Introduction
Antitumor agents are mostly cytotoxic drugs, which
inevitably lead to damage of normal tissue cells and organs, or
cause adverse reaction when killing cancer cells. Chemotherapeutic
agents may cause liver damage, mainly including necrosis or fatty
degeneration of liver cells, cholestasis and liver vessel damage
(1–4). Hepatic dysfunction tends to affect the
course of antitumor treatment, increasing patient discomfort and
the overall financial burden.
Hydrogen is a naturally existing colorless,
tasteless and odorless gas, and its protective effect against
oxidative damage to the brain, liver, kidney and other major organs
has been previously described (5).
Solubilized hydrogen (hydrogen-rich water) is a portable, easily
administered and safe means of delivering molecular hydrogen
(6). The aim of the present
prospective study was to administer hydrogen-rich water to
colorectal cancer (CRC) patients treated with mFOLFOX6 chemotherapy
by a randomized, single-blind, controlled clinical research method,
and compare the chemotherapy-induced liver damage between the
treatment and control groups.
Subjects and methods
Subjects
A total of 152 patients with CRC were recruited at
the Department of Oncology of Taishan Hospital (Taian, China)
between June 2010 and February 2016, among whom 146 met the
inclusion criteria. Subsequently, 144 patients were randomized into
the treatment (n=80) and placebo (n=64) groups. At the end of the
study, 76 patients in the hydrogen treatment group and 60 patients
in the placebo group were included in the final analysis (Fig. 1).
The 136 subjects were aged 41–86 years (mean age,
55.6±14.2 years) and included 56 men and 80 women and the number of
elderly subjects (aged ≥70 years) was 78.
Inclusion criteria
Stage ≥IIB CRC (after surgery or inoperable); need
for mFOLFOX6 chemotherapy; age >18 years; Eastern Cooperative
Oncology Group (ECOG) performance status (PS) score <1; all
cases had a definitive pathological diagnosis.
Exclusion criteria
Viral hepatitis, carriers of viral hepatitis,
alcoholic hepatitis, drug-induced hepatitis and autoimmune
hepatitis, hematological diseases, heart diseases, hyperthyroidism,
pregnancy, hepatic cirrhosis and neuropsychiatric disorders. This
was a controlled, randomized, single-blind study. The study
protocol was approved by the Ethics Committee of Shandong
Provincial Taishan Hospital, and written informed consent was
obtained from all the subjects prior to enrollment.
Preparation of hydrogen-rich
water
Hydrogen-rich water was prepared by increasing the
hydrogen pressure in the solution (7). First, the partial air pressure in the
water was reduced using a 1406 type vacuum pump (Shanghai Medical
Equipment Works Co., Ltd., Shanghai, China). The solution was then
passed through hydrogen cylinders with a 99.999% hydrogen purity
for 2 h to obtain a water solution rich in hydrogen. The amount of
dissolved hydrogen in hydrogen-rich water was measured using an
ENH-1000 portable meter (TRUSTLEX Co., Ltd., Tokyo, Japan), as this
water is only considered drinkable under the level of 0.27–0.4
ppm.
Chemotherapy regimen and
randomization
All the subjects received mFOLFOX6 chemotherapy.
Dosage: Oxaliplatin 85 mg/m2 on day 1 as an i.v. drip,
calcium folinate 400 mg/m2 on day 1 i.v., 5-fluorouracil
(5-FU) 2,400 mg/m2 as a continuous i.v. infusion over 46
h (National Comprehensive Cancer Network guidelines 2012, version
1) (8). The baseline characteristics
of patients in the two groups are summarized in Table I.
 | Table I.Baseline characteristics of
patients. |
Table I.
Baseline characteristics of
patients.
|
|
| Groups |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Total | Hydrogen-rich water
(n=76) | Control (n=60) | P-value |
|---|
| Age (years) |
|
|
| 0.919 |
|
<70 | 58 | 32 | 26 |
|
| ≥70 | 78 | 44 | 34 |
|
| Gender |
|
|
| 0.243 |
| Male | 56 | 36 | 20 |
|
|
Female | 80 | 40 | 40 |
|
| ECOG PS score |
|
|
| 0.340 |
| 0–1 | 82 | 42 | 40 |
|
| 2 | 54 | 34 | 20 |
|
| Smoking history |
|
|
| 0.174 |
| Yes | 76 | 48 | 28 |
|
| No | 60 | 28 | 32 |
|
| History of alcohol
intake |
|
|
| 0.457 |
| Yes | 38 | 18 | 20 |
|
| No | 94 | 54 | 40 |
|
| History of
hepatitis |
|
|
| 0.660 |
| Yes | 46 | 24 | 22 |
|
| No | 90 | 52 | 38 |
|
| Hepatic
metastases |
|
|
| 0.382 |
|
Yes | 48 | 24 | 24 |
|
| No | 72 | 36 | 36 |
|
| No. of chemotherapy
cycles |
|
|
| 0.265 |
|
1–3 | 80 | 40 | 40 |
|
|
3–6 | 40 | 22 | 18 |
|
|
>6 | 16 | 10 | 6 |
|
Intake of hydrogen-rich water
The patients in the hydrogen-rich water group
started drinking hydrogen-rich water 1 day prior to chemotherapy
until the end of the cycle, for a total of 4 days, with a daily
intake of 1,000 ml in 4 doses (250 ml each). Hydrogen-rich water
was consumed 0.5 h after a meal and before bedtime. The patients
did not discontinue consuming hydrogen-rich water during the entire
course of chemotherapy. The patients in the placebo group consumed
distilled water, with a daily intake of 1,000 ml in 4 doses (250 ml
each).
Assessment of liver function
Alanine aminotransferase (ALT), aspartate
transaminase (AST), alkaline phosphatase (ALP), direct bilirubin
(DBIL) and indirect bilirubin (IBIL) were measured with an
automatic biochemical analyzer (TBA-120R; Toshiba, Tokyo, Japan),
and the reagents (no. 12125) were purchased from Whiteman Biotech
Co., Ltd. (Nanjing, China). The measurements were performed
according to the manufacturer's instructions. Liver function
assessment was performed on the 10th day after chemotherapy. The
standard classification of liver toxicity following chemotherapy
was shown in Table II (WHO
standards).
 | Table II.Standard classification of liver
toxicity following chemotherapy according to the World Health
Organization guidelines. |
Table II.
Standard classification of liver
toxicity following chemotherapy according to the World Health
Organization guidelines.
| Classification | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
|---|
| BIL | N=1.71–17.1
µmol/l | <1.5*N | (1.5–3)*N | (3–10)*N | >10*N |
| AST/ALT | N=0–40 IU/l | ≤2.5*N | (2.6–5)*N | (5.1–20)*N | >20*N |
| ALP | N=25–90 IU/l |
|
|
|
|
| Hepatic coma | No changes
before/after treatment | – | – | Pre-hepatic
coma | Hepatic coma |
Statistical analysis
All data are presented as mean ± standard deviation.
Measurement data were analyzed using independent-samples t-test and
numerical data with the use of the rank-sum test. All data analyses
were performed with SPSS software, version 13.0 (SPSS Inc.,
Chicago, IL, USA). P<0.05 was considered to indicate
statistically significant differences.
Results
Patients
As shown in Table I,
a total of 136 patients were analyzed. There was no statistically
significant difference in the stratification of all factors (age,
gender, ECOG PS, smoking history, history of alcohol consumption,
hepatic metastases, chemotherapy cycles) between the two groups
(P>0.05); thus, randomization was considered as baseline
balance.
Effect of mFOLFOX6 on liver
function
As shown in Table
III, the effect of mFOLFOX6 chemotherapy on the liver was
mainly characterized by elevated ALT, AST and IBIL levels. The
number of cases with abnormal ALT, AST, ALP, DBIL and IBIL levels
following chemotherapy was 46, 40, 11, 6 and 17, respectively,
accounting for 33.82, 29.41, 8.09, 4.41 and 12.50%,
respectively.
 | Table III.Types of hepatic damage in patients
exhibiting mFOLFOX6-induced liver injury. |
Table III.
Types of hepatic damage in patients
exhibiting mFOLFOX6-induced liver injury.
| Abnormal marker,
patient no. (%) |
|---|
|
|---|
| AST | ALT | ALP | DBIL | IBIL |
|---|
| 46 (33.82) | 40 (29.41) | 11 (8.09) | 6 (4.41) | 17 (12.50) |
Comparison of liver damage between
hydrogen-rich water and placebo groups
The probability and degree of chemotherapy-induced
liver damage in the hydrogen-rich water group were lower compared
with those in the placebo group. The comparison of hepatic injury
following chemotherapy between the two groups was performed with
the use of the rank-sum test. As shown in Table IV, the patients in the hydrogen-rich
water group had a lower probability and degree of hepatic damage
compared with those in the placebo group (P<0.05).
 | Table IV.Comparison of hepatic damage between
the hydrogen-rich water and control groups. |
Table IV.
Comparison of hepatic damage between
the hydrogen-rich water and control groups.
| Groups | Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Mean-rank |
|---|
| Hydrogen-rich
water | 54 | 14 | 4 | 4 | 0 | 58.13 |
| Control | 24 | 16 | 10 | 8 | 2 | 81.63 |
| P-value |
|
|
|
|
|
0.00 |
Comparison of liver function tests
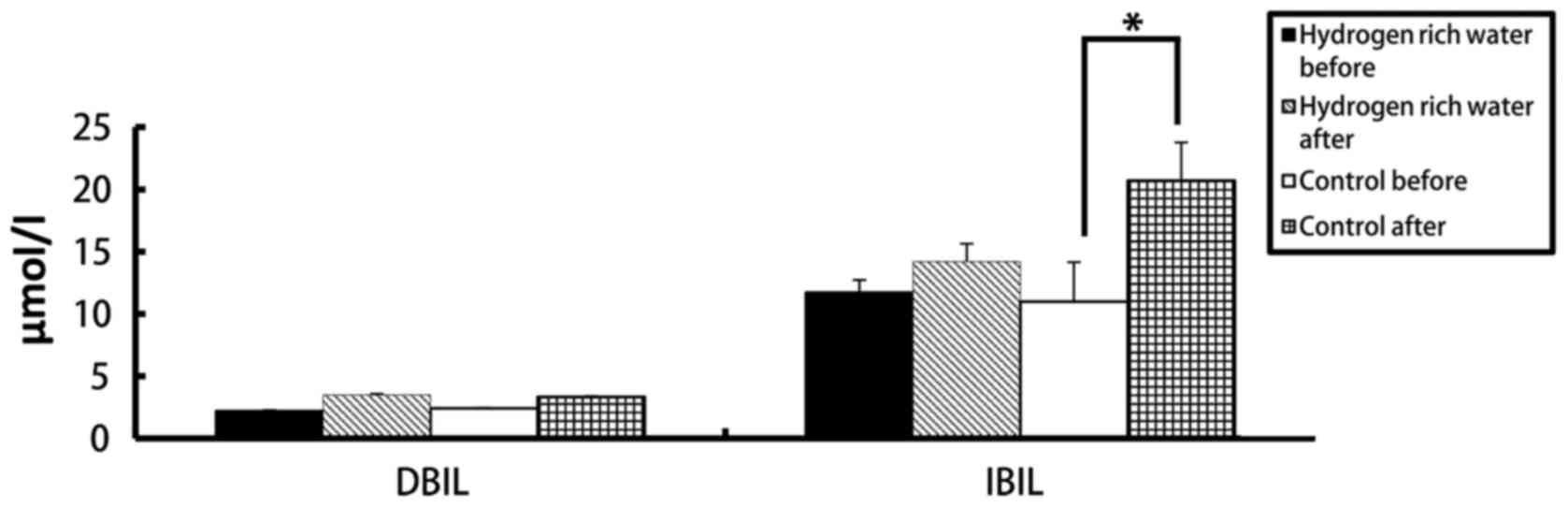
before and after treatment between the two groups
There were no statistically significant differences
in the levels of ALT, AST, ALP, DBIL and IBIL in the hydrogen-rich
water group before and after chemotherapy. However, the difference
in ALT, AST and DBIL levels before and after chemotherapy in the
control group was statistically significant (seen in Figs. 2 and 3). The ALT levels in the hydrogen-rich
water group before and after chemotherapy were 28.72±1.6 and
31.28±1.47 IU/l, respectively; the difference was not statistically
significant (P=0.46). The ALT levels in the control group before
and after chemotherapy were 26.78±3.8 and 58.22±2.46 IU/l,
respectively; the difference was statistically significant
(P=0.04). The AST levels in the hydrogen-rich water group before
and after chemotherapy were 22.74±2.74 and 23.43±2.66 IU/l,
respectively; the difference was not statistically significant
(P=0.67). The AST in the control group before and after
chemotherapy were 23.43±3.24 and 39.28±5.17 IU/l, respectively; the
difference was statistically significant (P=0.032). The ALP levels
in the hydrogen-rich water group before and after chemotherapy were
65.52±7.13 and 67.34±3.32 IU/l, respectively; the difference was
not statistically significant (P=0.45). The ALP levels in the
control group before and after chemotherapy were 63.44±4.70 and
70.52±5.22 IU/l, respectively; the difference was not statistically
significant (P=0.70). The DBIL levels in the hydrogen-rich water
group before and after chemotherapy were 2.2±0.07 and 3.48±0.10
µmol/l, respectively; the difference was not statistically
significant (P=0.44). The DBIL levels in the control group before
and after chemotherapy were 2.42±0.04 and 3.34±0.05 µmol/l
respectively; the difference was not statistically significant
(P=0.32). The IBIL levels in the hydrogen-rich water group before
and after chemotherapy were 11.70±1.02 and 14.20±1.44 µmol/l,
respectively; the difference was not statistically significant
(P=0.10). The IBIL levels in the control group before and after
chemotherapy were 10.98±3.17 and 20.70±3.07 µmol/l, respectively;
the difference was statistically significant (P=0.046).
Discussion
Chemotherapeutic agents and their metabolites may
directly induce damage to hepatic parenchymal cells; in addition,
they may also cause further damage to the hepatocytes through
cellular or humoral immunity mechanisms. There are two major
categories of hepatic tissue damage commonly occurring as a
consequence of chemotherapy: One is similar to non-alcoholic fatty
liver disease and is often referred to as chemotherapy-associated
steatohepatitis (CASH) (9); the
other results from injury to the sinusoids causing venous
congestion. Endothelial cells in the sinusoids become damaged,
leading to initiation of the coagulation cascade within the
subendothelial space of Disse and, ultimately, to sinusoidal
obstruction, as fibrotic changes occur in the central venules
(10).
Of the 136 patients included in the present study,
58 (42.6%) experienced post-chemotherapeutic hepatic function
compromise. In the majority of the cases (75.9%), the liver injury
was grade 1–2. The mechanism underlying chemotherapeutic
agent-induced liver injury is as follows: The majority of
chemotherapeutic agents are metabolized in the liver; when the
chemotherapeutic agent and its metabolites are beyond the hepatic
metabolic abilities, the electrophilic products and superoxide ions
generated via the metabolic process damage the hepatocellular,
hepatic mitochondrial and microsomal membranes, directly inducing
hepatocellular injury through covalent bonding and promotion of
lipid peroxidation; in addition, the drug metabolites form
oxygen-free radicals promoting lipid peroxidation, indirectly
inducing hepatocellular injury.
mFOLFOX6 chemotherapy consists of 5-FU, oxaliplatin
and calcium folinate, once every 2 weeks, which is associated with
high risk of hepatotoxicity. 5-FU is converted in vivo into
a triple complex, which is difficult to depolymerize, including
fluorodeoxyuridine phosphate, thymidylate synthase and
5,10-citrovorum factor; it inhibits thymidylate synthase and also
blocks the synthesis of deoxythymidylic acid, eventually affecting
the synthesis of deoxynucleotides. Oxaliplatin may act on DNA
through the generation of alkylation conjugates, forming intrachain
and interstrand cross-links, and generating cytotoxic effects. 5-FU
is more associated with steatosis (11), whereas oxaliplatin regimens may
result in sinusoidal injuries leading to sinusoidal obstruction
syndrome (12,13). 5-FU specifically is considered to
affect mitochondrial membranes, allowing for an increase in
reactive oxygen species and setting off a cascade of events leading
to lipid peroxidation, fibrosis and cell death (14,15).
Sinusoidal injury is also considered to result from reactive oxygen
species. Once endothelial cells were injured, the coagulation
cascade is activated and may lead to sinusoidal obstruction
(16).
A comparison of several indicators of the liver
function was performed; the post-chemotherapeutic liver injuries
mainly manifested as elevation in ALT, AST and IBIL levels, with 46
cases with ALT abnormalities, 40 cases of AST abnormalities, and 17
cases of elevation in IBIL levels. Considering that the patients
received mFOLFOX6 chemotherapy, post-chemotherapeutic liver injury
mainly manifested as elevation in ALT, AST and IBIL levels.
Chemotherapeutic agents injure hepatocytes, mainly
through interfering with hepatocellular metabolism and forming
oxygen free radicals, inducing hepatocellular necrosis and
inflammation, or the damages are induced by hepatic fibrosis, fatty
degeneration and sinusoidal obstruction. ALT and AST, which are
mainly distributed in the hepatocellular cytoplasm, are the most
sensitive indicators reflecting hepatocellular inflammatory injury.
ALT and AST are released from damaged cells into the blood.
Therefore, chemotherapy leads to an elevation in liver enzyme and
IBIL levels. ALP is elevated when the bile excretion is blocked
with hepatocellular injuries. TBIL is generated by the liver and
excreted through the biliary tract; an elevation in serum DBIL
indicates inhibition of bile excretion, or a disorder in liver
uptake and bilirubin secretion. Chemotherapy does not lead to
biliary tract inflammation or obstruction, nor to elevation in
serum DBIL and ALP levels. However, Nakano et al reported
that gemcitabine may lead to bile duct obstruction and cholestasis
(17).
Some studies suggest that >6 cycles of
chemotherapy is an independent prognostic factor of liver injury
(17). In addition, patients with
elevated body mass index, type 2 diabetes mellitus, or metabolic
syndrome, have an increased risk of steatosis, irrespective of
chemotherapy (18).
In 2007, Ohsawa et al (19) observed that molecular hydrogen
selectively reduced cytotoxic reactive oxygen species in
vitro and exerted a therapeutic antioxidant effect. It has been
demonstrated that H2 exerted preventive or therapeutic
effects on cerebral, myocardial and hepatic ischemia-reperfusion
injuries, intestinal, lung, renal and heart transplantation, and
acute graft-versus-host disease post-allogeneic hematopoietic stem
cell transplantation (20,21). Recent basic and clinical research
revealed that hydrogen is an important physiological regulatory
factor with antioxidant, anti-inflammatory and anti-apoptotic
protective effects on cells and organs (22–25).
Using hydrogen as a potential antioxidant has
multiple advantages: It may effectively neutralize hydroxy radicals
present in live cells, unlike most known antioxidants, which cannot
successfully enter the target organelles. Hydrogen has good
distribution characteristics, and it can penetrate biomembranes and
diffuse into the cytoplasm, mitochondrion and nucleus. Although
hydrogen's activity is mild, its rapid gas diffusion properties are
very effective in reducing the toxicity of free radicals in
cells.
Previous studies have indicated that inhalation of
1% hydrogen gas or oral administration of hydrogen-rich water may
reduce organ toxicity induced by cisplatin chemotherapy, improve
the quality of life and decelerate weight loss, without affecting
the effectiveness of chemotherapy (26). As reported, hydrogen-rich water may
reduce hepatic injury through reducing oxidative stress and high
mobility group box 1 (27). In
vitro experimental studies have indicated that hydrogen-rich
water may reduce the liver damage induced by obstructive jaundice
and endotoxins (28,29).
In the present study, patients in chemotherapy who
were treated with hydrogen had no significant differences in liver
function indicators, such as ALT, AST, ALP, IBIL or DBIL after
chemotherapy, indicating that hydrogen does exert a protective
effect on liver function.
Hydrogen-rich water is an easily-accessible, safe,
cost-effective and promising treatment. The hydrogen gas mainly
enters the blood circulation following entry into the human body
and is mainly excreted with respiration, it is not metabolized via
the liver or kidneys, and it has no toxicity on human body
(30).
It is unknown whether hydrogen-rich water exerts
anti-oxidative stress effects on tumor tissues, and the local
control rate, progression-free survival and overall survival must
be observed through patient follow-up to verify that hydrogen-rich
water does not compromise the effectiveness of chemotherapy.
In the present study, hydrogen-rich water exhibited
good efficacy and safety in protecting liver function. However, a
large number of randomized clinical trials are required to confirm
whether this treatment may be applied in the clinical setting and
whether it affects the efficacy of chemotherapy.
Acknowledgements
The authors would like to thank the Department of
Pathology, Taishan Medical University. The present study was
supported by the Taian Science and Technology Fund (grant no.
2015NS2098).
References
|
1
|
Christova TY, Gorneva GA, Taxirov SI,
Duridanova DB and Setchenska MS: Effect of cisplatin and cobalt
chloride on antioxidant enzymes in the livers of Lewis lung
carcinoma bearing mice: Protective role of heme oxygenase. Toxicol
Lett. 138:235–242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Koc A, Duru M, Ciralik H, Akcan R and
Sogut S: Protective agent, erdosteine, against cisplatin-induced
hepatic oxidant injury in rats. Mol Cell Biochem. 278:79–84. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robinson K, Lambiase L, Li J, Monteiro C
and Schiff M: Fatal cholestatic liver failure associated with
gemcitabine therapy. Dig Dis Sci. 48:1804–1808. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bibeau F, Azria D, Chateau MC, Borrelly C,
Ychou M, Quenet F and Rouanet P: Vascular hepatic injury following
neoadjuvant treatment for a cardial adenocarcinoma. Gastroenterol
Clin Biol. 30:611–613. 2006.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang CS, Kawamura T, Toyoda Y and Nakao
A: Recent advances in hydrogen research as a therapeutic medical
gas. Free Radic Res. 44:971–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cardinal JS, Zhan J, Wang Y, Sugimoto R,
Tsung A, McCurry KR, Billiar TR and Nakao A: Oral hydrogen water
prevents chronic allograft nephropathy in rats. Kidney Int.
77:101–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mao YF, Zheng XF, Cai JM, You XM, Deng XM,
Zhang JH, Jiang L and Sun XJ: Hydrogen-rich saline reduces lung
injury induced by intestinal ischemia/reperfusion in rats. Biochem
Biophys Res Commun. 381:602–605. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
National Comprehensive Cancer Network:
(NCCN) Clinical Practice Guidelines in Oncology. Colon Cancer.
Version 1. 2012 https://www.nccn.org/professionals/physician_gls/f_guidelines.aspAccessed.
August 30–2011.
|
|
9
|
Fong Y and Bentrem DJ: CASH
(chemotherapy-associated steatohepatitis) costs. Ann Surg. 243:8–9.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Maor Y and Malnick S: Liver injury induced
by anticancer chemotherapy and radiation therapy. Int J Hepatol.
2013:8151052013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pawlik TM, Olino K, Gleisner AL, Torbenson
M, Schulick R and Choti MA: Preoperative chemotherapy for
colorectal liver metastases: Impact on hepatic histology and
postoperative outcome. J Gastrointest Surg. 11:860–868. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vauthey JN, Pawlik TM, Ribero D, Wu TT,
Zorzi D, Hoff PM, Xiong HQ, Eng C, Lauwers GY, Mino-Kenudson M, et
al: Chemotherapy regimen predicts steatohepatitis and an increase
in 90-day mortality after surgery for hepatic colorectal
metastases. J Clin Oncol. 24:2065–2072. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tamandl D, Klinger M, Eipeldauer S,
Herberger B, Kaczirek K, Gruenberger B and Gruenberger T:
Sinusoidal obstruction syndrome impairs long-term outcome of
colorectal liver metastases treated with resection after
neoadjuvant chemotherapy. Ann Surg Oncol. 18:421–430. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laurent A, Nicco C, Van Nhieu Tran J,
Borderie D, Chéreau C, Conti F, Jaffray P, Soubrane O, Calmus Y,
Weill B and Batteux F: Pivotal role of superoxide anion and
beneficial effect of antioxidant molecules in murine
steatohepatitis. Hepatology. 39:1277–1285. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pessayre D, Berson A, Fromenty B and
Mansouri A: Mitochondria in steatohepatitis. Semin Liver Dis.
21:57–69. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rubbia-Brandt L, Audard V, Sartoretti P,
Roth AD, Brezault C, Le Charpentier M, Dousset B, Morel P, Soubrane
O, Chaussade S, et al: Severe hepatic sinusoidal obstruction
associated with oxaliplatin-based chemotherapy in patients with
metastatic colorectal cancer. Ann Oncol. 15:460–466. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nakano H, Oussoultzoglou E, Rosso E,
Casnedi S, Chenard-Neu MP, Dufour P, Bachellier P and Jaeck D:
Sinusoidal injury increases morbidity after major hepatectomy in
patients with colorectal liver metastases receiving preoperative
chemotherapy. Ann Surg. 247:118–124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dyson JK, McPherson S and Anstee QM:
Non-alcoholic fatty liver disease: Non-invasive investigation and
risk stratification. J Clin Pathol. 66:1033–1045. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hayashida K, Sano M, Ohsawa I, Shinmura K,
Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, et
al: Inhalation of hydrogen gas reduces infarct size in the rat
model of myocardial ischemia-reperfusion injury. Biochem Biophys
Res Commun. 373:30–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Buchholz BM, Kaczorowski DJ, Sugimoto R,
Yang R, Wang Y, Billiar TR, McCurry KR, Bauer AJ and Nakao A:
Hydrogen inhalation ameliorates oxidative stress in transplantation
induced intestinal graft injury. Am J Transplant. 8:2015–2024.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun Q, Kang Z, Cai J, Liu W, Liu Y, Zhang
JH, Denoble PJ, Tao H and Sun X: Hydrogen-rich saline protects
myocardium against ischemia/reperfusion injury in rats. Exp Biol
Med (Maywood). 234:1212–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fukuda K, Asoh S, Ishikawa M, Yamamoto Y,
Ohsawa I and Ohta SP: Inhalation of hydrogen gas suppresses hepatic
injury caused by ischemia/reperfusion through reducing oxidative
stress. Biochem Biophys Res Commun. 361:670–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cai J, Kang Z, Liu WW, Luo X, Qiang S,
Zhang JH, Ohta S, Sun X, Xu W, Tao H and Li R: Hydrogen therapy
reduces apoptosis in neonatal hypoxia-ischemia rat model. Neurosci
Lett. 441:167–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fu Y, Ito M, Fujita Y, Ito M, Ichihara M,
Masuda A, Suzuki Y, Maesawa S, Kajita Y, Hirayama M, et al:
Molecular hydrogen is protective against 6-hydroxydopamine-induced
nigrostriatal degeneration in a rat model of Parkinson's disease.
Neurosci Lett. 453:81–85. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakashima-Kamimura N, Mori T, Ohsawa I,
Asoh S and Ohta S: Molecular hydrogen alleviates nephrotoxicity
induced by an anti-cancer drug cisplatin without compromising
anti-tumor activity in mice. Cancer Chemother Pharmacol.
64:753–761. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Yang L, Tao K, Vizcaychipi MP,
Lloyd DM, Sun X, Irwin MG, Ma D and Yu W: Protective effects of
hydrogen enriched saline on liver ischemia reperfusion injury by
reducing oxidative stress and HMGB1 release. BMC Gastroenterol.
14:122014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Q, Shen WF, Sun HY, Fan DF, Nakao A,
Cai JM, Yan G, Zhou WP, Shen RX, Yang JM and Sun XJ: Hydrogen-rich
saline protects against liver injury in rats with obstructive
jaundice. Liver Int. 30:958–968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu XF and Zhang J: Saturated hydrogen
saline attenuates endotoxin-induced acute liver dysfunction in
rats. Physiol Res. 62:395–403. 2013.PubMed/NCBI
|
|
30
|
Levitt MD and Bond JH Jr: Volume,
composition, and source of intestinal gas. Gastroenterology.
59:921–929. 1970.PubMed/NCBI
|