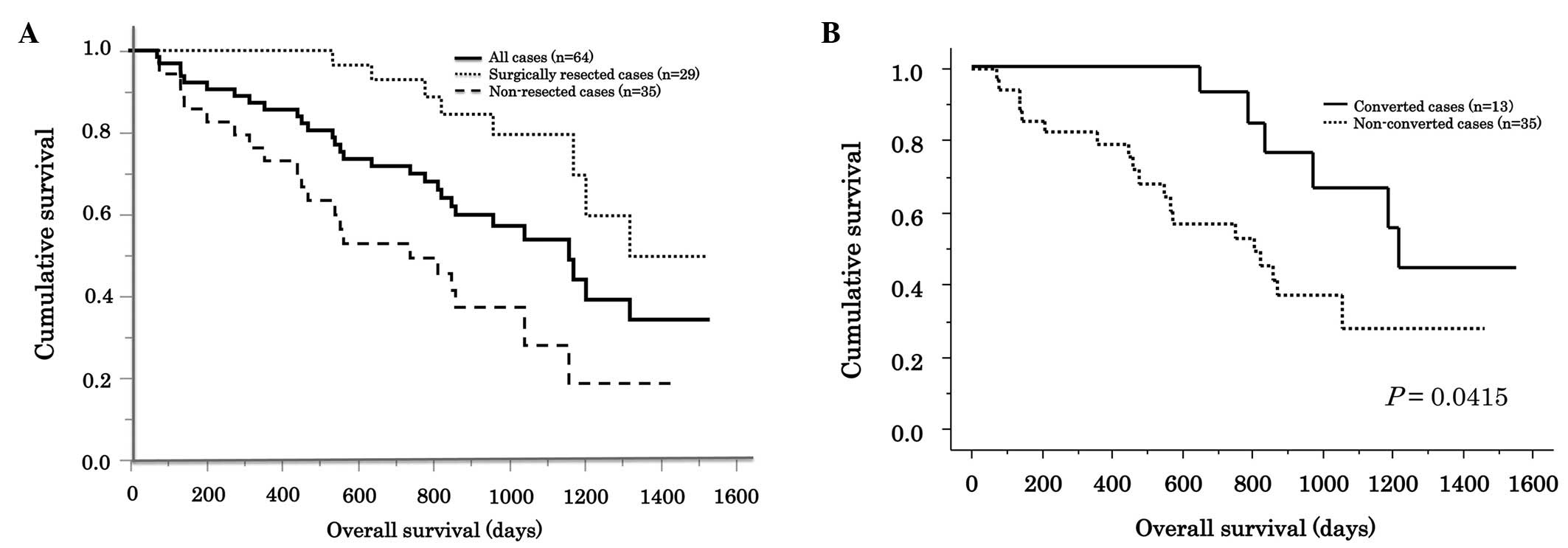
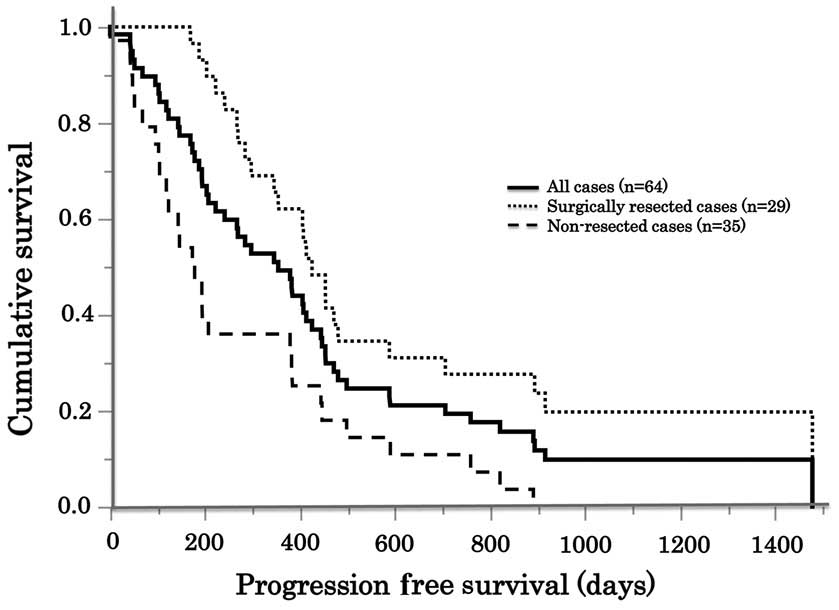
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Center MM, Jemal A and Ward E:
International trends in colorectal cancer incidence rates. Cancer
Epidemiol Biomarkers Prev. 18:1688–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Colorectal Cancer Estimated Incidence
Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx
|
|
5
|
Cassidy J, Twelves C, Van Cutsem E, Hoff
P, Bajetta E, Boyer M, Bugat R, Burger U, Garin A, Graeven U, et
al: First-line oral capecitabine therapy in metastatic colorectal
cancer: A favorable safety profile compared with intravenous
5-fluorouracil/leucovorin. Ann Oncol. 13:566–575. 2002. View Article : Google Scholar
|
|
6
|
Cunningham D, Pyrhönen S, James RD, Punt
CJ, Hickish TF, Heikkila R, Johannesen TB, Starkhammar H, Topham
CA, Awad L, et al: Randomised trial of irinotecan plus supportive
care versus supportive care alone after fluorouracil failure for
patients with metastatic colorectal cancer. Lancet. 352:1413–1418.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rougier P, Van Cutsem E, Bajetta E,
Niederle N, Possinger K, Labianca R, Navarro M, Morant R, Bleiberg
H, Wils J, et al: Randomised trial of irinotecan versus
fluorouracil by continuous infusion after fluorouracil failure in
patients with metastatic colorectal cancer. Lancet. 352:1407–1412.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saltz LB, Cox JV, Blanke C, Rosen LS,
Fehrenbacher L, Moore MJ, Maroun JA, Ackland SP, Locker PK, Pirotta
N, et al: Irinotecan plus fluorouracil and leucovorin for
metastatic colorectal cancer. Irinotecan study group. N Engl J Med.
343:905–914. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Douillard JY, Cunningham D, Roth AD,
Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J,
Alakl M, et al: Irinotecan combined with fluorouracil compared with
fluorouracil alone as first-line treatment for metastatic
colorectal cancer: A multicentre randomised trial. Lancet.
355:1041–1047. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goldberg RM, Sargent DJ, Morton RF, Fuchs
CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC and Alberts
SR: A randomized controlled trial of fluorouracil plus leucovorin,
irinotecan and oxaliplatin combinations in patients with previously
untreated metastatic colorectal cancer. J Clin Oncol. 22:23–30.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grothey A, Sargent D, Goldberg RM and
Schmoll HJ: Survival of patients with advanced colorectal cancer
improves with the availability of fluorouracil-leucovorin,
irinotecan and oxaliplatin in the course of treatment. J Clin
Oncol. 22:1209–1214. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uehara K and Nagino M: Neoadjuvant
treatment for locally advanced rectal cancer: A systematic review.
Surg Today. 2015.(Epub ahead of print).
|
|
13
|
Cunningham D, Humblet Y, Siena S, Khayat
D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype
C, et al: Cetuximab monotherapy and cetuximab plus irinotecan in
irinotecan-refractory metastatic colorectal cancer. N Engl J Med.
351:337–345. 2004. View Article : Google Scholar
|
|
14
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bokemeyer C, Bondarenko I, Makhson A,
Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G,
Stroh C, et al: Fluorouracil, leucovorin and oxaliplatin with and
without cetuximab in the first-line treatment of metastatic
colorectal cancer. J Clin Oncol. 27:663–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saeki H, Emi Y, Kumashiro R, Otsu H,
Kawano H, Ando K, Ida S, Kimura Y, Tokunaga E, Oki E, et al: Impact
of second-line and later cetuximab-containing therapy and KRAS
genotypes in patients with metastatic colorectal cancer: A
multicenter study in Japan. Surg Today. 44:1457–1464. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Folprecht G, Gruenberger T, Bechstein WO,
Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher
J, Weitz J, et al: Tumour response and secondary resectability of
colorectal liver metastases following neoadjuvant chemotherapy with
cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol.
11:38–47. 2010. View Article : Google Scholar : PubMed/NCBI
|