
|
|---|
|
|
|
|
|
|
|
|---|
| Study (Refs.) | Accrual years | Randomly assigned
patients, n | Median follow-up,
years | CCRT | CCT | CCRT without
CCT |
|---|
| Carter et al
(21) | – | 119 | At least 36
months | Induction
chemotherapy with PA 200 mg/m2 + C AUC = 6 every 3 weeks
for 2 cycles and then weekly PA 45 mg/m2 + C AUC = 2 for
7 weeks, with concurrent daily XRT to 66.6 Gy | PA (70
mg/m2 IV per week) for 7 weekly cycles | Induction
chemotherapy with PA 200 mg/m2 + C AUC = 6 every 3 weeks
for 2 cycles, and then weekly PA 45 mg/m2 + C AUC = 2
for 7 weeks, with concurrent daily XRT to 66.6 Gy |
| Hanna et al
(22) | 2002–2006 | 147 | 41.6 months | P 50
mg/m2 IV D1, D8, D29 and D36 and E 50 mg/m2
IV D1-5 and D29-33 concurrently with chest XRT to 59.40 Gy | D 75
mg/m2 IV every 21 days for 3 cycles | The same as
CCRT |
| Colin et al
(23) | – | 71 | At least 36
months | PA (45
mg/m2), C (AUC = 2), and XRT 60–66 Gy (5×2 Gy per
week) | 3 cycles of PA (175
mg/m2) and C (AUC = 5) D1, D22 and D43 | The same as
CCRT |
| Huber et al
(24) | 2005–2009 | 201 | At least 48
months | NVBo 50
mg/m2 D1, D8, D15 + P20 mg/m2 D1-D4 q4w/2
cycles + XRT (66 Gy/33 fractions) | NVBo 60–80
mg/m2 D1 and D8 + P 80 mg/m2 D1 q3w/2 cycles
+ BSC or BSC (non-CCT arm) | The same as
CCRT |
| Ahn et al
(25) | 2005–2011 | 420 | 50.7 months | D 20
mg/m2 IV and P 20 mg/m2 IV D1, D8, D15, D22,
D29 and D36 concurrently with chest XRT to 66.0 Gy | Three cycles of DP
(35 mg/m2 each on days 1 and 8, every 3 weeks) | The same as
CCRT |
 | Table II.Patient characteristics. |
Table II.
Patient characteristics.
|
| CCT after CCRT | CCRT |
|
|---|
|
|
|
|
|
|---|
|
Characteristics | No. | % | No. | % | P-value |
|---|
| Median age,
years | 61 | 61 | – |
|
Range | 31–86 | 33–86 | – |
| Gender
(female) | 92 | 24.3 | 82 | 21.0 | 0.27 |
| Performance
status |
| 0 | 90 | 31.9 | 95 | 33.5 | 0.72 |
|
≥1.0 | 191 | 67.7 | 190 | 66.7 | 0.79 |
| FEV1, 1 |
| 0.8 to
<2.0 | 113 | 40.0 | 93 | 32.6 | 0.07 |
|
≥2.0 | 169 | 59.9 | 192 | 67.4 | 0.07 |
| Stage |
|
IIIA | 91 | 24.1 | 102 | 26.2 | 0.51 |
|
IIIB | 286 | 75.7 | 287 | 73.6 | 0.51 |
| Toxicity |
|
Infection | 23 |
9.4 | 10 |
4.1 | 0.02 |
|
Pneumonitis | 30 | 12.2 | 11 |
4.5 |
0.003 |
|
Treatment-related death |
9 |
3.7 |
0 |
0.0 | 0.04 |
|
Esophagitis | 61 | 35.3 | 46 | 26.9 | 0.09 |
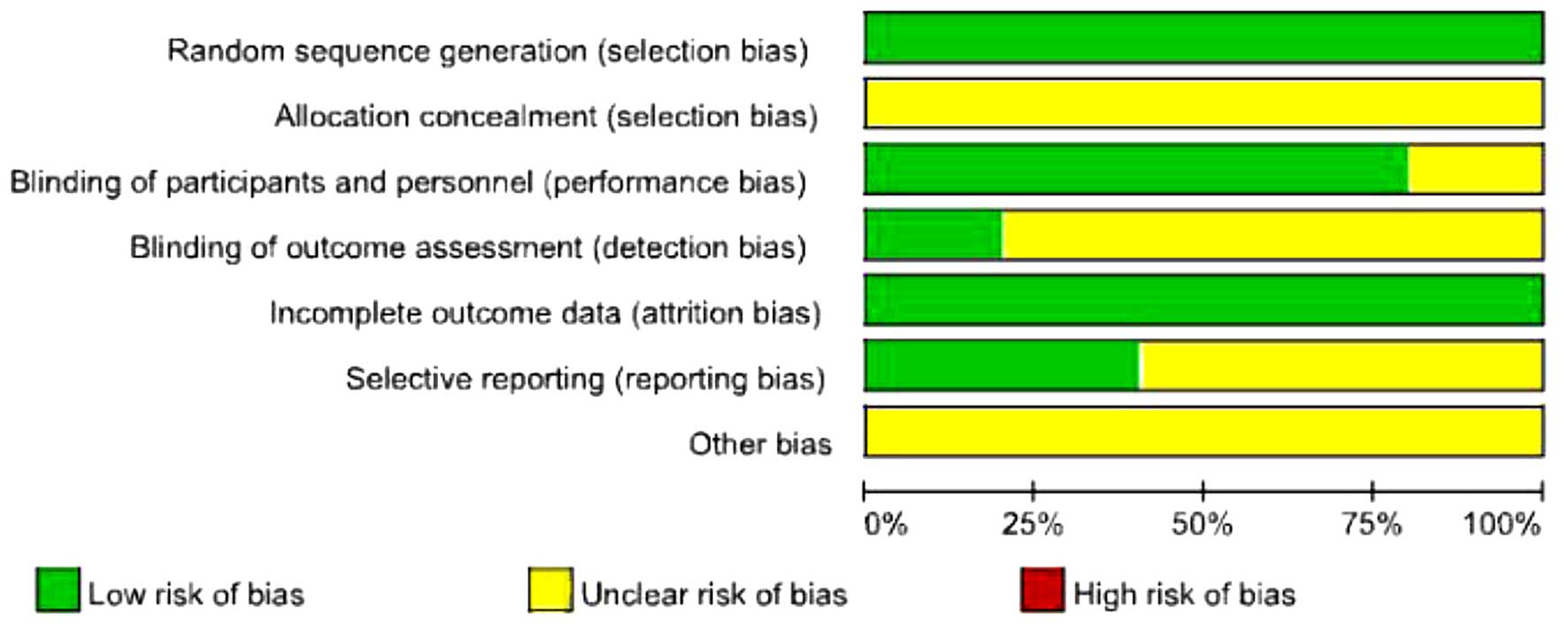
Study quality
The quality of the included trials is shown in
Table III. All the included studies
were found to have an unclear allocation concealment, but they were
conducted with a method of adequate randomization and with
intention-to-treat analysis. One trial clearly pointed out blinded
assessment of outcome (22), whereas
the remaining 4 trials did not describe the assessment method of
outcome. According to the methodological quality of 3 trials, we
reviewed the authors' judgement regarding each risk of bias item
(Fig. 2).
 | Table III.Methodological quality of included
trials. |
Table III.
Methodological quality of included
trials.
| Study (Refs.) | Allocation
concealment | Method of
randomization | Blinded assessment
of outcome | Description of
withdrawals | Intention to treat
analysis |
|---|
| Carter et al
(21) | Unclear | Adequate | None described | Yes | Yes |
| Hanna et al
(22) | Unclear | Adequate | Yes | Yes | Yes |
| Colin t al
(23) | Unclear | Adequate | None described | Yes | Yes |
| Huber et al
(24) | Unclear | Adequate | None described | Yes | Yes |
| Ahn et al
(25) | Unclear | Adequate | None described | Yes | Yes |
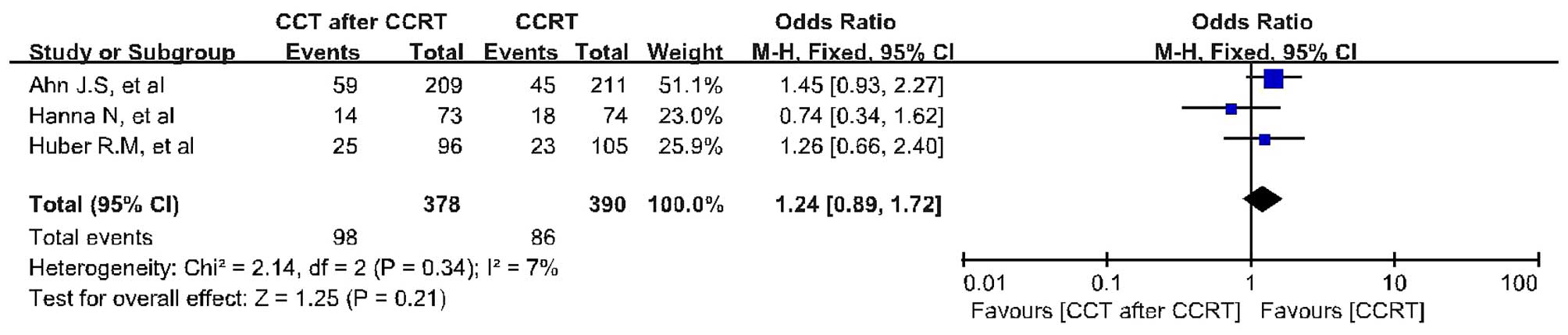
Survival analysis
The survival analysis was based on 3 trials with 768
patients. CCRT followed by CCT failed to result in significant
improvement in terms of 4-year OS (OR=1.24; 95% CI: 0.89–1.72;
P=0.21) compared with CCRT alone (Fig.
3). There was no evidence of significant statistical
heterogeneity with an I2 value of 7% (χ2 test
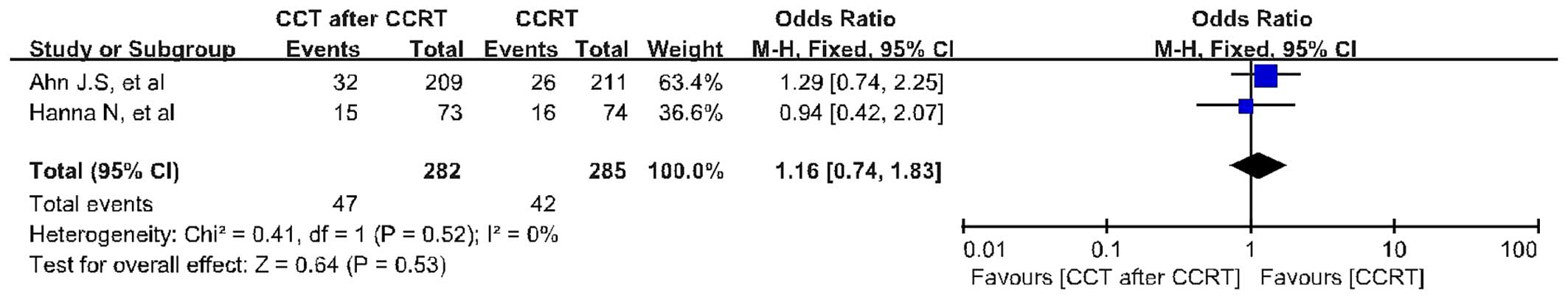
for heterogeneity P=0.34). The PFS analysis was based on 2 trials
including 567 patients. CCRT followed by CCT did not improve 3-year
PFS (OR=1.16; 95% CI: 0.74–1.83; P=0.53) compared with CCRT alone
(Fig. 4). There was no evidence of
significant statistical heterogeneity, with an I2 value
of 0% (χ2 test for heterogeneity P=0.52). Data were
available for 491 patients (51%) for infection (P=0.02), peumonitis
(P=0.003) and treatment-related death (P=0.04). Esophageal toxicity
was analyzed by only 1 available trial (25) due to sparsity of data (P=0.09)
(Table II).
Discussion
Despite the advances in the treatments for LA-NSCLC,
the multidisciplinary approach for the management of LA-NSCLC
remains controversial among clinicians. On the basis of large
clinical trials (6–15), the treatment of choice for stage III
unresectable NSCLC is CCRT. However, the main benefit of CCRT is
likely to be due to decreased locoregional progression, rather than
distant progression control and decreased acute toxicities
(16). Recently, clinical trials on
CCRT followed by CCT (18–25) or induction treatment followed by CCRT
(32–34) have became progressively more popular
in an attempt to improve distant disease control. However, there is
no clear evidence in terms of conferring survival benefits compared
with the current standard CCRT for LA-NSCLC patients. Against this
background, we conducted a meta-analysis to evaluate the efficacy
and toxicity of CCRT followed by CCT vs. CCRT alone in the
treatment of LA-NSCLC.
To the best of our knowledge, ours is the first
meta-analysis of CCRT followed by CCT compared with CCRT alone,
including 5 complete phase III RCTs. Although a pooled analysis
performed by Tsujino et al (35) demonstrated the inefficiency of CCT
after CCRT for LA-NSCLC, their subsequent letters to the editor
(36) pointed out several limitations
that may have affected their study results. First, the authors
failed to assess the heterogeneity at the individual patient level,
indicating that they did not analyze the specific characteristics
of the patients. In addition, the diversity of the CCT regimens
among trials is another important factor that may affect their
study results. Two patterns were included: Continuous CCT, which
continues the same chemotherapy as CCRT, and switch CCT, which
changes to different agents in the consolidation phase. However, in
our meta-analysis, we overcame these limitations by selecting
complete phase III RCTs with specific patient characteristics. In
addition, the trials included only investigated continuous CCT,
suggesting that our study significantly decreased publication
bias.
Our meta-analysis revealed no significant survival
benefit in terms of OS (OR=1.24; 95% CI: 0.89–1.72; P=0.21) and PFS
(OR=1.16; 95% CI: 0.74–1.83; P=0.53) for CCRT followed by CCT
compared with CCRT alone. In accordance with the results from the 5
included phase III RCTs, the difference in OS and PFS was not
significant between patients receiving CCRT followed by CCT and
those receiving CCRT alone. In addition, the OS and PFS synthesis
included 3 trials (22,24,25), which
were based on cisplatin combined with one other drug as
chemotherapeutic agents, and used almost the same radiation
technique and doses for treatment. This way, treatment
heterogeneity may be lowered to the minimum degree. Collectively,
our meta-analysis provided clinicians with highly persuasive
evidence that the survival benefit of CCT after CCRT is moderate.
Moreover, Kelly et al (37)
conducted a progressive phase III trial of maintenance gefitinib or
placebo after CCRT and docetaxel cosolidation in inoperable stage
III NSCLC. Although treatment with epidermal growth factor
receptor-tyrosine kinase inhibitors as maintenance after CCT was
delivered to the patients, gefitinib still failed to improve
distant progression and survival, suggesting that the concept of
CCT requires further investigation. However, a number of
oncologists still treat LA-NSCLC patients with CCT after CCRT. It
appears that clinicians reached a plateau in survival benefit using
the current treatment (CCRT followed by CCT as well as CCRT alone)
against stage III NSCLC.
With regards to our meta-analysis, we noted
significant differences in toxicities, such as infection (P=0.02),
pneumonitis (P=0.003) and treatment-related death (P=0.04). By
contrast, the pooled analysis (35)
indicated that no difference was observed in toxicity between the
two groups, mainly as several included studies were phase I/II
clinical trials without sufficient available toxicity data. Our
meta-analysis included all phase III RCTs with specific data, so
that we were able to analyze the differences in toxicity between
patients who received CCT after CCRT and those who received CCRT
alone. Schild et al (38)
reported that older patients experienced higher rates of grade 4
toxicity (81 vs. 62%, P=0.007), hematological toxicity (78 vs. 56%,
P=0.003) and pneumonitis (6 vs. 1%, P=0.02). Additionally, based on
the HOG LUN 01–24 phase III trial (22), Jalal et al (39) published undated survival and outcome
data that also support grade 3 and 4 toxicity noted during the
induction and consolidation phases of the trial, particularly for
patients aged ≥70 years vs. younger patients (87 vs. 73%,
respectively; P=0.02). However, KCSG-LU05-04 (25) reported a significant benefit with CCT
after CCRT in patients aged >60 years, suggesting that a more
gradual strategy may be more appropriate for the elderly
population. This results were consistent with a population-based
study from the National Cancer Institute's Surveillance,
Epidemiology and End Results database (40). There are two possible reasons for this
discrepancy: First, for CCT after CCRT, several patients were
unable to complete all the CCT cycles; thus, we could not exclude
the possibility that the patients who did complete CCT after CCRT
were aged ≥60 years. Furthermore, although the radiation was
delivered under the same conditions, cisplatin combined with weekly
docetaxel as second-line chemotherapy may be superior to the
first-line chemotherapy due to the acceptable toxicity profile. In
addition, several phase III trials and a meta-analysis demonstrated
a significant benefit in grade 3–4 neutropenia compared with
docetaxel every 3 weeks (41–44).
There were two limitations in this meta-analysis.
First, since we included published trials, our analysis may include
heterogeneous studies. For example, eligible patients were not
selected based on rigid inclusion criteria. A number of patients
who were unable to complete all the cycles of CCT after CCRT were
included in the analysis. Second, 3 abstract meetings were included
in our analysis, for which not all survival data were available;
our meta-analysis may be updated following publication of their
specific data.
On the basis of our meta-analysis, CCT after CCRT,
as compared with CCRT alone, failed to improve the OS and PFS
rates; in addition, CCT after CCRT was associated with increased
toxicity. Thus, further clinical trials are warranted to seek novel
breakthrough treatment options to improve the prognosis of patients
with LA-NSCLC.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
American Cancer Society: Cancer facts and
figures 2007. Atlanta: American Cancer Society. 2007.
|
|
3
|
Goldstraw P, Crowley J, Chansky K, Giroux
DJ, Groome PA, Rami-Porta R, Postmus PE, Rusch V and Sobin L:
International Association for the Study of Lung Cancer
International Staging Committee; Participating Institutions: The
IASLC Lung Cancer Staging Project: Proposals for the revision of
the TNM stage groupings in the forthcoming (seventh) edition of the
TNM Classification of Malignant Tumours. J Thorac Oncol. 2:706–714.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perez CA, Pajak TF, Rubin P, Simpson JR,
Mohiuddin M, Brady LW, Perez-Tamayo R and Rotman M: Long-term
observations of the patterns of failure in patients with
unresectable non-oat cell carcinoma of the lung treated with
definitive radiotherapy. Report by the Radiation Therapy Oncology
Group. Cancer. 59:1874–1881. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jonhnson DH, Einhorn LH, Bartolucci A,
Birch R, Omura G, Perez CA and Greco FA: Thoracic radiotherapy does
not prolong survival in patients with locally advanced,
unresectable non-small cell lung cancer. Ann Intern Med. 113:33–38.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dillman RO, Herndon J, Seagren SL, Eaton
WL Jr and Green MR: Improved survival in stage III non-small-cell
lung cancer: Seven-year follow-up of Cancer and Leukemia Group B
(CALGB) 8433 trial. J Natl Cancer Inst. 88:1210–1215. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schaake-Koning C, van den Bogaert W,
Dalesio O, Festen J, Hoogenhout J, van Houtte P, Kirkpatrick A,
Koolen M, Maat B, Nijs A, et al: Effects of concomitant cisplatin
and radiotherapy on inoperable non-small-cell lung cancer. N Engl J
Med. 326:524–530. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sause W, Kolesar P, Taylor S IV, Johnson
D, Livingston R, Komaki R, Emami B, Curran W Jr, Byhardt R, Dar AR
and Turrisi A III: Final results of phase III trial in regionally
advanced unresectable non-small cell lung cancer: Radiation Therapy
Oncology Group, Eastern Cooperative Oncology Group and Southwest
Oncology Group. Chest. 117:358–364. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Clamon G, Herndon J, Eaton W, Rosenman J,
Maurer LH, Cooper MR and Green MR: A feasibility study of extended
chemotherapy for locally advanced non-small cell lung cancer: A
phase II trial of Cancer and Leukemia Group B. Can Invest.
12:273–282. 1994. View Article : Google Scholar
|
|
10
|
Furuse K, Fukuoka M, Kawahara M, Nishikawa
H, Takada Y, Kudoh S, Katagami N and Ariyoshi Y: Phase III study of
concurrent versus sequential thoracic radiotherapy in combination
with mitomycin, vindesine, and cisplatin in unresectable stage III
non-small cell lung cancer. J Clin Oncol. 17:2692–2699.
1999.PubMed/NCBI
|
|
11
|
Curran WJ, Scott CB, Langer CJ, et al:
Long-term benefit is observed in a phase III comparison of
sequential versus concurrent chemo-radiation forpatients with
unresected stage III NSCLC: RTOG 9410. Proc Am Soc Clin Oncol.
22:6212003.
|
|
12
|
Ulutin HC, Güden M, Oysul K, Sürenkök S
and Pak Y: Split-course radiotherapy with or without concurrent or
sequential chemotherapy in non-small cell lung cancer. Radiat Med.
18:93–96. 2000.PubMed/NCBI
|
|
13
|
Fournel P, Robinet G, Thomas P, Souquet
PJ, Léna H, Vergnenégre A, Delhoume JY, Le Treut J, Silvani JA,
Dansin E, et al: Randomized phase III trial of sequential
chemoradiotherapy compared with concurrent chemoradiotherapy in
locally advanced non-small-cell lung cancer: Groupe
Lyon-Saint-Etienne d'Oncologie Thoracique-Groupe Francais de
Pneumo-Cance´rologie NPC 95-01 study. J Clin Oncol. 23:5910–5917.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zatloukal P, Petruzelka L, Zemanova M,
Havel L, Janku F, Judas L, Kubik A, Krepela E, Fiala P and Pecen L:
Concurrent versus sequential chemoradiotherapy with cisplatin and
vinorelbine in locally advanced non-small cell lung cancer: A
randomized study. Lung Cancer. 46:87–98. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Belderbos J, Uitterhoeve L, van Zandwijk
N, Belderbos H, Rodrigus P, van de Vaart P, Price A, van Walree N,
Legrand C, Dussenne S, et al: Randomised trial of sequential versus
concurrent chemo-radiotherapy in patients with inoperable non-small
cell lung cancer (EORTC 08972–22973). Eur J Cancer. 43:114–121.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aupérin A, Péchoux CL, Rolland E, Curran
WJ, Furuse K, Fournel P, Belderbos J, Clamon G, Ulutin HC, Paulus
R, et al: Meta-analysis of concomitant versus sequential
radiochemotherapy in locally advanced non-small-cell lung cancer. J
Clin Oncol. 28:2181–2190. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aupérin A, Le Péchoux C, Pignon JP, Koning
C, Jeremic B, Clamon G, Einhorn L, Ball D, Trovo MG, Groen HJ, et
al: Concomitant radio-chemotherapy based on platin compounds in
patients with locally advanced non-small cell lung cancer (NSCLC):
A meta-analysis of individual data from 1764 patients. Ann Oncol.
17:473–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Davies AM, Chansky K, Lau DH, Leigh BR,
Gaspar LE, Weiss GR, Wozniak AJ, Crowley JJ and Gandara DR: SWOG
S9712: Phase II study of consolidation paclitaxel after concurrent
chemoradiation in poor-risk stage III non-small-cell lung cancer:
SWOG S9712. J Clin Oncol. 24:5242–5246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Edelman MJ, Chansky K, Gaspar LE, Leigh B,
Weiss GR, Taylor SA, Crowley J, Livingston R and Gandara DR: Phase
II trial of cisplatin/etoposide and concurrent radiotherapy
followed by paclitaxel/carboplatin consolidation for limited
small-cell lung cancer: Southwest Oncology Group 9713. J Clin
Oncol. 22:127–132. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sakai H, Yoneda S, Kobayashi K, Komagata
H, Kosaihira S, Kazumoto T and Saito Y: Phase II study of bi-weekly
docetaxel and carboplatin with concurrent thoracic radiation
therapy followed by consolidation chemotherapy with docetaxel plus
carboplatin for stage III unresectable non-small cell lung cancer.
Lung Cancer. 43:195–201. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carter DL, Keller AM, Tolley RC, et al: A
randomized phase III trial of combined paclitaxel, carboplatin and
radiation therapy followed by either weekly paclitaxel or
observation in patients with stage III non-small cell lung cancer.
J Clin Oncol. 20(suppl, abstr 7076): 15s2004.
|
|
22
|
Hanna N, Neubauer M, Yiannoutsos C,
McGarry R, Arseneau J, Ansari R, Reynolds C, Govindan R, Melnyk A,
Fisher W, et al: Phase III study of cisplatin, etoposide, and
concurrent chest radiation with or without consolidation docetaxel
in patients with inoperable stage III non-small-cell lung cancer:
The Hoosier Oncology Group and US Oncology. J Clin Oncol.
26:5755–5760. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Colin P, Jovenin N, Ganem G, et al: Effect
of paclitaxel-carboplatin (PC) consolidation chemotherapy after
weekly PC concurrent chemo-radiotherapy (CCR) for patients with
locally advanced non-small cell lung cancer (LA-NSCLC): 3-year
definitive results of the B001-phase III GERCOR-study. J Clin
Oncol;. 24(suppl, abstr 7112): 18s2006.
|
|
24
|
Huber RM, Engel-Riedel W, Kollmeier J, et
al: GILT study: Oral vinorelbine (NVBo) and cisplatin (P) with
concomitant radiotherapy (RT) followed by either consolidation (C)
with NVBo plus P plus best supportive care (BSC) or BSC alone in
stage (st) III non-small cell lung cancer (NSCLC): Final results of
a phase (ph) III study. J Clin Oncol. 30:452s(suppl, abstr 7001).
2012.
|
|
25
|
Ahn JS, Ahn YC, Kim JH, Lee CG, Cho EK,
Lee KC, Chen M, Kim DW, Kim HK, Min YJ, et al: Multinational
randomized phase III trial with or without consolidation
chemotherapy using docetaxel and cisplatin after concurrent
chemoradiation in inoperable stage III non-small-cell lung cancer:
KCSG-LU05-04. J Clin Oncol. 33:2660–2666. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate health care
interventions: Explanation and elaboration. PLoS Med.
6:e10001002009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rami-Porta R, Crowley JJ and Goldstraw P:
The revised TNM staging system for lung cancer. Ann Thorac
Cardiovasc Surg. 15:4–9. 2009.PubMed/NCBI
|
|
28
|
Higgins JP and Green S: Cochrane Handbook
for Systematic Reviews of Interventions. Version 5.1.0 (updated
March 2011). The Cochrane Collaboration. 2011.simplewww.Cochrane-handbook.org
|
|
29
|
Jadad A, Moore RA, Carroll D, Jenkinson C,
Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Montori V and Guyatt GH:
Intention-to-treat principle. CMAJ. 165:1339–1341. 2001.PubMed/NCBI
|
|
31
|
Fergusson D, Aaron SD, Guyatt G and Hébert
P: Post-randomisation exclusions: The intention to treat principle
and excluding patients from analysis. BMJ. 325:652–654. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu D, Zheng X, Chen J, Liu G, Xu Y, Shen
Y, Xie L, Zhao W, Jiang G and Fan M: Induction chemotherapy with
cetuximab, vinorelbine-cisplatin followed by thoracic radiotherapy
and concurrent cetuximab, vinorelbine-cisplatin in patients with
unresectable stage III non-small cell lung cancer. Lung Cancer.
89:249–254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vokes EE, Herndon JE II, Kelley MJ,
Cicchetti MG, Ramnath N, Neill H, Atkins JN, Watson DM, Akerley W
and Green MR: Cancer and Leukemia Group B: Induction chemotherapy
followed by chemoradiotherapy compared with chemoradiotherapy alone
for regionally advanced unresectable stage III Non-small-cell lung
cancer: Cancer and Leukemia Group B. J Clin Oncol. 25:1698–1704.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Divers SG, Spencer SA, Carey D, Busby EM,
Hyatt MD and Robert F: Phase I/IIa study of cisplatin and
gemcitabine as induction chemotherapy followed by concurrent
chemoradiotherapy with gemcitabine and paclitaxel for locally
advanced non-small-cell lung cancer. J Clin Oncol. 23:6664–6673.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsujino K, Kurata T, Yamamoto S, Kawaguchi
T, Kubo A, Isa S, Hasegawa Y, Ou SH, Takada M and Ando M: Is
consolidation chemotherapy after concurrent chemo-radiotherapy
beneficial for patients with locally advanced non-small-cell lung
cancer? A pooled analysis of the literature. J Thorac Oncol.
8:1181–1189. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tsujino K, Kurata T, Kawaquchi T, Kubo A,
Takada M and Ando M: Role of consolidation chemotherapy after
concurrent chemo-radiotherapy in locally advanced non-small-cell
lung cancer. J Thorac Oncol. 9:e7–e8. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kelly K, Chansky K, Gaspar LE, Albain KS,
Jett J, Ung YC, Lau DH, Crowley JJ and Gandara DR: Phase III trial
of maintenance gefitinib or placebo after concurrent
chemoradiotherapy and docetaxel consolidation in inoperable stage
III non-small-cell lung cancer: SWOG S0023. J Clin Oncol.
26:2450–2456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schild SE, Stella PJ, Geyer SM, Bonner JA,
McGinnis WL, Mailliard JA, Brindle J, Jatoi A and Jett JR: North
Central Cancer Treatment Group: The outcome of combined-modality
therapy for stage III non-small-cell lung cancer in the elderly. J
Clin Oncol. 21:3201–3206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jalal SI, Riggs HD, Melnyk A, Richards D,
Agarwala A, Neubauer M, Ansari R, Govindan R, Bruetman D, Fisher W,
et al: Updated survival and outcomes for older adults with
inoperable stage III non-small-cell lung cancer treated with
cisplatin, etoposide, and concurrent chest radiation with or
without consolidation docetaxel: Analysis of a phase III trial from
the Hoosier Oncology Group (HOG) and US Oncology. Ann Oncol.
23:1730–1738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Davidoff AJ, Gardner JF, Seal B and
Edelman MJ: Population-based estimates of survival benefit
associated with combined modality therapy in elderly patients with
locally advanced non-small cell lung cancer. J Thorac Oncol.
6:934–941. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bria E, Cuppone F, Ciccarese M, Nisticò C,
Facciolo F, Milella M, Izzo F, Terzoli E, Cognetti F and
Giannarelli D: Weekly docetaxel as second line chemotherapy for
advanced non-small-cell lung cancer: Meta-analysis of randomized
trials. Cancer Treat Rev. 32:583–587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Camps C, Massuti B, Jiménez A, Maestu I,
Gómez RG, Isla D, González JL, Almenar D, Blasco A, Rosell R, et
al: Randomized phase III study of 3-weekly versus weekly docetaxel
in pretreated advanced non-small-cell lung cancer: A Spanish Lung
Cancer Group trial. Ann Oncol. 17:467–472. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gridelli C, Gallo C, Di Maio M, Barletta
E, Illiano A, Maione P, Salvagni S, Piantedosi FV, Palazzolo G,
Caffo O, et al: A randomised clinical trial of two docetaxel
regimens (weekly vs 3 week) in the second-line treatment of
non-small-cell lung cancer. The DISTAL 01 study. Br J Cancer.
91:1996–2004. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schuette W, Nagel S, Blankenburg T,
Lautenschlaeger C, Hans K, Schmidt EW, Dittrich I, Schweisfurth H,
von Weikersthal LF, Raghavachar A, et al: Phase III study of
second-line chemotherapy for advanced non-small-cell lung cancer
with weekly compared with 3-weekly docetaxel. J Clin Oncol.
23:8389–8395. 2005. View Article : Google Scholar : PubMed/NCBI
|