Introduction
Malignant lymphoma (ML) is a hematological
malignancy originating from lymphocytes. In Japan, the incidence of
ML is estimated to be ~22,000 individuals per year (1). ML commonly occurs in adults, with a
peak incidence between the seventh and ninth decades of life
(2). Although ML usually occurs
primarily in the lymph nodes, it rarely occurs primarily in the
bone (primary malignant lymphoma of bone; PLB). PLB accounts for
~5% of extranodal lymphomas and 3–7% of primary malignant bone
tumors (3,4). PLB usually occurs between the ages of
40 and 60 years and displays a slight male predominance (male:
female ratio, 1.5:1) (3,5,6).
Patients with PLB frequently lack systemic symptoms (i.e., fever,
night sweats and weight loss; B symptoms); conversely, they
commonly complain of local symptoms, such as pain, swelling and
pathological fractures (7).
Therefore, clinically diagnosing PLB without B symptoms is
difficult. The most frequent site of PLB is the femur, followed by
the pelvis, tibia, humerus and spine (7,8); small
bones of the hand and foot, particularly the talus, are rare sites
(4,5). We herein report an exceedingly rare
case of primary diffuse large B-cell lymphoma (DLBCL) occurring in
the talus and discuss it with reference to the literature.
Case report
A 74-year-old Japanese man, with a previous medical
history of diabetes, hypertension and prostatic hypertrophy,
presented to the primary hospital with a 5-month history of pain,
fever, swelling of the right ankle and a right inguinal lump. Plain
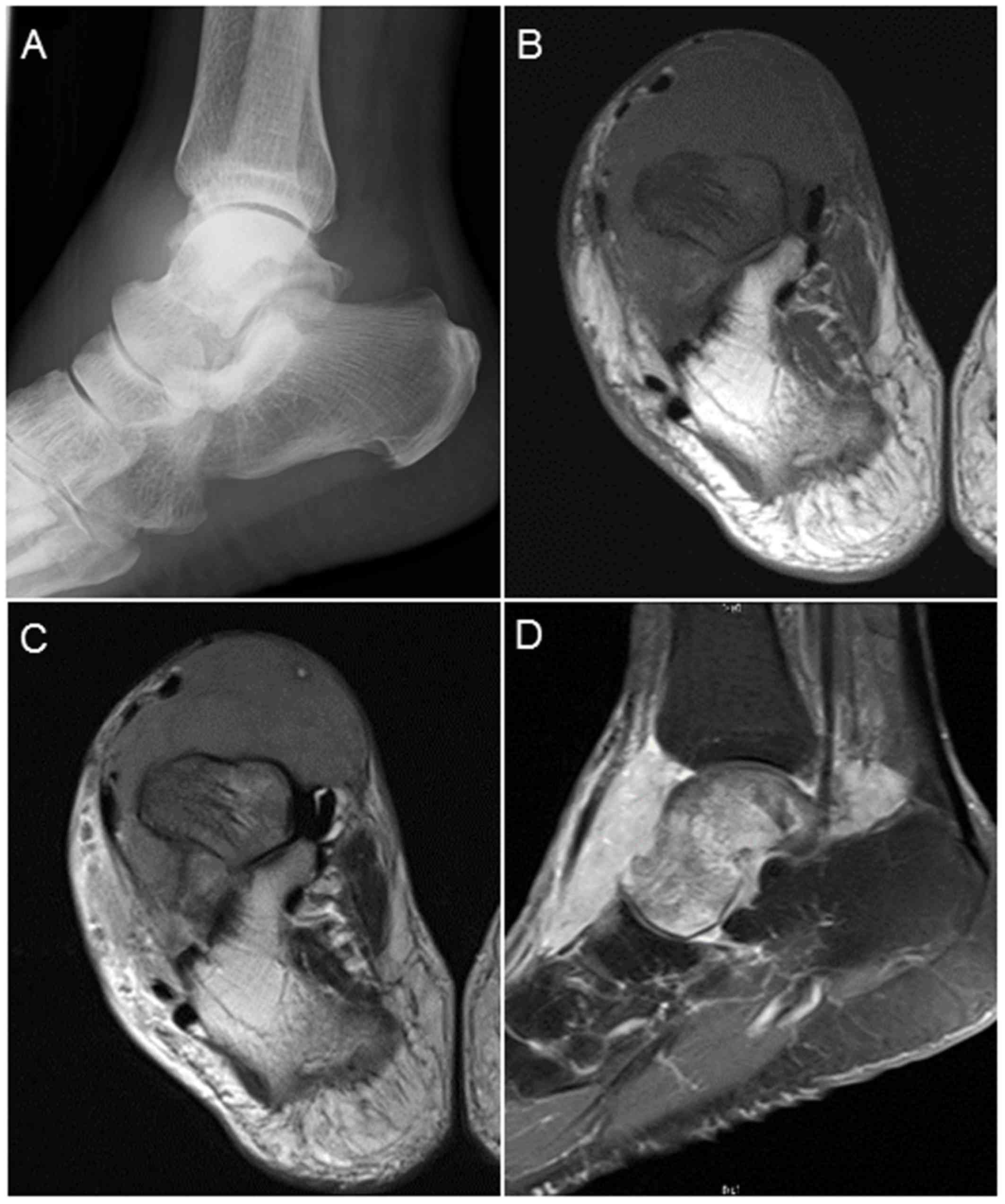
radiography of the right ankle revealed no abnormality (Fig. 1A). Magnetic resonance imaging (MRI)
revealed a well-circumscribed intraosseous tumor (8.0×6.9×4.3 cm)
extending to the surrounding soft tissue in front of the talus,
exhibiting low intensity on both T1-and T2-weighted imaging (WI),
and uniform enhancement on gadolinium-enhanced T1-weighted
fat-suppression imaging (Fig. 1B-D).
A primary soft tissue tumor was initially suspected and the patient
was referred to the Fukushima Medical University Hospital
(Fukushima, Japan). The laboratory findings at initial presentation
indicated mild anemia (hemoglobin: 12.9 g/dl; normal range,
13.5–17.5 g/dl) and elevated lactate dehydrogenase (LDH) level (310
IU/l; normal range, 120–242 IU/l). The liver and renal function
tests showed no abnormalities. Computed tomography (CT) of the
chest, abdomen and pelvis showed bilateral renal cysts and right
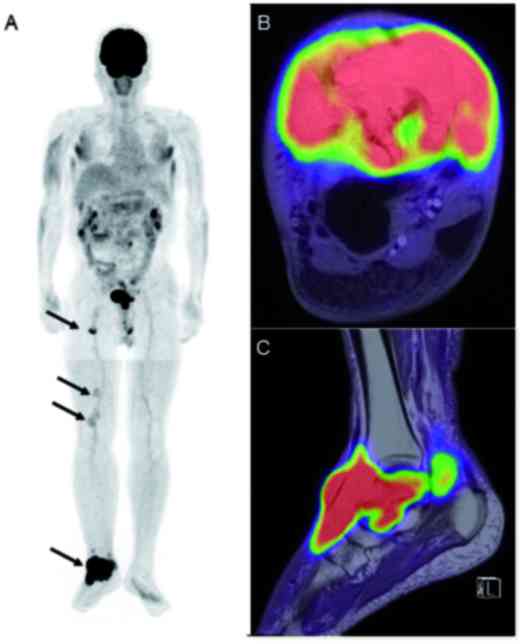
inguinal lymphadenopathy. Whole-body fluorine-18-fluorodeoxyglucose
(18F-FDG), positron emission tomography (PET)/MRI
revealed increased FDG uptake in the right talus and its
surrounding soft tissue [maximum standardized uptake value
(SUVmax): 28.7], the right inguinal lymph node (5.9),
and the right popliteal lymph nodes (3.5 and 2.2) (Fig. 2A-C). Since a malignant bone tumor was
initially suspected, core needle biopsy of the right talus was
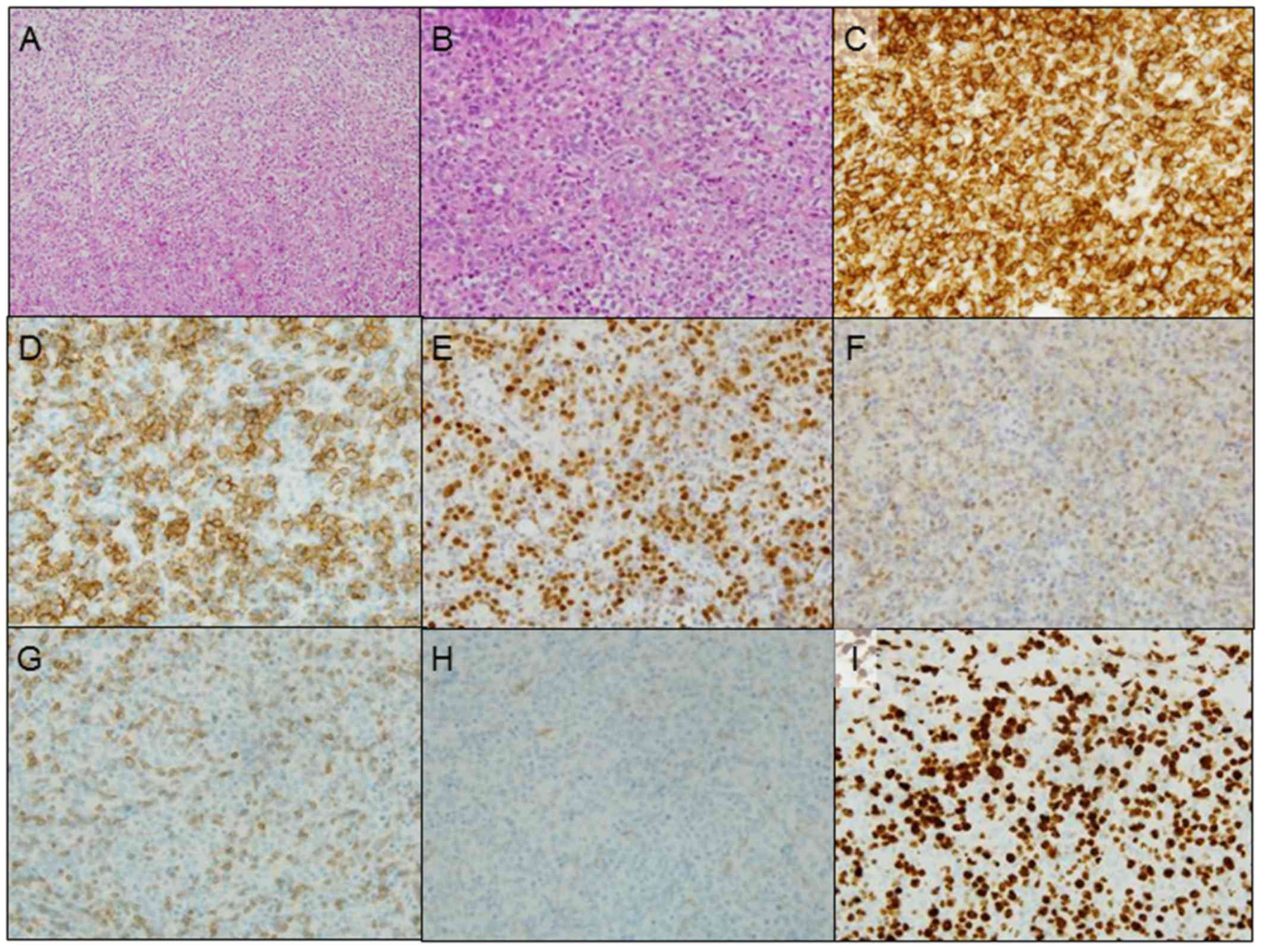
performed. The histopathological examination of the tumor revealed
diffuse growth of large anaplastic cells with round or oval nuclei.
Immunohistochemically, the tumor cells were positive for CD45,
CD20, B-cell lymphoma (bcl)-6, vimentin, Forkhead box protein-P1
and multiple myeloma oncogene-1, but negative for CD3, CD5, CD10,
CD34, bcl-2, and Ebstein-Barr virus-encoded RNA (Fig. 3A-H). The MIB-1 labeling index was
~70% (Fig. 3I). Additionally, the
level of serum soluble interleukin-2 receptor (sIL-2R) was markedly
elevated (1,890 U/ml; normal range, 122–496 U/ml).
Histopathological and imaging examinations led to the diagnosis of
primary DLBCL of the talus with metastasis to the right inguinal
and popliteal lymph nodes, or stage IIE according to the Ann Arbor
classification. The patient received eight courses of R-CHOP
chemotherapy (rituximab 250 mg/m2/course,
cyclophosphamide 430 mg/m2/course, doxorubicin 25
mg/m2/course, vincristine 0.7 mg/m2/course
and oral prednisolone 60 mg on days 1–5, every 3 weeks) without
complications at the local hospital. Although chemotherapy was
effective and the tumor was reduced in size, a pathological
fracture of the talus occurred during the chemotherapy; thus, the
patient was forced to walk in a non-weight-bearing manner using
crutches for 6 weeks. Six months after the first treatment,
additional radiotherapy (a total of 40 Gy in 20 fractions) was
performed on the right ankle. Eight months after the radiotherapy,
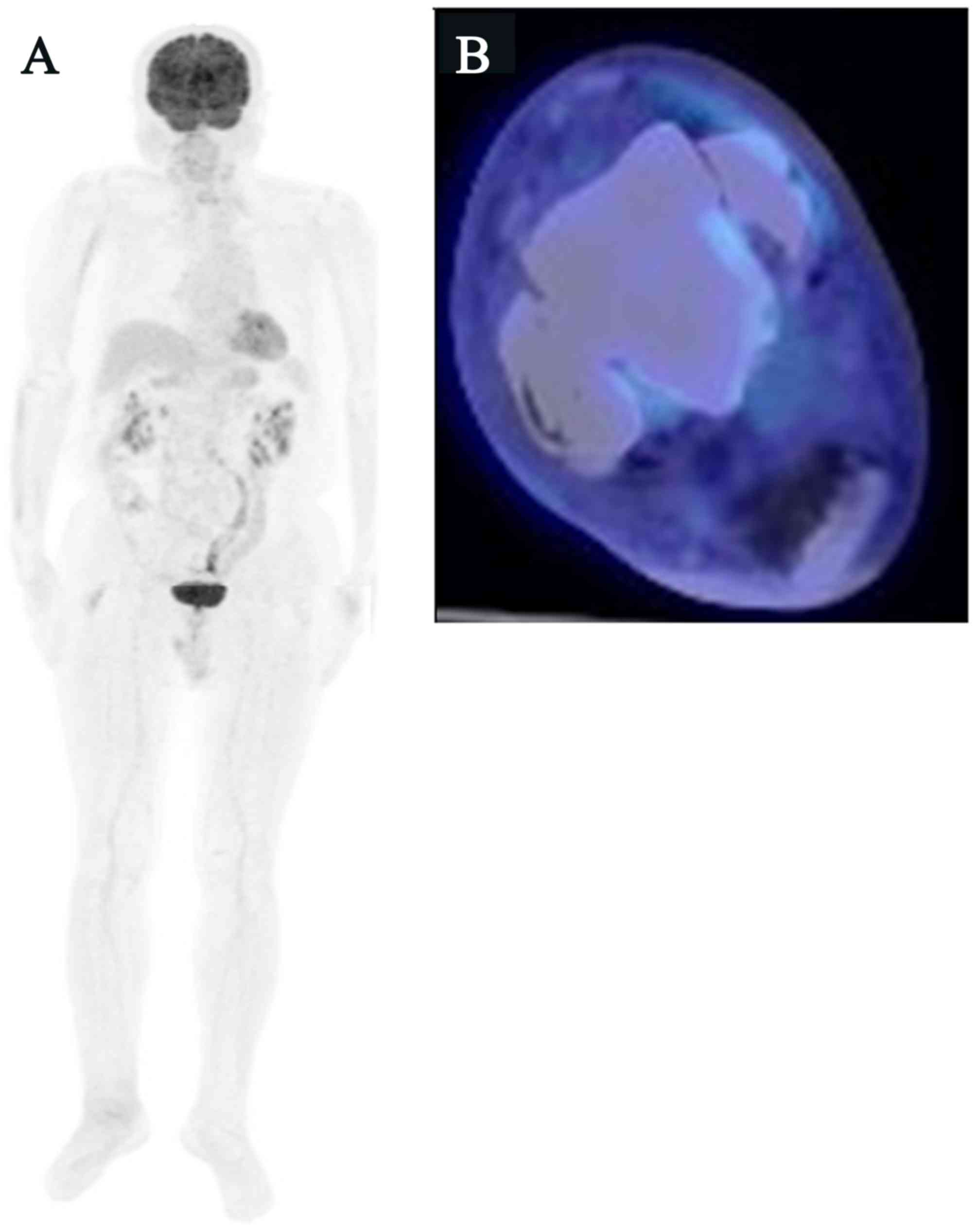
whole-body 18F-FDG PET/CT detected no FDG uptake in the
right inguinal and popliteal lymph nodes or the right talus,
indicating a complete response (CR), and the patient showed no
symptoms or signs of local recurrence or metastasis (Fig. 4A and B).
Discussion
PLB is defined as a neoplasm composed of malignant
lymphoid cells, producing one or more masses within the bone,
without any supraregional lymph node involvement or other
extranodal lesions, excluding regional lymph node involvement
(9). In the present case, the talus
tumor was diagnosed as primary, as the size of the tumor mass was
the largest and the FDG uptake of the talus was the highest; it
exhibited the mean uptake of PLB as described by Wang et al
(10), with an SUVmax of
15±11.82. The inguinal and popliteal lymph nodes were considered to
be compatible with regional lymph node metastasis from the talus
tumor. To the best of our knowledge, only 5 cases of PLB of the
talus have been previously reported in the English literature,
namely 1 case of multifocal and 4 cases of unifocal lesions, as in
the present case (Table I) (3,8,11–13).
 | Table I.Summary of previously reported cases
of PLB of the talus. |
Table I.
Summary of previously reported cases
of PLB of the talus.
| Study (Refs.) | Age
(years)/gender | Treatment | Histological
type | Prognosis | Follow-up duration
(months) |
|---|
| Present case | 74/M | CT + RT | DLBCL | CR | 8 |
| Bansal et al
(11) | 32/M | CT + RT | DLBCL | CR | 3 |
| Patel et al
(12) | 6/M | CT | DLBCL | CR | 18 |
| Nickisch et al
(8) | 58/M | CT + RT | DLBCL | CR | 18 |
| Kobayashi et
al (13) | 68/M | RT + CT | DLBCL | NA | 6 |
The radiological characteristics of PLB are variable
and non-specific (12,14,15).
Imaging usually shows an osteolytic lesion permeated with a
moth-eaten pattern of destruction (4,16). Mixed
lytic and sclerotic lesions are less common, and sclerotic-only
lesions are rare (16); if the
cortex is uninvolved, plain radiographs may show no abnormality
(9,16). MRI is very useful for evaluating the
extent of surrounding soft tissue and bone marrow involvement
(12,16). MRI in PLB usually shows an
abnormality of the bone marrow exhibiting low intensity on T1- and
high intensity on T2-WI. Although reactive changes, including
peritumoral edema of the bone marrow, exhibit high intensity on
T2-WI, the lesion including fibrosis typically shows low intensity.
Contrast-enhanced MRI shows enhancement within the lesion (4,16). The
differential diagnosis for these radiological findings have been
reported to include Ewing's sarcoma, multiple myeloma,
osteomyelitis, osteonecrosis and Paget's disease of the bone
(5,8,12,16). As
MRI findings are also variable and non-specific, imaging
examinations alone may lead to misdiagnosis (4,16,17). For
osteolytic lesions, however, serum sIL-2R has been reported to be a
useful marker that distinguishes PLB from other bone lesions
(18). As serum sIL-2R has shown
higher sensitivity (0.95) and specificity (0.70) compared with
other laboratory data, such as LDH and C-reactive protein (18), this marker should be measured when
PLB is suspected.
PET scans play an important role in the diagnosis,
staging and evaluation of the response to treatment of PLB
(10,19). The diagnostic sensitivity of PET/CT
has been reported to be significantly higher compared with that of
CT (98.9 vs. 43.2%, respectively) (8). As PET/MRI has been reported to show
higher sensitivity for detecting bone marrow involvement of ML
compared with PET/CT and bone scintigraphy (19,20),
PET/MRI is useful for detecting osseous involvement in ML,
including PLB. Although 3 of the 5 previously reported cases did
not undergo FDG PET scans (11–13),
PET/MRI or PET/CT is quite useful for accurate staging of PLB, as
well as evaluation of the therapeutic effects.
Among PLBs, DLBCL is the most common subtype and
accounts for 68–80% of the cases (7,21–23). The
current standard chemotherapy for patients with DLBCL consists of
cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) or
CHOP with rituximab (R-CHOP) (24).
As regards the treatment outcome of PLB, combination chemotherapy
with local radiotherapy has been reported to be superior to
radiotherapy alone (7,25,26).
Conversely, surgical resection, as a local treatment, has a limited
indication only for spinal cases with progressive neurological
disorder or cases with pathological fractures in the extremities
(27). In the present case, combined
chemotherapy with R-CHOP followed by irradiation was performed and
the tumor exhibited a CR; therefore, these treatments were
considered to be beneficial.
In conclusion, PLB is exceedingly rare and its
radiological findings are variable and non-specific; therefore,
accurate diagnosis without pathology is quite difficult in the
majority of the cases. Since PLB in the early stages is relatively
curable by appropriate multimodal treatment using chemo- and
radiotherapy, correct diagnosis and staging by histological and
imaging examinations are crucial. When radiologically diagnosing
bone tumors, including those of the talus, clinicians should
consider the possibility of PLB.
References
|
1
|
Matsuda A, Matsuda T, Shibata A, Katanoda
K, Sobue T and Nishimoto H: Japan Cancer Surveillance Research
Group: Cancer incidence and incidence rates in Japan in 2008: A
study of 25 population-based cancer registries for the Monitoring
of Cancer Incidence in Japan (MCIJ) project. Jpn J Clin Oncol.
44:388–396. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katanoda K, Hori M, Matsuda T, Shibata A,
Nishino Y, Hattori M, Soda M, Ioka A, Sobue T and Nishimoto H: An
updated report on the trends in cancer incidence and mortality in
Japan, 1958–2013. Jpn J Clin Oncol. 45:390–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mikhaeel NG: Primary bone lymphoma. Clin
Oncol (R Coll Radiol). 24:366–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mulligan ME, McRae GA and Murphey MD:
Imaging features of primary lymphoma of bone. AJR Am J Roentgenol.
173:1691–1697. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ibáñez M, Cortina B, Gómez V,
Alvaro-Gracia JM, Reina T and Castañeda S: Aggressive
transformation of a quiescent primary bone lymphoma simulating
Paget's disease. Clin Exp Rheumatol. 26:133–135. 2008.PubMed/NCBI
|
|
6
|
Bayrakci K, Yildiz Y, Saglik Y, Altay M,
Ogüt H, Samur M and Erekul S: Primary lymphoma of bones. Int
Orthop. 25:123–126. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Beal K, Allen L and Yahalom J: Primary
bone lymphoma: Treatment results and prognostic factors with
long-term follow-up of 82 patients. Cancer. 106:2652–2656. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nickisch F, Tashjian RZ, Ritter M, Terek
RM and DiGiovanni CW: Primary malignant non-Hodgkin lymphoma of the
talus: A case report. Foot Ankle Int. 26:568–571. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Unni KK and Hogendoorn PCW: Malignant
lymphoma. Fletcher CDM, Unni KK and Mertens F: Pathology and
Genetics: Tumours of Soft Tissue and Bone Lyon, France: IARC Press;
pp. pp306–pp308. 2002
|
|
10
|
Wang LJ, Wu HB, Wang M, Han YJ, Li HS,
Zhou WL and Wang QS: Utility of F-18 FDG PET/CT on the evaluation
of primary bone lymphoma. Eur J Radiol. 84:2275–2279. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bansal S and Dharra N: Primary malignant
non-hodgkin lymphoma of the talus. J Cancer Res Ther. 11:6492015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Patel S, Sudesh P, John R and Gupta P:
Primary non Hodgkin's lymphoma of talus in a child-a rare
presentation. Foot (Edinb). 24:210–212. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi H, Kato Y, Hakamada M, Hattori
Y, Sato A, Shimizu N, Imamura A, Mihara H, Kato H, Oki Y, et al:
Malignant lymphoma of the bone associated with systemic
sarcoidosis. Intern Med. 40:435–438. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gill P, Wenger DE and Inwards DJ: Primary
lymphomas of bone. Clin Lymphoma Myeloma. 6:140–142. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Camargo OP, dos Santos Machado TM,
Croci AT, de Oliveira CR, Giannotti MA, Baptista AM, Caiero MT,
Alves VA and Matsumoto LA: Primary bone lymphoma in 24 patients
treated between 1955 and 1999. Clin Orthop Relat Res. 271–280.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krishnan A, Shirkhoda A, Tehranzadeh J,
Armin AR, Irwin R and Les K: Primary bone lymphoma: Radiographic-MR
imaging correlation. Radiographics. 23:1371–1387. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heyning FH, Kroon HM, Hogendoorn PC,
Taminiau AH and van der Woude HJ: MR imaging characteristics in
primary lymphoma of bone with emphasis on non-aggressive
appearance. Skeletal Radiol. 36:937–944. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akahane T, Shimizu T, Isobe K, Yoshimura Y
and Kato H: Serum soluble interleukin-2 receptor levels in patients
with malignant lymphoma of bone. J Orthop Sci. 14:248–252. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moog F, Kotzerke J and Reske SN: FDG PET
can replace bone scintigraphy in primary staging of malignant
lymphoma. J Nucl Med. 40:1407–1413. 1999.PubMed/NCBI
|
|
20
|
Heacock L, Weissbrot J, Raad R, Campbell
N, Friedman KP, Ponzo F and Chandarana H: PET/MRI for the
evaluation of patients with lymphoma: Initial observations. AJR Am
J Roentgenol. 204:842–848. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ramadan KM, Shenkier T, Sehn LH, Gascoyne
RD and Connors JM: A clinicopathological retrospective study of 131
patients with primary bone lymphoma: A population-based study of
successively treated cohorts from the British Columbia Cancer
Agency. Ann Oncol. 18:129–135. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jacobs AJ, Michels R, Stein J and Levin
AS: Socioeconomic and demographic factors contributing to outcomes
in patients with primary lymphoma of bone. J Bone Oncol. 4:32–36.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Maruyama D, Watanabe T, Beppu Y, Kobayashi
Y, Kim SW, Tanimoto K, Makimoto A, Kagami Y, Terauchi T, Matsuno Y
and Tobinai K: Primary bone lymphoma: A new and detailed
characterization of 28 patients in a single-institution study. Jpn
J Clin Oncol. 37:216–223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Coiffier B, Lepage E, Briere J, Herbrecht
R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G,
Gaulard P, et al: CHOP chemotherapy plus rituximab compared with
CHOP alone in elderly patients with diffuse large-B-cell lymphoma.
N Engl J Med. 346:235–242. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barbieri E, Cammelli S, Mauro F, Perini F,
Cazzola A, Neri S, Bunkheila F, Ferrari S, Brandoli V, Zinzani P,
et al: Primary non-Hodgkin's lymphoma of the bone: Treatment and
analysis of prognostic factors for Stage I and Stage II. Int J
Radiat Oncol Biol Phys. 59:760–764. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baar J, Burkes RL, Bell R, Blackstein ME,
Fernandes B and Langer F: Primary non-Hodgkin's lymphoma of bone. A
clinicopathologic study. Cancer. 73:1194–1199. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Unni KK and Inwards CY: Malignant lymphoma
of bone. In: Unni KK (ed): Dahlin's Bone Tumors, General aspects
and data on 10,165 cases. (6th edition). (Philadelphia). Wolters
Kluwer Health/Lippincott Williams & Wilkins. 201–210. 2010.
|