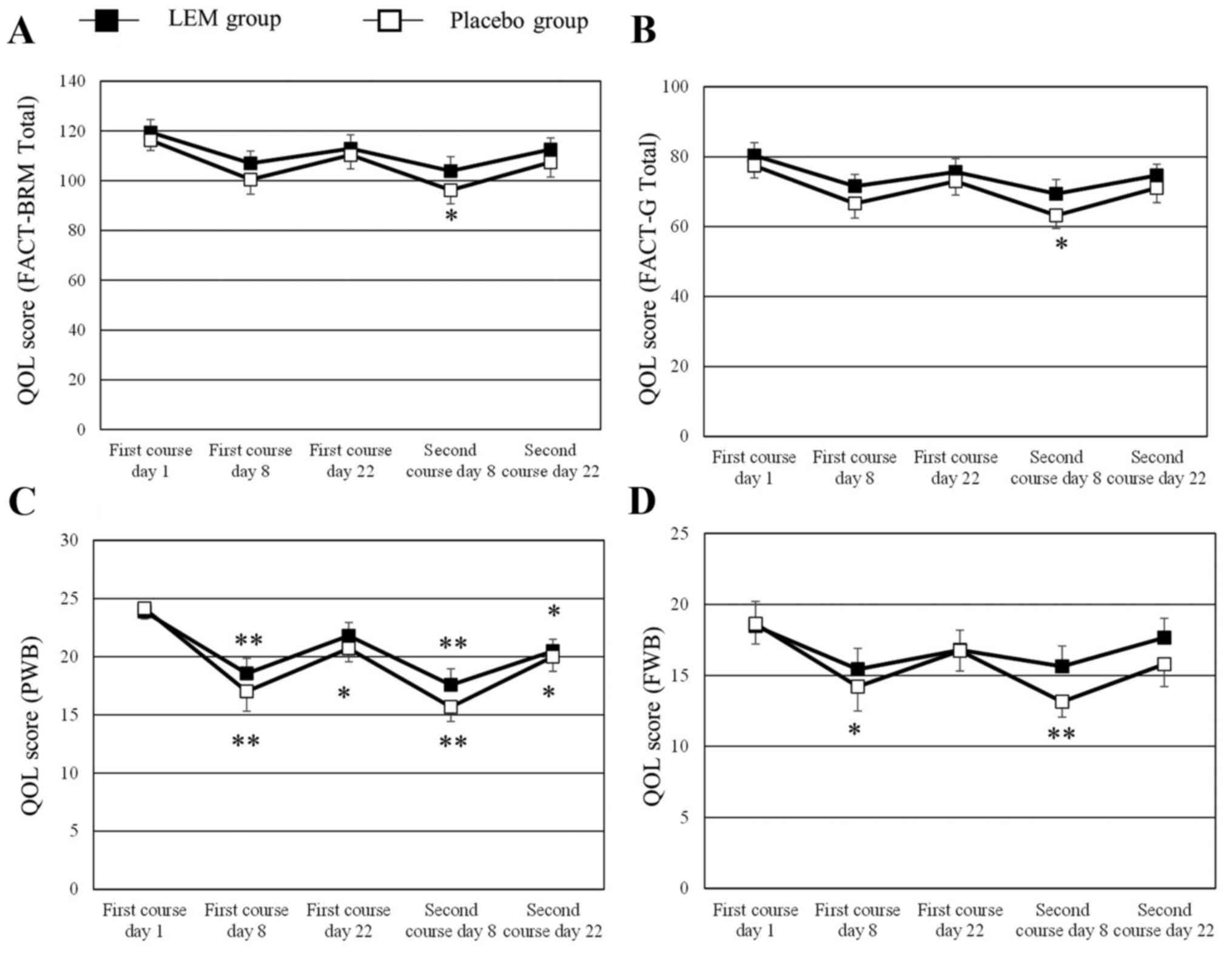
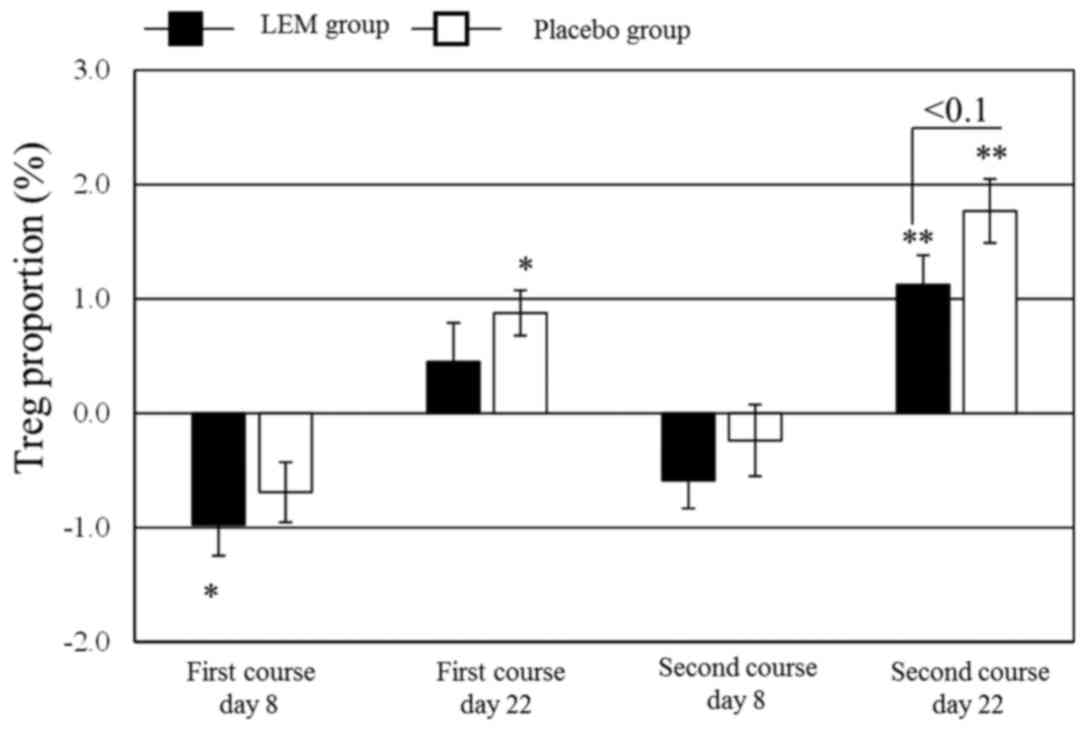
|
1
|
Group (EBCTCG): Effects of chemotherapy
and hormonal therapy for early breast cancer on recurrence and
15-year survival: An overview of the randomised trials. Lancet.
365:1687–1717. 2005. View Article : Google Scholar
|
|
2
|
French Adjuvant Study Group: Benefit of a
high-dose epirubicin regimen in adjuvant chemotherapy for
node-positive breast cancer patients with poor prognostic factors:
5-year follow-up results of French Adjuvant Study Group 05
randomized trial. J Clin Oncol. 19:602–611. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Turner N, Biganzoli L and Di Leo A:
Continued value of adjuvant anthracyclines as treatment for early
breast cancer. Lancet Oncol. 16:e362–e369. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Riccardi A, Brugnatelli S, Giordano M,
Danova M, Pugliese P, Tinelli C, Klersy C, Richetti A, Fava S,
Nastasi G, et al: Myeloprotective effect of early primary
granulocyte-colony stimulating factor during six courses of
intensified 5-fluorouracil, epirubicin and cyclophosphamide
(120FEC) chemotherapy for advanced breast cancer. Cooperative group
of study and treatment of breast cancer. Tumori. 84:540–546.
1998.PubMed/NCBI
|
|
5
|
Baltali E, Günel N, Onat DA, Atahan IL,
Akçali Z, Büyukünal E and Firat D: Neoadjuvant chemotherapy in
locally advanced breast cancer: A preliminary report. Turkish
oncology study group. Tumori. 85:483–487. 1999.PubMed/NCBI
|
|
6
|
Takeuchi H, Saeki T, Aiba K, Tamura K,
Aogi K, Eguchi K, Okita K, Kagami Y, Tanaka R, Nakagawa K, et al:
Japanese society of clinical oncology clinical practice guidelines
2010 for antiemesis in oncology: Executive summary. Int J Clin
Oncol. 21:1–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohzawa H, Miki A, Hozumi Y, Miyazaki C,
Sagara Y, Tanaka Y, Shiba S, Joutoku H, Sakuragi M, Takehara M, et
al: Comparison between the antiemetic effects of palonosetron and
granisetron in breast cancer patients treated with
anthracycline-based regimens. Oncol Lett. 9:119–124.
2015.PubMed/NCBI
|
|
8
|
Rapoport BL, Jordan K, Boice JA, Taylor A,
Brown C, Hardwick JS, Carides A, Webb T and Schmoll HJ: Aprepitant
for the prevention of chemotherapy-induced nausea and vomiting
associated with a broad range of moderately emetogenic
chemotherapies and tumor types: A randomized, double-blind study.
Support Care Cancer. 18:423–431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berger AM, Lockhart K and Agrawal S:
Variability of patterns of fatigue and quality of life over time
based on different breast cancer adjuvant chemotherapy regimens.
Oncol Nurs Forum. 36:563–570. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kornblith AB, Lan L, Archer L, Partridge
A, Kimmick G, Hudis C, Winer E, Casey R, Bennett S, Cohen HJ and
Muss HB: Quality of life of older patients with early-stage breast
cancer receiving adjuvant chemotherapy: A companion study to cancer
and leukemia group B 49907. J Clin Oncol. 29:1022–1028. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Martín M, Lluch A, Seguí MA, Ruiz A, Ramos
M, Adrover E, Rodriguez-Lescure A, Grosse R, Calvo L,
Fernandez-Chacón C, et al: Toxicity and health-related quality of
life in breast cancer patients receiving adjuvant docetaxel,
doxorubicin, cyclophosphamide (TAC) or 5-fluorouracil, doxorubicin
and cyclophosphamide (FAC): Impact of adding primary prophylactic
granulocyte-colony stimulating factor to the TAC regimen. Ann
Oncol. 17:1205–1212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Apetoh L, Ghiringhelli F, Tesniere A,
Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E,
Saulnier P, et al: Toll-like receptor 4-dependent contribution of
the immune system to anticancer chemotherapy and radiotherapy. Nat
Med. 13:1050–1059. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mozaffari F, Lindemalm C, Choudhury A,
Granstam-Björneklett H, Helander I, Lekander M, Mikaelsson E,
Nilsson B, Ojutkangas ML, Osterborg A, et al: NK-cell and T-cell
functions in patients with breast cancer: effects of surgery and
adjuvant chemo- and radiotherapy. Br J Cancer. 97:105–111. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawanishi T, Ikeda-Dantsuji Y and Nagayama
A: Effects of two basidiomycete species on interleukin 1 and
interleukin 2 production by macrophage and T cell lines.
Immunobiology. 215:516–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sugano N, Hibino Y, Choji Y and Maeda H:
Anticarcinogenic actions of water-soluble and alcohol-insoluble
fractions from culture medium of Lentinus edodes mycelia. Cancer
Lett. 17:109–114. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sugano N, Choji Y, Hibino Y, Yasumura S
and Maeda H: Anticarcinogenic action of an alcohol-insoluble
fraction (LAP1) from culture medium of Lentinus edodes mycelia.
Cancer Lett. 27:1–6. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu M, Li J, Kong F, Lin J and Gao Y:
Induction of immunomodulating cytokines by a new
polysaccharide-peptide complex from culture mycelia of Lentinus
edodes. Immunopharmacology. 40:187–198. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tanaka K, Matsui Y, Ishikawa S, Kawanishi
T and Harada M: Oral ingestion of Lentinula edodes mycelia extract
can restore the antitumor T cell response of mice inoculated with
colon-26 cells into the subserosal space of the cecum. Oncol Rep.
27:325–332. 2012.PubMed/NCBI
|
|
19
|
Tanaka K, Ishikawa S, Matsui Y, Tamesada
M, Harashima N and Harada M: Oral ingestion of Lentinula edodes
mycelia extract inhibits B16 melanoma growth via mitigation of
regulatory T cell-mediated immunosuppression. Cancer Sci.
102:516–521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamaguchi Y, Miyahara E and Hihara J:
Efficacy and safety of orally administered Lentinula edodes mycelia
extract for patients undergoing cancer chemotherapy: A pilot study.
Am J Chin Med. 39:451–459. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okuno K and Uno K: Efficacy of orally
administered Lentinula edodes mycelia extract for advanced
gastrointestinal cancer patients undergoing cancer chemotherapy: A
pilot study. Asian Pac J Cancer Prev. 12:1671–1674. 2011.PubMed/NCBI
|
|
22
|
Suzuki N, Takimoto Y, Suzuki R, Arai T,
Uebaba K, Nakai M, Strong JM and Tokuda H: Efficacy of oral
administration of Lentinula edodes mycelia extract for breast
cancer patients undergoing postoperative hormone therapy. Asian Pac
J Cancer Prev. 14:3469–3472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagashima Y, Maeda N, Yamamoto S, Yoshino
S and Oka M: Evaluation of host quality of life and immune function
in breast cancer patients treated with combination of adjuvant
chemotherapy and oral administration of Lentinula edodes mycelia
extract. Onco Targets Ther. 6:853–859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kurihara M, Shimizu H, Tsuboi K, Kobayashi
K, Murakami M, Eguchi K and Shimozuma K: Development of quality of
life questionnaire in Japan: Quality of life assessment of cancer
patients receiving chemotherapy. Psychooncology. 8:355–363. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bacik J, Mazumdar M, Murphy BA, Fairclough
DL, Eremenco S, Mariani T, Motzer RJ and Cella D: The functional
assessment of cancer therapy-BRM (FACT-BRM): A new tool for the
assessment of quality of life in patients treated with biologic
response modifiers. Qual Life Res. 13:137–154. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmidt ME, Wiskemann J, Armbrust P,
Schneeweiss A, Ulrich CM and Steindorf K: Effects of resistance
exercise on fatigue and quality of life in breast cancer patients
undergoing adjuvant chemotherapy: A randomized controlled trial.
Int J Cancer. 137:471–480. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mozaffari F, Lindemalm C, Choudhury A,
Granstam-Björneklett H, Lekander M, Nilsson B, Ojutkangas ML,
Osterborg A, Bergkvist L and Mellstedt H: Systemic immune effects
of adjuvant chemotherapy with 5-fluorouracil, epirubicin and
cyclophosphamide and/or radiotherapy in breast cancer: A
longitudinal study. Cancer Immunol Immunother. 58:111–120. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sato E, Olson SH, Ahn J, Bundy B,
Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone
C, et al: Intraepithelial CD8+ tumor-infiltrating
lymphocytes and a high CD8+/regulatory T cell ratio are
associated with favorable prognosis in ovarian cancer. Proc Natl
Acad Sci USA. 102:18538–18543. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamaguchi T and Sakaguchi S: Regulatory T
cells in immune surveillance and treatment of cancer. Semin Cancer
Biol. 16:115–123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, et al: Specific recruitment of regulatory T cells in
ovarian carcinoma fosters immune privilege and predicts reduced
survival. Nat Med. 10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tanigawa K, Ito Y, Sakai M and Kobayashi
Y: Evaluation of quality of life and immune function in cancer
patients receiving combined immunotherapy and oral administration
of lentinula edodes mycelia extract. Gan To Kagaku Ryoho.
39:1779–1781. 2012.(In Japanese). PubMed/NCBI
|
|
32
|
Maeda K, Hazama S, Tokuno K, Kan S, Maeda
Y, Watanabe Y, Kamei R, Shindo Y, Maeda N, Yoshimura K, et al:
Impact of chemotherapy for colorectal cancer on regulatory T-cells
and tumor immunity. Anticancer Res. 31:4569–4574. 2011.PubMed/NCBI
|
|
33
|
Bates GJ, Fox SB, Han C, Leek RD, Garcia
JF, Harris AL and Banham AH: Quantification of regulatory T cells
enables the identification of high-risk breast cancer patients and
those at risk of late relapse. J Clin Oncol. 24:5373–5380. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ercolini AM, Ladle BH, Manning EA,
Pfannenstiel LW, Armstrong TD, Machiels JP, Bieler JG, Emens LA,
Reilly RT and Jaffee EM: Recruitment of latent pools of
high-avidity CD8(+) T cells to the antitumor immune response. J Exp
Med. 201:1591–1602. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu S, Foulkes WD, Leung S, Gao D, Lau S,
Kos Z and Nielsen TO: Prognostic significance of FOXP3+
tumor-infiltrating lymphocytes in breast cancer depends on estrogen
receptor and human epidermal growth factor receptor-2 expression
status and concurrent cytotoxic T-cell infiltration. Breast Cancer
Res. 16:4322014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aruga T, Suzuki E, Saji S, Horiguchi S,
Horiguchi K, Sekine S, Kitagawa D, Funata N, Toi M, Sugihara K and
Kuroi K: A low number of tumor-infiltrating FOXP3-positive cells
during primary systemic chemotherapy correlates with favorable
anti-tumor response in patients with breast cancer. Oncol Rep.
22:273–278. 2009.PubMed/NCBI
|
|
37
|
Rech AJ, Mick R, Kaplan DE, Chang KM,
Domchek SM and Vonderheide RH: Homeostasis of peripheral FoxP3(+)
CD4 (+) regulatory T cells in patients with early and late stage
breast cancer. Cancer Immunol Immunother. 59:599–607. 2010.
View Article : Google Scholar : PubMed/NCBI
|