Introduction
Renal cell carcinoma (RCC) is highly resistant to
conventional chemotherapeutic agents (1). Previously, cytokine therapies were the
only available treatment approach for patients with metastatic RCC
(m-RCC); however, the efficacy of these treatments was low with a
median overall survival of ~1 year (2). Based on the increased knowledge of the
molecular mechanisms involved in the progression of RCC, several
types of novel molecular-targeted agents have been developed, and
their introduction into clinical practice has resulted in a marked
paradigm shift regarding therapeutic strategies for m-RCC (3).
Of several molecular-targeted agents, sunitinib, an
orally available inhibitor of multiple tyrosine kinases, exhibits
one of the strongest antitumor activities against m-RCC (4). In preclinical experimental studies,
sunitinib has been demonstrated to exert inhibitory effects on
tumor cell proliferation and angiogenesis (5). Furthermore, the significantly superior
efficacy of sunitinib over interferon-α (IFN-α) as a first-line
therapy for m-RCC has been demonstrated in a clinical setting, with
a median progression-free survival (PFS) of 11 months, compared
with 5 months compared for the IFN-α arm (6). However, several limitations are
associated with the use of sunitinib for patients with m-RCC,
including the comparatively short interval of a durable response
and the low proportion of patients showing a complete response (CR)
(7). In order to provide
individualized risk-directed therapies for m-RCC patients, it is
advantageous to identify novel markers predicting their
susceptibility to sunitinib treatment.
To date, various model systems have been developed
to predict the clinical course of m-RCC patients receiving
molecular-targeted agents (8,9). However, it may be difficult to predict
the prognosis of patients with m-RCC based on conventional
clinicopathological parameters alone, since RCC has been
characterized by unique biological features as well as
heterogeneous genetic backgrounds (10). Therefore, the present study evaluated
the expression levels of multiple potential molecular markers
involved in the Hedgehog signaling pathway, which has been
demonstrated to have an important role in the progression of a
large variety of malignant tumor types via the regulation of
numerous target genes (11), in
addition to major molecular targets of sunitinib, in radical
nephrectomy specimens from a total of 39 consecutive metastatic
clear cell RCC (m-ccRCC) patients treated with sunitinib by
immunohistochemical staining, and analyzed their association with
the outcome based on several conventional parameters.
Patients and methods
Patients
The present study included a total of 39 consecutive
patients who underwent radical nephrectomy for ccRCC and were
diagnosed with metastatic disease. These patients were subsequently
treated with sunitinib as a first-line systemic therapy between
April 2009 and March 2011 at Kobe University Hospital (Kobe,
Japan). Informed consent was obtained from each patient prior to
enrolment in the present study, and the study design was approved
by the Research Ethics Committee of Kobe University Hospital.
Treatment with sunitinib
All the patients included in the present study
initially received 50 mg sunitinib once daily in repeated 6-week
cycles, consisting of 4 weeks of treatment followed by a break of 2
weeks. Sunitinib was continuously administered until disease
progression or intolerable adverse events (AEs). In cases with
treatment-associated AEs corresponding to grade ≥3, the dose of
sunitinib was modified by initial dose reduction from 50 to 37.5
mg/day and subsequently to 25 mg/day.
Patient evaluation
As baseline evaluations, the performance status (PS)
was assessed and clinicopathological examinations were performed
based on the Karnofsky PS scale (12)
and the Union for International Cancer Control
Tumor-Nodes-Metastasis classification system (13), respectively, while risk classification
was performed using the Memorial Sloan-Kettering Cancer Center
(MSKCC) (8) and Heng's risk
classification systems (9). Prior to
the initiation of sunitinib treatment, radiological evaluations
were performed for all patients by computed tomography (CT) of the
brain, chest and abdomen as well as a radionuclide bone scan. In
general, tumor measurements were repeated by CT at least every 12
weeks after the initiation of sunitinib treatment.
Immunohistochemical staining
Immunohistochemical staining of radical nephrectomy
specimens was performed as previously described (14). In brief, formaldehyde-fixed,
paraffin-embedded tissue sections were deparaffinized, rehydrated
and incubated with 5% normal blocking serum for 20 min at room
temperature. The sections were incubated at 4°C overnight with
1:100 diluted antibodies targeting human GLI1 or GLI2 rabbit
polyclonal antibody (cat. nos. ab92611 and ab26056, respectively;
both from Abcam, Cambridge, MA, USA), cyclin D1 rabbit polyclonal
antibody (cat. no. 2922; Cell Signaling Technology, Inc., Danvers,
MA, USA), cyclin E rabbit polyclonal antibody (cat. no. sc-481;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), transforming
growth factor (TGF)-β rabbit polyclonal antibody (cat. no. ab66043;
Abcam), vascular endothelial growth factor receptor-1 (VEGFR-1)
rabbit polyclonal antibody (cat. no. ab2350; Epitomics, Burlingame,
CA, USA), VEGFR-2 rabbit monoclonal antibody (cat. no. 9698; Cell
Signaling Technology, Inc.), platelet-derived growth factor
receptor-α (PDGFR-α) rabbit polyclonal antibody or PDGFR-β rabbit
polyclonal antibody (cat. nos. sc-338 and sc-432, respectively;
both from Santa Cruz Biotechnology, Inc.), followed by incubation
with biotinylated immunoglobulin G (Vector Laboratories,
Burlingame, CA, USA). After incubation in an avidin-biotin
peroxidase complex for 30 min, the samples were exposed to
diaminobenzidine tetrahydrochloride solution (Nacalai Tesque, Inc.,
Kyoto, Japan) and counterstained with methyl green (Wako Pure
Chemical Industries, Ltd., Osaka, Japan).
Staining results were interpreted by two independent
investigators blinded to the clinicopathological findings of the
included patients. Discrepancies between results were resolved by
joint review and/or consultation with a third investigator familiar
with the immunohistochemical pathology. For each protein, the
highest immunohistochemical staining intensity was visually scored
in several fields of each section and classified as negative, weak,
moderate or strong. According to previous studies, either moderate
or strong staining intensity in >10% of tumor cells was
classified as strong expression (15,16).
Western blot analysis
In our previous study, a human RCC cell line
resistant to sunitinib (ACHN/R) was generated by culturing parental
ACHN cells (ACHN/P) in the presence of sunitinib at serially
increased doses (17). ACHN/P and
ACHN/R cells cultured for 24 h in either standard medium or medium
containing 5 µM sunitinib were lysed and equal amounts of protein
(25 µg) measured by the Bradford protein assay from lysates were
subjected to 10% of sodium dodecyl sulfate polyacrylamide gel
electrophoresis and transferred onto a nitrocellulose membrane. The
membrane was then incubated at 4°C overnight with 1:1,000 diluted
antibodies against GLI2 (Abcam) and 1:5,000 diluted β-actin (Santa
Cruz Biotechnology, Inc.), and further incubated for 30 min with
horseradish peroxide-conjugated secondary antibodies (Santa Cruz
Biotechnology, Inc.). Specific proteins were detected using an
enhanced chemiluminescence Western blot analysis system (GE
Healthcare, Little Chalfont, UK).
Statistical analysis
All statistical analyses were performed using
Statview 5.0 software (Abacus Concepts, Inc., Berkeley, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference. PFS rates were calculated using the Kaplan-Meier method
and differences were determined by the log-rank test. The Cox
proportional hazards regression model was used to assess the
prognostic significance of certain factors.
Results
Patient response to sunitinib
The clinicopathological characteristics and outcomes
of the 39 m-ccRCC patients included in the present study and the
expression of various molecular markers in their resected primary
tumors are listed in Table I.
Treatment with sunitinib achieved a CR in 1 patient, while 5
patients showed a partial response, 23 had stable disease and 10
had progressive disease. The overall response rate to sunitinib was
16.7% and the median duration of the objective response in the 6
responders was 9.4 months.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variable | Patients |
|---|
| Age, years
(range) | 61 (36–77) |
| Gender, n (%) |
|
| Male | 27 (69.2) |
|
Female | 12 (30.8) |
| Pathological tumor
stage, n (%) |
|
| pT1 | 8 (20.5) |
| pT2 | 6 (15.4) |
| pT3 | 23 (59.2) |
| pT4 | 2 (5.1) |
| Tumor grade, n
(%) |
|
| 2 | 24 (61.5) |
| 3 | 15 (38.5) |
| Microvascular
invasion, n (%) |
|
|
Negative | 6 (15.4) |
|
Positive | 33 (84.6) |
| Metastatic sites, n
(%) |
|
|
Single | 21 (53.8) |
|
Multiple | 18 (46.2) |
| MSKCC classification,
n (%) |
|
|
Favorable | 7 (17.9) |
|
Intermediate | 24 (61.5) |
| Poor | 8 (20.6) |
| Heng's risk
classification, n (%) |
|
|
Favorable | 2 (5.1) |
|
Intermediate | 13 (33.3) |
| Poor | 24 (61.6) |
| C-reactive protein
(mg/dl) (range) | 1.9
(<0.1–18.3) |
| GLI1 expression, n
(%) |
|
| Weak | 15 (38.5) |
|
Strong | 24 (61.5) |
| GLI2 expression, n
(%) |
|
| Weak | 25 (64.1) |
|
Strong | 14 (35.9) |
| Cyclin D1 expression,
n (%) |
|
| Weak | 21 (53.8) |
|
Strong | 18 (46.2) |
| Cyclin E expression,
n (%) |
|
| Weak | 18 (46.2) |
|
Strong | 21 (53.8) |
| TGF-β expression, n
(%) |
|
| Weak | 18 (46.2) |
|
Strong | 21 (53.8) |
| VEGFR-1 expression, n
(%) |
|
Weak | 17 (43.6) |
|
Strong | 22 (56.4) |
| VEGFR-2 expression,
n (%) |
|
|
Weak | 17 (43.6) |
|
Strong | 22 (56.4) |
| PDGFR-α expression,
n (%) |
|
|
Weak | 6 (15.4) |
|
Strong | 33 (84.6) |
| PDGFR-β expression,
n (%) |
|
|
Weak | 13 (33.3) |
|
Strong | 26 (66.7) |
GLI2 is an independent predictor of
PFS
During the follow-up period of 15.1 months from the
initiation of sunitinib treatment, 26 patients (66.7%) showed
disease progression and the median duration of PFS was 13.2 months.
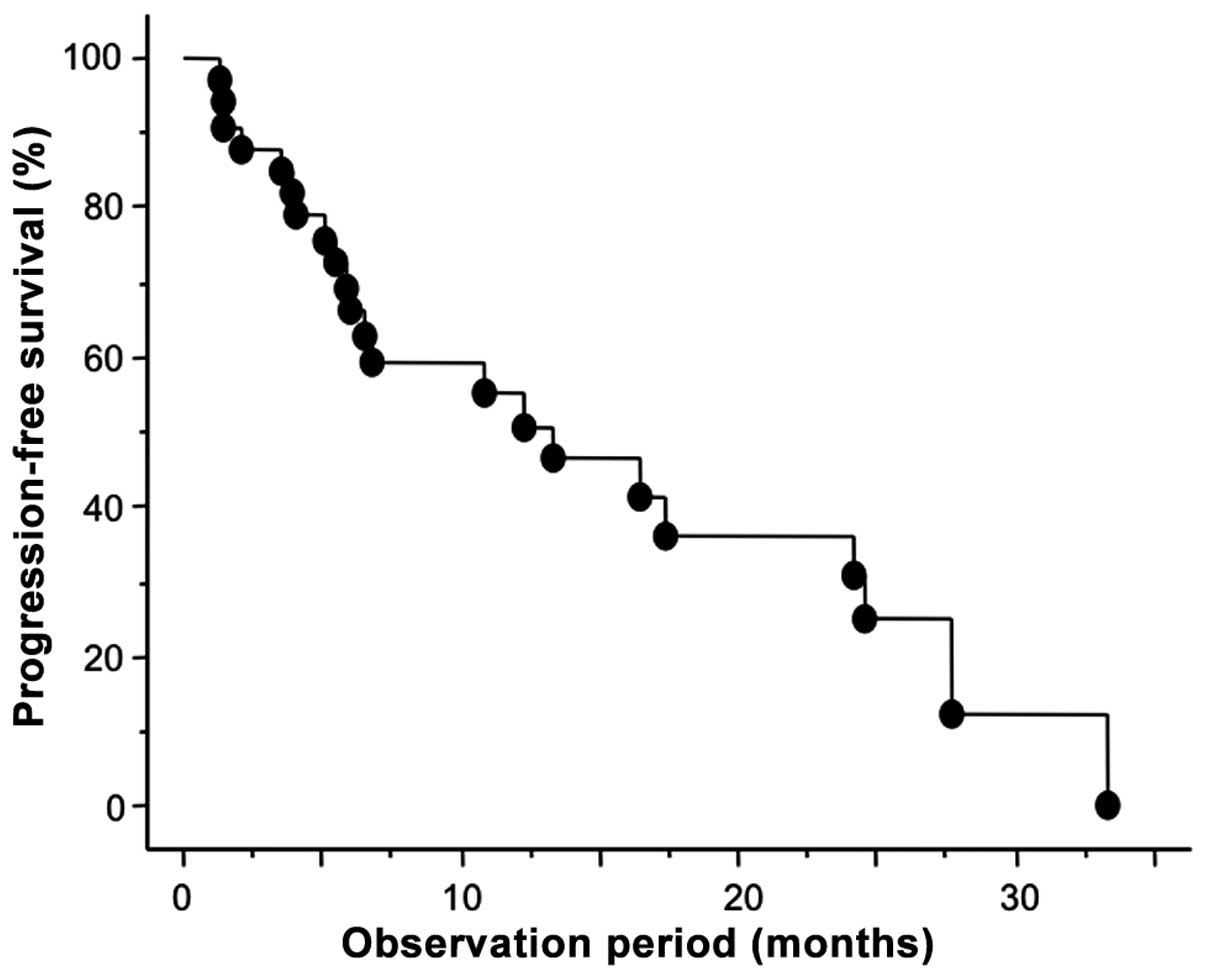
As shown in Fig. 1, the 1- and 2-year
PFS rates were 55.5 and 31.0%, respectively. To identify parameters
associated with PFS in m-ccRCC patients treated with sunitinib,
uni- and multivariate analyses were performed using the Cox
proportional hazard regression model. Of the 9 molecular markers
analyzed in the present study, the expression levels of GLI2,
VEGFR1 and VEGFR2 were identified as significant predictors of PFS
by univariate analysis (Table II).
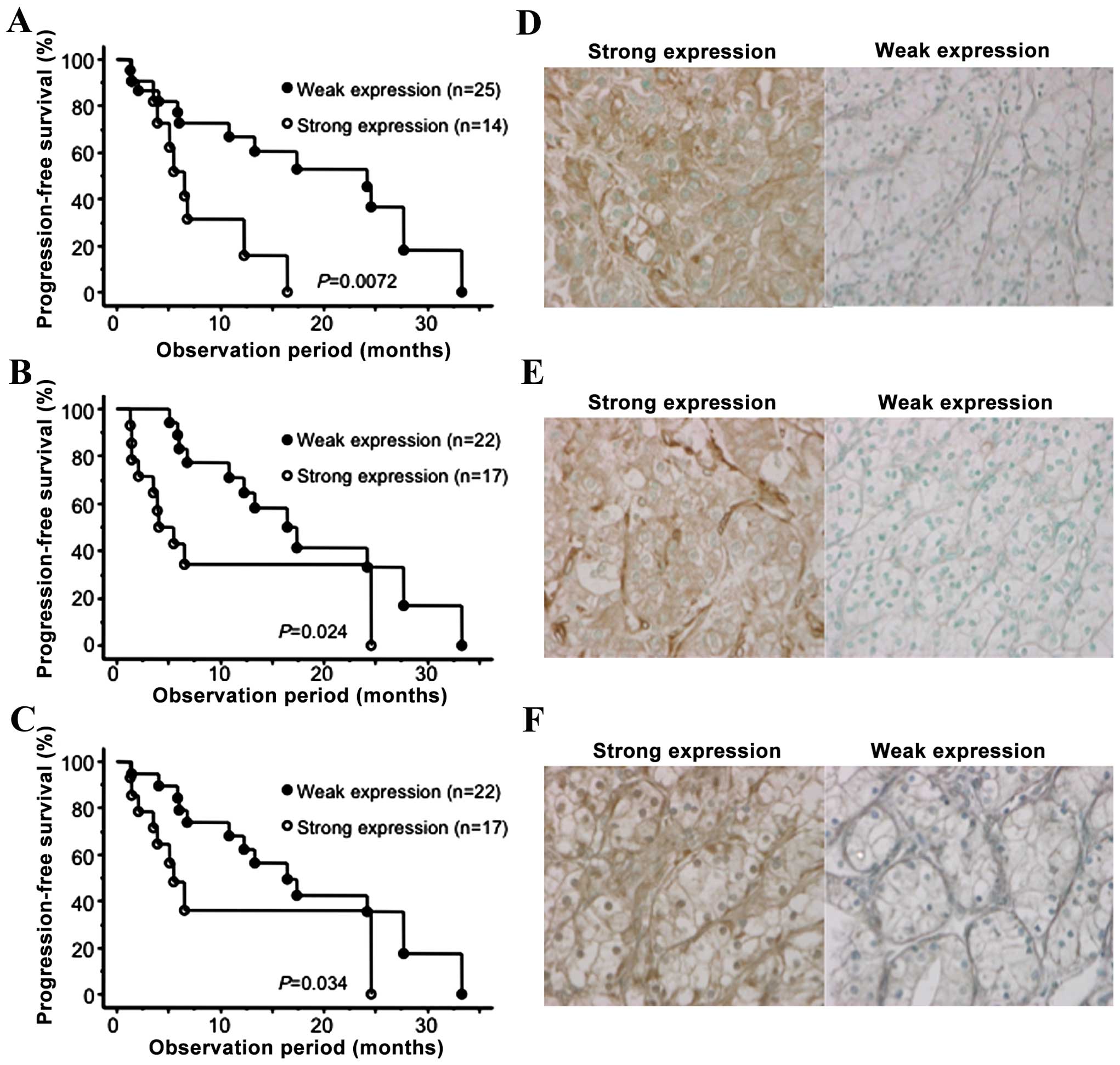
In Fig. 2, the PFS curves according
to the expression status of GLI2, VEGFR1 and VEGFR2 are presented
in addition to the representative immunohistochemical images for
the expression levels of these molecular markers. In addition to
these molecular markers, the MSKCC and baseline C-reactive protein
(CRP) levels were also significantly correlated with PFS among
several conventional factors examined. Furthermore, multivariate
analysis of these 5 significant predictors of PFS on univariate
analysis revealed that only the expression status of GLI2 was
independently correlated with the other factors included (Table II).
 | Table II.Uni- and multivariate analyses of the
association between various parameters with progression-free
survival. |
Table II.
Uni- and multivariate analyses of the
association between various parameters with progression-free
survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Parameter | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age, years (<70
vs. ≤70) | 1.50
(0.65–3.4) | 0.33 |
|
|
| Gender (male verses
female) | 1.46
(0.48–4.38) | 0.49 |
|
|
| Karnofsky
performance scale (≥80 vs. <80) | 1.17
(0.39–7.40) | 0.77 |
|
|
| Pathological tumor
stage (pT1/pT2 vs. pT3/pT4) | 2.55
(0.74–8.77) | 0.13 |
|
|
| Tumor grade (2 vs.
3) | 1.72
(0.67–4.40) | 0.25 |
|
|
| Microvascular
invasion (negative verses positive) | 1.71
(0.39–7.40) | 0.47 |
|
|
| Metastatic sites
(single verses multiple) | 1.14
(0.47–2.73) | 0.76 |
|
|
| MSKCC
classification (favorable/intermediate verses poor) | 2.57
(1.03–7.19) | 0.042 | 2.15
(0.23–19.8) | 0.49 |
| Heng's risk
classification (favorable/intermediate verses poor) | 1.27
(0.33–1.86) | 0.58 |
|
|
| Baseline C-reactive
protein (normal verses abnormal) | 2.69
(1.07–6.73) | 0.034 | 2.24
(0.68–7.43) | 0.18 |
| GLI1 (low verses
high expression) | 1.04
(0.43–2.53) | 0.91 |
|
|
| GLI2 (low verses
high expression) | 3.57
(1.33–9.52) | 0.011 | 3.86
(1.11–13.3) | 0.038 |
| Cyclin D1 (low
verses high expression) | 2.04
(0.85–4.90) | 0.10 |
|
|
| Cyclin E (low
verses high expression) | 1.12
(0.47–2.83) | 0.79 |
|
|
| TGF-β (low verses
high expression) | 1.56
(0.64–3.78) | 0.32 |
|
|
| VEGFR-1 (low verses
high expression) | 2.69
(1.10–6.58) | 0.029 | 5.17
(0.38–69.8) | 0.21 |
| VEGFR-2 (low verses
high expression) | 2.66
(1.04–6.79) | 0.040 | 3.55
(0.23–52.6) | 0.35 |
| PDGFR-α (low verses
high expression) | 1.27
(0.41–3.92) | 0.67 |
|
|
| PDGFR-β (low verses
high expression) | 1.45
(0.58–3.64) | 0.41 |
|
|
GLI2 expression is involved in the
resistance of RCC to sunitinib in vitro
To further characterize the significance of GLI2
expression in RCC tissues with regard to the efficacy of sunitinib,
changes in GLI2 expression in ACHN/P and ACHN/R cells cultured in
the absence or presence of sunitinib were examined. As shown in
Fig. 3, despite the lack of a
significant difference in GLI2 expression between ACHN/P and ACHN/R
in the absence of sunitinib, administration of sunitinib resulted
in a marked downregulation of GLI2 in ACHN/P, but not in
ACHN/R.
Discussion
Due to the results of a pivotal randomized phase-III
clinical trial (6), sunitinib is
currently used as standard first-line treatment of m-ccRCC.
Furthermore, Gore et al (18)
previously reported the acceptable efficacy and safety profiles of
sunitinib in a global expanded-access trial of patients with m-RCC.
Our recent retrospective study comprehensively evaluated the
clinical outcomes in a total of 110 Japanese patients who received
sunitinib as a first-line therapy for m-RCC and reported
encouraging findings with respect to cancer control as well as
tolerability in a clinical setting (19). However, the use of sunitinib has
several limitations. Therefore, the patients with m-RCC who are
likely to respond to sunitinib treatment should be selected prior
to its administration.
To date, various studies have indicated the
efficiency of several types of biomarker to assess the prognosis of
patients with m-RCC treated with sunitinib (20). Our previous study reported that an
imbalance between the serum levels of matrix metalloproteinase-9
and tissue expression levels of inhibitors of matrix
metalloproteinase-2 levels may serve as a novel biomarker to
predict the disease progression in patients with m-RCC undergoing
treatment with sunitinib (21).
However, to date, no such markers have been introduced into
clinical practice. A number of studies have suggested the important
role of the molecules associated with the Hedgehog signaling
pathway in the progression of a wide variety of malignant tumor
types, including RCC (11,22–24). For
example, Dormoy et al (22)
reported that inactivation of the Hedgehog pathway by a specific
inhibitor, cyclopamine, induced the regression of ccRCC tumors in
nude mice through the inhibition of tumor cell proliferation and
neo-vascularization. Furthermore, D'Amato et al (23) showed the involvement of Hedgehog
signaling in the resistance of RCC cells to molecular-targeted
agents, including sunitinib. Considering these findings, the
present study evaluated the expression levels of Hedgehog
signaling-related proteins in addition to major molecular targets
of sunitinib in primary tumor specimens in order to identify
prognostic factors that are significantly correlated with the
outcome for patients with m-ccRCC treated by sunitinib.
In the present study, a total of 39 patients with
m-ccRCC who underwent radical nephrectomy and subsequently received
sunitinib as a first-line systemic therapy were included. All 9
molecular markers examined were detectable by immunohistochemical
staining in the majority of primary ccRCC tissues. Of these, only
GLI2, VEGFR1 and VEGFR2 were identified as significant predictors
of PFS on univariate analysis. Several previous studies reported
the significance of VEGFR and its associated proteins as biomarkers
in RCC patients treated with sunitinib (25,26). For
instance, Deprimo et al (25)
reported that changes in plasma VEGF and VEGFR levels in patients
showing an objective response to sunitinib were greater compared
with those in patients with stable disease or disease progression
(25). To the best of our knowledge,
the present study was the first to report the prognostic value of a
Hedgehog signaling-related protein (GLI2) in m-ccRCC patients
receiving sunitinib.
In addition to the 3 molecular markers, the MSKCC
and baseline CRP levels were also significantly correlated with PFS
on univariate analysis. Multivariate analysis of these 5 parameters
compared with the outcome of the 39 m-ccRCC patients revealed a
significant correlation between the expression levels of GLI2 and
PFS, indicating the independent prognostic value of GLI2. GLI2 was
initially regarded as having essential functions as an effector of
the Hedgehog signaling pathway, while a number of studies have
demonstrated the ubiquitous induction of GLI2 by TGF-β, resulting
in the enhanced development of solid tumors (27–29). For
instance, the overexpression of GLI2 in mouse skin using keratin 5
promoter was shown to be sufficient to initiate basal cell
carcinomas (28), whereas GLI2
knockdown in prostate cancer cells delayed the growth of xenograft
tumors and enhanced their sensitivity to chemotherapeutic agents
(29). However, the current knowledge
on the effect of GLI2 expression on the phenotype of RCC remains
limited and the results of the present study should be confirmed in
another cohort with a prospective setting.
It is of interest to investigate whether GLI2
expression in RCC cells mediates the acquisition of a resistant
phenotype to sunitinib. Several previous studies have assessed the
mechanisms underlying the acquired resistance of RCC cells to
sunitinib (17,30,31). In
the present study GLI2 expression was maintained in
sunitinib-resistant cells, but was decreased in parental cells
following culture in the presence of sunitinib. This finding
suggested the possible involvement of GLI2 expression in the
resistance of RCC cells to sunitinib. Further experiments are
required to determine the precise mechanism of the acquired
resistance involving GLI2 regulation in RCC cells. Taken together,
it may be worthwhile examining the role of additional treatment
with an agent capable of inactivating GLI2, such as NVP-LDE225
(23), to overcome resistance to
sunitinib in RCC patients.
Of note, the present study had certain limitations.
First, it was a retrospective study and the cohort of 39
consecutive patients with m-ccRCC treated with sunitinib was not of
sufficient size to draw definitive conclusions, particularly
regarding their prognosis. Furthermore, the expression levels of
the molecular markers was assessed in radical nephrectomy specimens
only; however, it may have been suitable to also examine their
expression in metastatic tissues to obtain results more closely
reflecting the clinical outcomes. Finally, the present study
focused on only 9 selected molecules as potential biomarkers for
predicting the response of m-ccRCC to sunitinib; however, other
molecules more significantly correlated with the prognosis of
m-ccRCC patients receiving sunitinib may exist.
In conclusion, by simultaneous evaluation of several
clinicopathological parameters along with expression levels of
multiple Hedgehog signaling-related proteins as well as major
molecular targets of sunitinib, only GLI2 expression was identified
as an independent factor associated with PFS in m-ccRCC patients
treated with sunitinib. Therefore, assessment of the expression
levels of GLI2 in resected primary tumors in addition to
conventional prognostic factors may aid in the careful selection of
patients with m-ccRCC who are most likely to benefit from sunitinib
treatment.
Acknowledgements
The present study was supported by JSPS KAKENHI
(grant no. 15K20090).
References
|
1
|
Rini BI, Rathmell WK and Godley P: Renal
cell carcinoma. Curr Opin Oncol. 20:300–306. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parton M, Gore M and Eisen T: Role of
cytokine therapy in 2006 and beyond for metastatic renal cell
cancer. J Clin Oncol. 24:5584–5592. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Figlin R, Sternberg C and Wood CG: Novel
agents and approaches for advanced renal cell carcinoma. J Urol.
188:707–715. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gan HK, Seruga B and Knox JJ: Sunitinib in
solid tumors. Expert Opin Investig Drugs. 18:821–834. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mendel DB, Laird AD, Xin X, Louie SG,
Christensen JG, Li G, Schreck RE, Abrams TJ, Ngai TJ, Lee LB, et
al: In vivo antitumor activity of SU11248, a novel tyrosine kinase
inhibitor targeting vascular endothelial growth factor and
platelet-derived growth factor receptors: Determination of a
pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res.
9:327–337. 2003.PubMed/NCBI
|
|
6
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hutson TE, Figlin RA, Kuhn JG and Motzer
RJ: Targeted therapies for metastatic renal cell carcinoma: An
overview of toxicity and dosing strategies. Oncologist.
13:1084–1096. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Motzer RJ, Bacik J, Murphy BA, Russo P and
Mazumdar M: Interferon-alfa as a comparative treatment for clinical
trials of new therapies against advanced renal cell carcinoma. J
Clin Oncol. 20:289–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heng DY, Xie W, Regan MM, Warren MA,
Golshayan AR, Sahi C, Eigl BJ, Ruether JD, Cheng T, North S, et al:
Prognostic factors for overall survival in patients with metastatic
renal cell carcinoma treated with vascular endothelial growth
factor-targeted agents: Results from a large, multicenter study. J
Clin Oncol. 27:5794–5792. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su D, Singer EA and Srinivasan R:
Molecular pathways in renal cell carcinoma: Recent advances in
genetics and molecular biology. Curr Opin Oncol. 27:217–223. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ruch JM and Kim EJ: Hedgehog signaling
pathway and cancer therapeutics: Progress to date. Drugs.
73:613–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karnofsky DA, Abelmann WH, Craver LF and
Burchenal JH: The use of nitrogen mustards in the palliative
treatment of carcinoma. With particular reference to bronchogenic
carcinoma. Cancer. 1:634–656. 1948. View Article : Google Scholar
|
|
13
|
Greene FL, Page DL, Fleming ID, Fritz A,
Balch CM, Haller DG and Morrow M: AJCC Cancer Staging Manual (6th).
Springer. New York, NY: 2002. View Article : Google Scholar
|
|
14
|
Kususda Y, Miyake H, Gleave ME and
Fujisawa M: Clusterin inhibition using OGX-011 synergistically
enhances antitumour activity of sorafenib in a human renal cell
carcinoma model. Br J Cancer. 106:1945–1952. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miyake H, Muramaki M, Kurahashi T,
Takenaka A and Fujisawa M: Expression of potential molecular
markers in prostate cancer: Correlation with clinicopathological
outcomes in patients undergoing radical prostatectomy. Urol Oncol.
28:145–151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Wu T, Lu J, Cao Y, Song N, Yang T,
Dong R, Yang Y, Zang L, Du X and Wang S: Immunohistochemical
evidence of the prognostic value of hedgehog pathway components in
primary gallbladder carcinoma. Surg Today. 42:770–775. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sakai I, Miyake H and Fujisawa M: Acquired
resistance to sunitinib in human renal cell carcinoma cells is
mediated by constitutive activation of signal transduction pathways
associated with tumour cell proliferation. BJU Int. 112:E211–E220.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gore ME, Szczylik C, Porta C, Bracarda S,
Bjarnason GA, Oudard S, Hariharan S, Lee SH, Haanen J, Castellano
D, et al: Safety and efficacy of sunitinib for metastatic
renal-cell carcinoma: An expanded-access trial. Lancet Oncol.
10:757–763. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miyake H, Miyazaki A, Harada K and
Fujisawa M: Assessment of efficacy, safety and quality of life of
110 patients treated with sunitinib as first-line therapy for
metastatic renal cell carcinoma: Experience in real-world clinical
practice in Japan. Med Oncol. 31:9782014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuasa T, Takahashi S, Hatake K, Yonese J
and Fukui I: Biomarkers to predict response to sunitinib therapy
and prognosis in metastatic renal cell cancer. Cancer Sci.
102:1949–1957. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miyake H, Nishikawa M, Tei H, Furukawa J,
Harada K and Fujisawa M: Significance of circulating matrix
metalloproteinase-9 to tissue inhibitor of metalloproteinases-2
ratio as a predictor of disease progression in patients with
metastatic renal cell carcinoma receiving sunitinib. Urol Oncol.
32:584–588. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dormoy V, Danilin S, Lindner V, Thomas L,
Rothhut S, Coquard C, Helwig JJ, Jacqmin D, Lang H and Massfelder
T: The sonic hedgehog signaling pathway is reactivated in human
renal cell carcinoma and plays orchestral role in tumor growth. Mol
Cancer. 8:1232009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
D'Amato C, Rosa R, Marciano R, D'Amato V,
Formisano L, Nappi L, Raimondo L, Di Mauro C, Servetto A, Fulciniti
F, et al: Inhibition of Hedgehog signalling by NVP-LDE225
(Erismodegib) interferes with growth and invasion of human renal
cell carcinoma cells. Br J Cancer. 111:1168–1179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou J, Wu K, Gao D, Zhu G, Wu D, Wang X,
Chen Y, Du Y, Song W, Ma Z, et al: Reciprocal regulation of
hypoxia-inducible factor 2α and GLI1 expression associated with the
radioresistance of renal cell carcinoma. Int J Radiat Oncol Biol
Phys. 90:942–951. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deprimo SE, Bello CL, Smeraglia J, Baum
CM, Spinella D, Rini BI, Michaelson MD, Motzer RJ, et al:
Circulating protein biomarkers of pharmacodynamic activity of
sunitinib in patients with metastatic renal cell carcinoma:
Modulation of VEGF and VEGF-related proteins. J Transl Med.
5:322007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kontovinis LF, Papazisis KT, Touplikioti
P, Andreadis C, Mouratidou D and Kortsaris AH: Sunitinib treatment
for patients with clear-cell metastatic renal cell carcinoma:
Clinical outcomes and plasma angiogenesis markers. BMC Cancer.
9:822009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Javelaud D, Alexaki VI, Dennler S,
Mohammad KS, Guise TA and Mauviel A: TGF-β/SMAD/GLI2 signaling axis
in cancer progression and metastasis. Cancer Res. 71:5606–5610.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki
H, Hui CC and Dlugosz AA: Basal cell carcinomas in mice
overexpressing Gli2 in skin. Nat Genet. 24:216–217. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Narita S, So A, Ettinger S, Hayashi N,
Muramaki M, Fazli L, Kim Y and Gleave ME: GLI2 knockdown using an
antisense oligonucleotide induces apoptosis and chemosensitizes
cells to paclitaxel in androgen-independent prostate cancer. Clin
Cancer Res. 14:5769–5777. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang D, Ding Y, Zhou M, Rini BI, Petillo
D, Qian CN, Kahnoski R, Futreal PA, Furge KA and Teh BT:
Interleukin-8 mediates resistance to antiangiogenic agent sunitinib
in renal cell carcinoma. Cancer Res. 70:1063–1071. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hammers HJ, Verheul HM, Salumbides B,
Sharma R, Rudek M, Jaspers J, Shah P, Ellis L, Shen L, Paesante S,
et al: Reversible epithelial to mesenchymal transition and acquired
resistance to sunitinib in patients with renal cell carcinoma:
Evidence from a xenograft study. Mol Cancer Ther. 9:1525–1535.
2010. View Article : Google Scholar : PubMed/NCBI
|