Introduction
Invasive mucinous adenocarcinoma (IMA), formerly
referred to as mucinous bronchioloalveolar carcinoma, is distinct
from non-mucinous adenocarcinoma and has been re-classified as a
variant of invasive adenocarcinoma in the International Association
for the Study of Lung Cancer/American Thoracic Society/European
Respiratory Society lung adenocarcinoma classification system, due
to its distinct clinical, radiological and pathological
characteristics, as well as its distinct genetic background
(frequent KRAS mutations) (1). The typical computed tomography (CT)
findings in IMA include pneumonic consolidation, ground-glass
opacity and nodules; by contrast, cystic lesions are rare. We
herein describe a rare case of IMA presenting as a large cystic
lesion.
Case report
A 75-year-old man was admitted to the Hino Municipal
Hospital due to a productive cough and mucous sputum lasting for 6
months. The patient had no previous illness or history of cigarette
smoking. A chest radiograph obtained on admission revealed
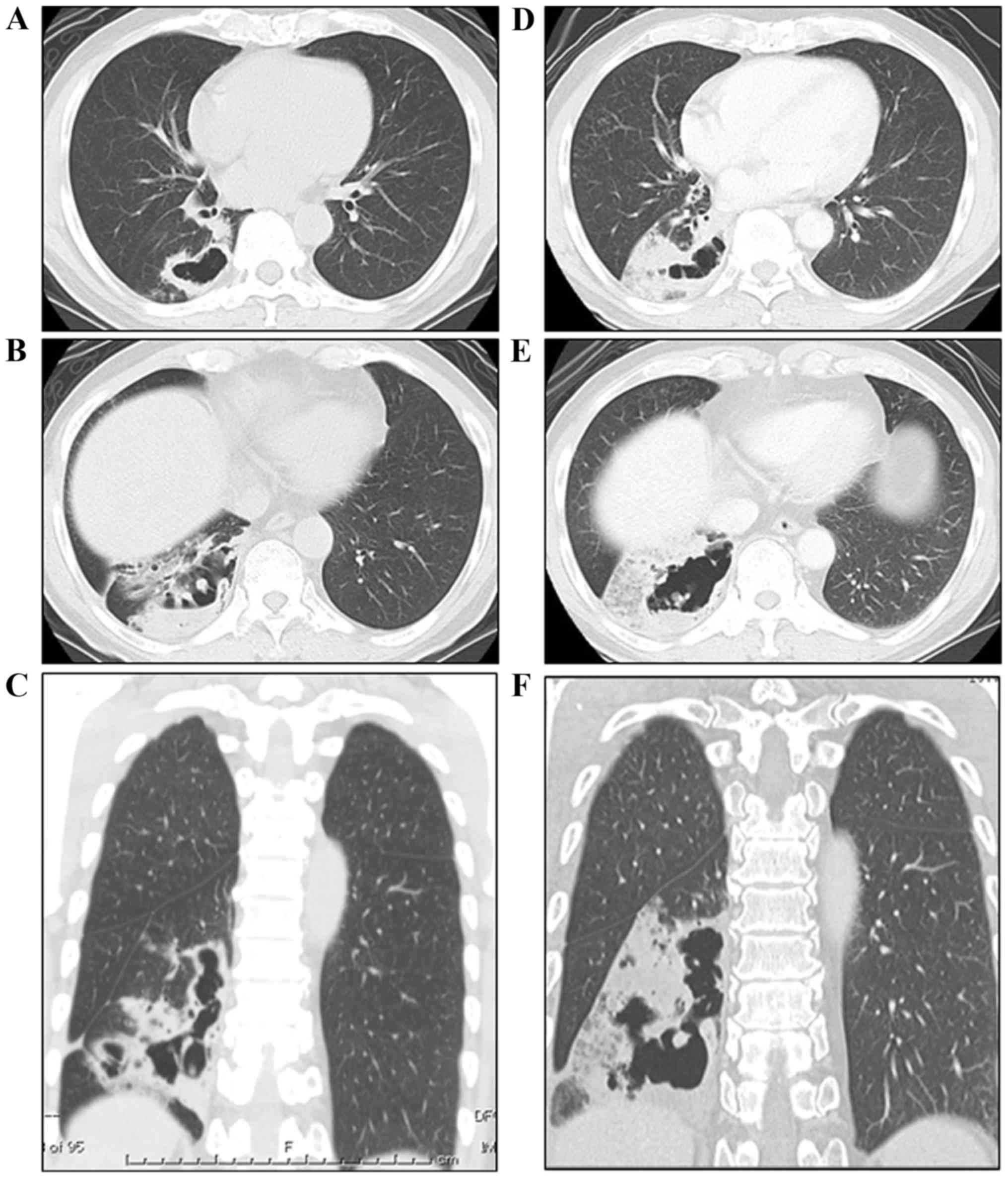
infiltration in the lower lobe of the right lung. A chest CT scan
revealed an irregularly shaped cystic lesion comprising thin walls
in the lower lobe of the right lung (9 cm in maximum diameter) and
a mixed, dense and ground-glass opacity occupying a portion of the
pericystic parenchyma (Fig. 1A, C and
E). The tissue specimens obtained during bronchoscopy revealed
non-specific inflammatory findings, without any neoplasm or
vasculitis. The tissue culture was positive for Streptococcus
anginosus. As treatment with antibiotics was ineffective, the
CT scan was repeated one and a half months later, showing a rapid
increase in the size of the cyst and progression of the parenchymal
opacity in the lower lobe of the patient's right lung (Fig. 1B, D and F). In addition, centriacinar
small nodules, some of which were cystic, appeared in the right
middle and left upper lobes. The second-chance bronchoscopy
revealed an atypical epithelium with abundant cytoplasmic mucin in
the lung specimen obtained from the right lower lobe. The patient
was diagnosed with clinical stage IV (T4N0M1a) IMA of the lung and
was transferred to the Tachikawa Hospital to undergo right lower
lobectomy prior to anticancer chemotherapy, due to major concerns
of complications, such as infection, hemorrhage, or rupture of the
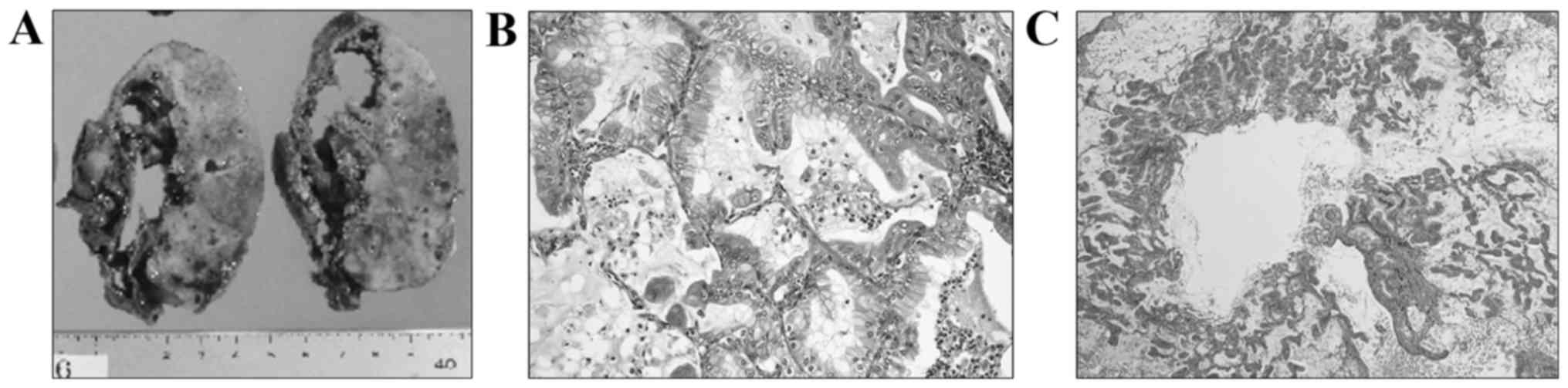
large cyst. The macroscopic findings of the resected right lower
lobe included a sizeable cystic lesion (9×6×2 cm) and a
white-colored solid nodule adjacent to the cyst (Fig. 2A). Microscopically, the large cystic
space, situated just beneath the fibrous thickened visceral pleura,
was lined by non-neoplastic bronchial epithelial cells on the
pleural side, and it directly faced the pulmonary parenchyma with
carcinoma invasion on the other side. In the pericystic parenchyma,
atypical columnar epithelium with intracytoplasmic mucin
proliferated chiefly in a lepidic growth pattern, occasionally
invading the interstitium (Fig. 2B).
In addition, emphysema-like airspace enlargement was observed at
the opening portion of the bronchiole, with mucinous material
filling the conductive airways in a portion of the
carcinoma-invading areas (Fig. 2C).
Tissue necrosis was not evident in the pericystic area or inside
the cyst.
Following thoracic surgery, the patient was
readmitted to Hino Municipal Hospital for further treatment. The
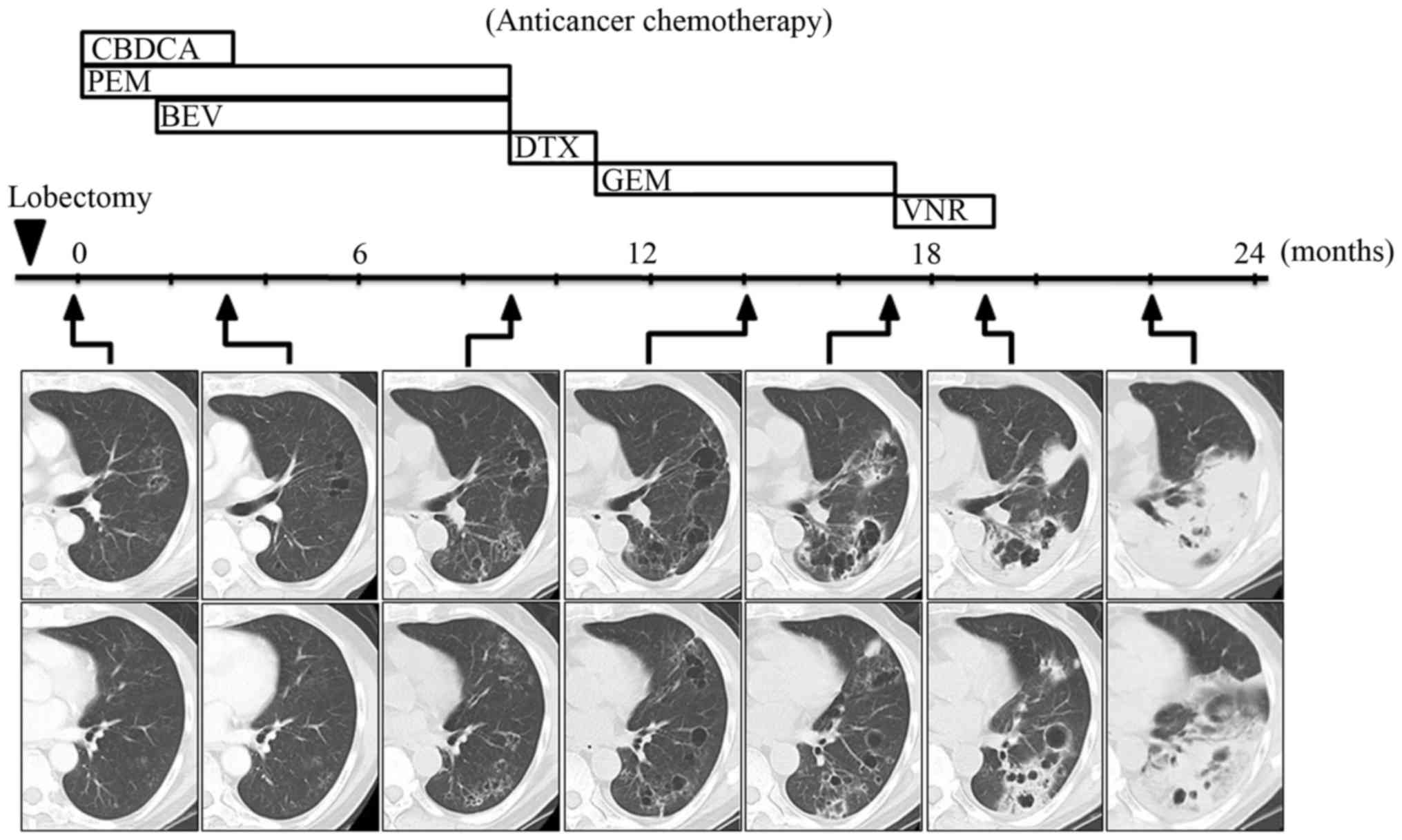
timeline of the anticancer chemotherapy and the CT scans are shown
in Fig. 3. As the cancer lesion
contained little solid material at an earlier time point, the
response to each chemotherapy regimen could not be determined. The
chemotherapy regimen, comprising carboplatin and pemetrexed, was
initiated 1 month after the surgery, and bevacizumab was added from
the third cycle onwards. Following completion of 4 cycles of this
regimen, the overall cyst size was not affected, but the cyst wall
was found to be thinner at 3 months (Fig. 3). Continuous maintenance therapy with
pemetrexed and bevacizumab was conducted until disease progression,
which occurred after 7 cycles of the regimen, with enlargement of
the pre-existing cystic lesions and the appearance of new lesions
after 9 months (Fig. 3). The
right-sided pleural effusion also increased (not shown), and the
presence of adenocarcinoma cells was cytologically confirmed.
Second- and third-line chemotherapy were sequentially administered
using docetaxel and gemcitabine for 3 and 6 cycles, respectively.
Chemotherapy with gemcitabine achieved stable disease for 3 months,
but the cyst gradually increased in size and, interestingly,
parenchymal opacity appeared in the area surrounding the cyst at 17
months (Fig. 3). Thereafter, the
opacity expanded rapidly over 19 months, despite continued
administration of vinorelbine (Fig.
3). The radiological changes observed on CT scans between 17
and 19 months resembled what had been initially observed 2 months
prior to diagnosis (Fig. 1),
suggesting that the primary lesion developed through a similar
formative process. The anticancer chemotherapy ceased at 19 months.
The lung infiltration had significantly worsened at 22 months
(Fig. 3) and the patient succumbed
to respiratory failure 2 months later.
Discussion
IMA is a rare variant of invasive adenocarcinoma,
accounting for 2.2–3.9% of resected adenocarcinoma cases (2–4). A
pictorial review of IMA elucidated the typical CT findings, such as
consolidation, ground-glass opacity and nodules (5–8). The
bubble-like lucency of pseudocavitation formation in the
consolidation or nodule was also a major finding, which was
observed in 40–78% of IMA cases (5–8). By
contrast, a thin-walled cystic lesion, particularly a large cyst,
is rare. In the present case, the initial CT findings demonstrated
a mixture of two major components: An irregular-shaped large cystic
lesion with thin walls, and a parenchymal opacity next to the cyst.
It was unclear at diagnosis whether the cyst had developed
primarily or formed secondarily in the pre-existing consolidation;
however, the longitudinal CT observations throughout the entire
course of the disease strongly suggested that the cystic lesion was
the primary lesion.
A thin-walled cyst in the lungs is generally
associated with benign disease, and it may delay the diagnosis of
cancer (9,10). Guo et al reported 15 cases of
lung cancer presenting as a thin-walled cyst and reviewed the
literature describing similar cases over the last two decades
(11); they found adenocarcinoma to
be the most common histological type (11 of 15 original cases and
11 of 19 reviewed cases) in a study including two cases of IMA
(12). Prichard et al
reported two original cases of IMA with a thin-walled cavity and
found that none of the 10 previously reported cases of cystic lung
cancer was histologically diagnosed as IMA in a review of the
literature between 1947 and 1979 (12). Taken together, these studies suggest
that cystic lesions are a rare radiological finding as a primary
lesion in IMA.
We comprehensively reviewed the literature from
another angle to identify the cases of IMA presenting as a
large-size cyst or cavity (>5 cm in maximum diameter). A search
was conducted through PubMed and the Ichu-shi website (Japanese
database) and a total of 5 cases were identified, which are
summarized in Table I. The ages of
the patients ranged between 28 and 82 years, and the genders were
equally distributed. All the subjects except 1 were never-smokers.
The primary lesions had cysts or cavities of 6–11 cm in maximum
diameter and were predominantly located in the right lower lobe (5
of 6 cases). In 1 case, the primary lesion was in the left lower
lobe. The lower lobe predominance was not previously observed in
the review series of all IMA cases (17 of 36 cases) (5), or of lung cancers (regardless of the
histological type) presenting as thin-walled cysts (6 of 15 cases)
(11). By contrast, Manning et
al reported a trend toward lower lobe predominance only in
cases of IMA with solitary nodules (4 of 5 cases) (17). The cyst or cavity was described as
‘thin-walled’ in the primary lesion or the metastases in the
majority of the cases (5 of 6 cases). The patients underwent
surgical resection in all cases, and lobectomy was selected in 5
cases. Surgical resection was not curative in the present case;
similarly, postoperative recurrence was also observed in two
previous studies (12,14). Of note, the patient described herein,
as well as one of the two previously described patient (14), exhibited similar radiological changes
on postoperative CT scans: i) The primary lesion was a large cystic
lesion with peripheral opacity in the right lower lobe; ii) the
transbronchial metastases were originally observed as small
nodules, which enlarged and transformed into a multiloculated large
cyst with thin walls; and iii) peripheral opacity was not observed
until late in the clinical course.
 | Table I.Invasive mucinous adenocarcinoma
presenting as large cysts or cavities. |
Table I.
Invasive mucinous adenocarcinoma
presenting as large cysts or cavities.
| Age, years | Gender | Smoking status | Cavity size, cm
(location) | Radiological
description | Treatment | Mechanism | Refs. |
|---|
| 41 | F | Never | 6 (LLL) | Solitary thin-walled
cyst | Pneumonectomy | ND | (12) |
|
|
|
|
| Adjacent ill-defined
mass | (recurrence
→BSC) |
|
|
| 28 | M | Current | 11 (RLL) | Solitary thin-walled
cavity | Lobectomy | ND | (13) |
| 82 | M | Never | 10 (RLL) | Mass lesion with
thick-walled cavity | Lobectomy | Check-bulb | (14) |
|
|
|
|
| Thin-walled cystic
cavity (metastases) | (recurrence
→BSC) | Necrosis |
|
|
| 68 | F | Never | 7 (RLL) | Cavity surrounded by
an irregular wall | Lobectomy | Neutrophilic
infiltrate | (15) |
| 51 | F | Never | 6.5 (RLL) | Solitary thin-walled
cavity | Lobectomy | Check-bulb | (16) |
| 75 | M | Never | 9 (RLL) | Solitary thin-walled
large cyst with opacity | Lobectomy | Check-bulb | Present case |
|
|
|
|
| Thin-walled
multiloculated cyst (metastases) | Chemotherapy |
|
|
Several mechanisms through which lung cancer forms a
cavity or cyst have been proposed, such as ischemic tumor necrosis,
check-bulb, or destruction of alveoli by a direct invasion,
proteolysis, or excessive mucus retention. As IMA is generally
considered to be less invasive and exhibits reduced necrotic
tendency, the check-bulb mechanism is the most likely explanation
of the formative process of the thin-walled cyst. As shown in
Table I, the check-bulb mechanism
was hypothesized to underlie cyst formation in 3 of the 4 cases,
although necrosis and neutrophil infiltration were also suspected.
In the present case, the check-bulb mechanism was indirectly
supported by the histological findings of emphysema-like airspace
enlargement and by the presence of an intraluminal mucous plug in
the conductive airway. In the present case, as well as in another
report (14), the air-filled cyst
increased in size and its walls appeared thinner on postoperative
CT scans, strongly suggesting the check-bulb mechanism. A recent
study by Nakamura et al suggested a pivotal role of mucus
retention in cavity formation in lung adenocarcinoma (18); however, cases of non-mucinous lung
adenocarcinoma were also reported to display thin-walled large
cysts (11,19,20).
The explanation as to why the parenchymal opacity
rapidly appeared late in the clinical course also remains unknown.
Two possible explanations include accelerated tumor growth caused
by a decreased effectiveness of the antitumor chemotherapy, or
transformation of the tumor to another histological type. As
discussed above, similar radiological changes were also observed in
patients with IMA receiving only supportive care (14). In addition, the pathological findings
observed in the pericystic lesion in our case showed a typical
histology for IMA, growing in a lepidic pattern without
transformation to another histological type.
In summary, we presented a rare case of IMA
presenting as a large cyst with pericystic consolidation. This case
was not unique, but it revealed the formative process of these rare
radiological findings. Clinicians should be aware of thin-walled
cystic lesions as they may represent an unusual radiological
manifestation of IMA.
Acknowledgements
The authors would like to thank Editage (www.editage.jp) for the English language editing.
Glossary
Abbreviations
Abbreviations:
|
IMA
|
invasive mucinous adenocarcinoma
|
|
CT
|
computed tomography
|
References
|
1
|
Travis WD, Brambilla E, Noguchi M,
Nicholson AG, Geisinger KR, Yatabe Y, Beer DG, Powell CA, Riely GJ,
Van Schil PE, et al: International association for the study of
lung cancer/american thoracic society/european respiratory society
international multidisciplinary classification of lung
adenocarcinoma. J Thorac Oncol. 6:244–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoshizawa A, Sumiyoshi S, Sonobe M,
Kobayashi M, Fujimoto M, Kawakami F, Tsuruyama T, Travis WD, Date H
and Haga H: Validation of the IASLC/ATS/ERS lung adenocarcinoma
classification for prognosis and association with EGFR and KRAS
gene mutations: Analysis of 440 Japanese patients. J Thorac Oncol.
8:52–61. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yanagawa N, Shiono S, Abiko M, Ogata SY,
Sato T and Tamura G: The correlation of the international
association for the study of lung cancer (IASLC)/American Thoracic
Society (ATS)/European Respiratory Society (ERS) classification
with prognosis and EGFR mutation in lung adenocarcinoma. Ann Thorac
Surg. 98:453–458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mansuet-Lupo A, Bobbio A, Blons H, Becht
E, Ouakrim H, Didelot A, Charpentier MC, Bain S, Marmey B, Bonjour
P, et al: The new histologic classification of lung primary
adenocarcinoma subtypes is a reliable prognostic marker and
identifies tumors with different mutation status: The experience of
a French cohort. Chest. 146:633–643. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akira M, Atagi S, Kawahara M, Iuchi K and
Johkoh T: High-resolution CT findings of diffuse bronchioloalveolar
carcinoma in 38 patients. AJR Am J Roentgenol. 173:1623–1629. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aquino SL, Chiles C and Halford P:
Distinction of consolidative bronchioloalveolar carcinoma from
pneumonia: Do CT criteria work? AJR Am J Roentgenol. 171:359–363.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patsios D, Roberts HC, Paul NS, Chung T,
Herman SJ, Pereira A and Weisbrod G: Pictorial review of the many
faces of bronchioloalveolar cell carcinoma. Br J Radiol.
80:1015–1023. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim TH, Kim SJ, Ryu YH, Chung SY, Seo JS,
Kim YJ, Choi BW, Lee SH and Cho SH: Differential CT features of
infectious pneumonia versus bronchioloalveolar carcinoma (BAC)
mimicking pneumonia. Eur Radiol. 16:1763–1768. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wigh R and Gilmore FR: Solitary pulmonary
necrosis; a comparison of neoplastic and inflammatory conditions.
Radiology. 56:708–717. 1951. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Woodring JH, Fried AM and Chuang VP:
Solitary cavities of the lung: Diagnostic implications of cavity
wall thickness. AJR Am J Roentgenol. 135:1269–1271. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo J, Liang C, Sun Y, Zhou N, Liu Y and
Chu X: Lung cancer presenting as thin-walled cysts: An analysis of
15 cases and review of literature. Asia Pac J Clin Oncol.
12:e105–e112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Prichard MG, Brown PJ and Sterrett GF:
Bronchioloalveolar carcinoma arising in longstanding lung cysts.
Thorax. 39:545–549. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
De Jong PM, Busscher DL and Bakker W:
Bronchioloalveolar carcinoma presenting as a thin walled cavity in
a young man. Thorax. 44:230–231. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Isobe K, Hata Y, Iwata M, Ishida F,
Kaburaki K, Gocho K, Kobayashi M, Sakaguchi S, Satou D, Sano G, et
al: An autopsied case of mucinous bronchioloalveolar carcinoma
associated with multiple thin-walled cavities. Nihon Kokyuki Gakkai
Zasshi. 47:512–517. 2009.(In Japanese). PubMed/NCBI
|
|
15
|
Tekeuchi Y, Kuwahara O, Tani Y, Ohta M,
Obunai S and Hanada M: A case of the bronchiolo-alveolar cell
carcinoma presenting multiple cavities and synchronous gastric
cancer. Jpn J Lung Cancer. 32:397–402. 1992. View Article : Google Scholar
|
|
16
|
Kataoka K, Nakamura I, Sumiyoshi H,
Fujiwara1 T, Matsuura M and Seno N: A case of bronchioloalveolar
carcinoma with a thin-walled cavity associated with high uptake of
18F-fluorodeoxyglucose on positron emission tomography. Jpn J Lung
Cancer. 48:861–865. 2008. View Article : Google Scholar
|
|
17
|
Manning JT Jr, Spjut HJ and Tschen JA:
Bronchioloalveolar carcinoma: The significance of two
histopathologic types. Cancer. 54:525–534. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura S: CT Findings of pneumonic
adenocarcinoma: Comparison between invasive mucinous adenocarcinoma
and nonmucinous adenocarcinoma. GJMR-D. 14:Version 1.0. 2014.
|
|
19
|
Matsushima H, Oda T, Hasejima N, Kou E,
Kadoyama C and Takezawa S: Pulmonary adenocarcinoma with
multiloculated cystic change. Nihon Kokyuki Gakkai Zasshi.
45:556–559. 2007.(In Japanese). PubMed/NCBI
|
|
20
|
Yoshida T, Harada T, Fuke S, Konishi J,
Yamazaki K, Kaji M, Morikawa T, Ota S, Itoh T, Dosaka-Akita H and
Nishimura M: Lung adenocarcinoma presenting with enlarged and
multiloculated cystic lesions over 2 years. Respir Care.
49:1522–1524. 2004.PubMed/NCBI
|