Introduction
Gastrinoma is a rare neuroendocrine tumor (NET) with
an annual incidence of 0.05-0.2 cases per 100,000 population
overall (1). When Zollinger-Ellison
described the eponymous syndrome (Zollinger-Ellison syndrome, ZES)
in 1955(2), the pancreas seemed the
most frequent location of gastrinomas. Over time, however, studies
have found that gastrinoma occurs far more commonly in the duodenum
than in the pancreas (2). Gastrinoma
is the cause of 0.1% of peptic ulcers and 2-5% of recurrent ulcers
(3). The tumors are often
misdiagnosed because of their relative rarity and lack of specific
symptoms. Typical symptoms include abdominal pain, secretory
diarrhea, esophagitis, and hypercalcemia (4,5). The
prognosis of sporadic and multiple endocrine neoplasia type 1
(MEN1)-associated duodenal gastrinoma is better than that of
pancreatic gastrinoma, as the former slowly progresses to
metastasis (6). Surgical management
is the only curative treatment for gastrinoma (7). Malignancy in gastrinoma is graded by
assessing resected specimens according to the World Health
Organization (WHO) 2017 criteria (1). Ki-67 scoring in cytology is an
alternative approach for establishing the gastrinoma grade
(8). However, the feasibility of
such grading using cytopathological specimens remains unclear
(1).
Cytopathological analysis of the Ki-67 index of
duodenal gastrinoma has never been described previously (9,10).
Therefore, this study determined the optimal method of measuring
the Ki-67 index and the role of cytomorphology in cytological and
histopathological specimens, using resected duodenal gastrinomas as
the standard criterion.
Case report
Materials
For cytological examination, to simulate the
clinical practice of sampling methods by fine needle aspiration, we
sampled freshly removed surgical specimens by aspiration biopsy
using an 18G needle mounted on a syringe. Several smears were
obtained which were wet fixed with 95% ethanol for at least 15 min
and dried in cold air.
Methods
Pap and May-Grünwald-Giemsa stains were performed,
and immunocytochemistry (ICC) staining was done using chromogranin
A (Nichirei Biosciences Inc.), cytokeratin AE1/AE3 (Agilent
Technologies Japan, Ltd.), gastrin (Agilent Technologies Japan),
Ki-67 antigen (Agilent Technologies Japan), and synaptophysin
(Agilent Technologies Japan). For dilutions in immunohistochemistry
(IHC) and ICC, we used the commercial base of antibodies. Retrieval
methods were followed in accordance with the IMMUNOSAVER protocol
(Nissin EM Co. Ltd.).
To precisely evaluate Ki-67 positive nuclei in both
samples, ‘hot spots’ with more frequent positive nuclei were
identified on both cytopathological and histopathological
preparations. Digital images of the latter were taken, and positive
nuclei were manually counted in 10 different microscopic images
taken in equivalent high power magnification, (magnification, x400)
microscopic fields.
For histological examination, tissues fixed in 10%
buffered formalin were dehydrated and embedded in paraffin. Then,
5-µm-thick sections were cut and stained with hematoxylin and eosin
and Verhoeff-van Gieson elastic stain. IHC was performed in the
same manner as ICC, except deparaffinization.
For electron microscopy, specimens were immersed in
a fixative containing 1.5% glutaraldehyde buffered with a 0.1 M
phosphate buffer (pH 7.4), postfixed, with osmium tetroxide,
dehydrated in a graded series of ethanol baths, and embedded in
epoxy resin. Ultrathin sections were cut, double-stained with
uranyl acetate and lead citrate, and examined under an 80 kv H-7650
electron microscope (Hitachi).
The length of tumor cell nuclei, nuclear cleavage,
and neurosecretory granules were measured using Image J software
and then statistically compared.
Statistical analysis
All statistical analyses were performed using EZR
version 1.27 (Saitama Medical Center, Jichi Medical University).
Between-group differences at specific stages were assessed using
Student's t-test (unpaired t-test assuming equal variances).
P<0.001 was considered statistically significant.
Case 1
A 56-year-old man was admitted to Amagasaki Chuo
Hospital, Hyogo, Japan, for abdominal pain, increased serum
hepatobiliary enzymes, and biliary sludge and dilatation of the
common bile duct on computed tomography (CT). Upper
gastrointestinal (GI) endoscopic examination showed a duodenal
submucosal tumor, and incisional biopsy revealed gastrinoma.
Laparoscopic partial gastrectomy around the pyloric ring was
performed, and postoperative course was uneventful.
Postoperatively, the patient's serum gastrin was 84 pg/ml, and the
serum intact parathyroid hormone (PTH) level was 37 pg/ml. After 3
years of follow-up, the patient is alive and has not had any
complaints. Macroscopically, the resected tumor located at the
duodenal gastrinoma triangle measured 12x10 mm, with a fibrous
capsule showing partial invasion into neighboring tissue. The cut
surface had a light- yellow tinge similar in color to the
functioning tumor's cut surface. Pap staining showed a highly
cellular neoplasm with a clean background (Fig. 1). Neoplastic cells were found both in
loosely cohesive clusters and as dispersed elements. The nuclei
were uniform and round to oval and contained coarsely granular
chromatin, referred to as a ‘salt and pepper pattern.’ An organoid
pattern was observed, in the form of rosette-like groups, a finding
that is suspicious for gastrinoma. Optically recognizable mitotic
figures were not observed.
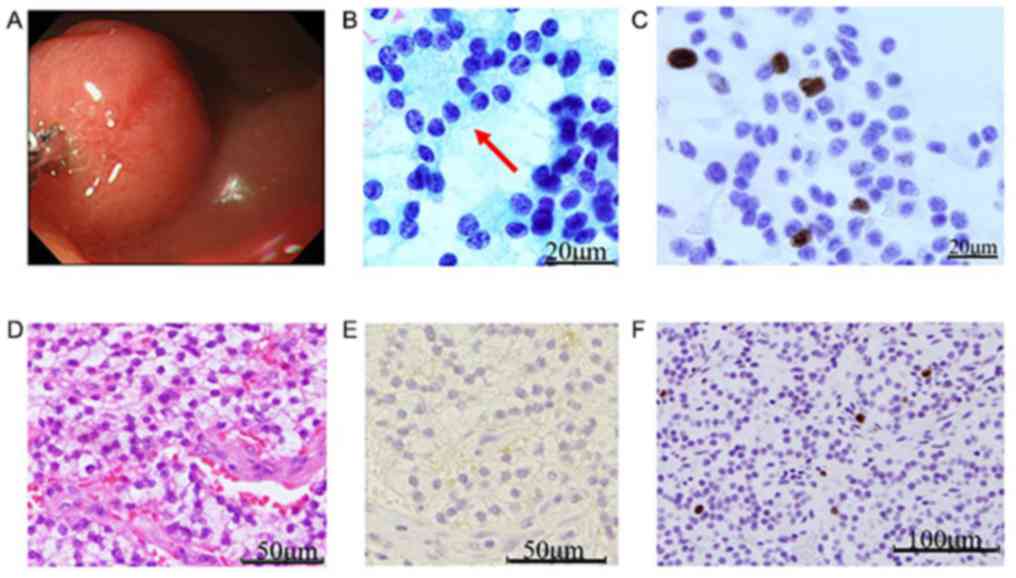 | Figure 1Case 1. (A) Endoscopic view showing
duodenal gastrinoma in the gastrinoma triangle, measuring 12x10 mm
with a smooth surface and a light yellow-tinged cut surface. (B)
Pap staining of a cytologic smear, showing a highly cellular
organoid neoplasm with a clean background. Neoplastic cells
presented as loosely cohesive clusters or as singly dispersed
elements. Plasmacytoid configuration was characteristic. Uniform,
round-to-oval nuclei contain coarsely granular chromatin, also
defined as a ‘salt-and-pepper pattern’. Rosette-like structures
(arrow), characteristic of gastrinomas could also be observed.
Mitotic figures were not observed (Pap stain; magnification,
x1,000). (C) ICC detecting expression of Ki-67 antigen
(magnification, x400). (D) Tumor cells displayed an organoid
pattern, with oval-shaped nuclei and clear cytoplasms. Rosette-like
arrangements could occasionally be observed. Definite atypia or
mitotic figures are both rare (hematoxylin and eosin stain;
magnification, x400). (E) IHC staining for gastrin (magnification,
x400). (F) Positive expression of Ki-67 antigen (IHC;
magnification, x400). Pap, Papanicolou; ICC, immunocytochemistry;
IHC, immunohistochemistry. |
Table I shows ICC
results. IHC results were identical to ICC results. Therefore, a
diagnosis of gastrinoma of the duodenum, NET G1, was made (4).
 | Table IICC and IHC results of Cases 1 and
2. |
Table I
ICC and IHC results of Cases 1 and
2.
| Parameters | Case 1 | Case 2 | P-value |
|---|
| Salt and pepper
pattern | + | + | |
| Rosette-like
arrangement | + | + | |
| Chromogranin A
(ICC) | + | + | |
| Cytokeratin AE1/AE3
(ICC) | + | + | |
| Gastrin (ICC) | + | + | |
| Ki-67 antigen (ICC),
% | 2 | 14 | |
| Ki-67 antigen (IHC),
% | 2 | 10 | |
| Synaptophysin
(ICC) | + | + | |
| Mitosis | <2/10 HPF | <2/10 HPF | |
| Level of tumor cell
nuclear cleavage, % | 0.10 | 7.65 | <0.001 (tumor
cells, n=954) |
| Mean of major axis of
tumor cell nuclei, µm | 6.13 | 6.46 | <0.001 (tumor
cells, n=486) |
| Mean of major axis of
neurosecretory granules, nm | 207.94 | 427.94 | <0.001 (granules,
n=49) |
| Focal margin
invasion | - | + | |
| Lymphovascular
invasion | - | partial + | |
| Histology | NET G1 | NET G2 | |
Case 2
A 66-year-old woman with epigastric distress 44
months prior to admission to our hospital underwent upper GI
tract-endoscopic examination; duodenal bulb biopsy revealed a NET.
Chest and abdominal CT showed no particular changes; however, we
detected, calcification of the right thyroid lobe. Cystic lesions
of the liver and kidney were also observed, while cranial magnetic
resonance imaging did not reveal pituitary adenoma. Her parathyroid
glands showed no swelling. Abdominal exploratory subtotal resection
of the stomach including the pyloric ring, was performed, using the
Roux-en-Y method. The patient's serum gastrin was 684 pg/ml before
surgery and 80 pg/ml after surgery, and her serum intact PTH level
was 15 pg/ml. After 2 years of follow-up, the patient has not
complained of GI tract symptoms. The submucosal tumor at the
duodenal bulb measured 10x10x7 mm, and its cut surface, too,
revealed a light-yellow tinge. Pap staining of the obtained smears
showed tumor cells arranged in loosely cohesive clusters or
individually; both contained slightly coarse cytoplasmatic
granules. The tumor cell chromatin was finely granular and showed a
salt-and-pepper or focally dispersed pattern. Nuclear atypia was
present, but mitotic figures were rare (Fig. 2). Table
I shows ICC results. The tumor capsule/pseudocapsule showed
focal invasion but was mostly intact, while partial lymphovascular
invasion was observed. IHC results were similar to ICC results.
Therefore, a diagnosis of gastrinoma of the duodenum, NET G2, was
made. Table I also shows comparisons
of the Ki-67 index and nuclear cleavage between Cases 1 and 2. The
Ki-67 indexes of Cases 1 and 2 were cytologically 2 and 14%,
respectively. Alternatively, histopathologically they were 2 and
10%, respectively. The optical mitotic count was <2/10 high
power fields (HPF) in both cases. The tumor cell nuclear cleavage
level was statistically significant (P<0.001; n=954) between
Cases 1 and 2. In addition, the major axis of tumor cell nuclei was
statistically significant (P<0.001 0.001; n=486) between Cases 1
and 2 (Table I). We found variable
numbers of membrane-bound electron-dense neurosecretory granules
(Fig. 3), either nonpolarized within
the cytoplasm or oriented near the basal surfaces facing the
capillaries. The major axis of neurosecretory granules on
ultrastructural findings was statistically significant (P<0.001;
n=49) between Cases 1 and 2 (Table
I).
 | Figure 2Case 2. (A) Submucosal tumor of the
duodenal bulb measuring 10x10x7 mm with a light-yellow-tinged cut
surface. (B) Pap staining revealed that tumor cells were arranged
in loosely cohesive acinar clusters with slightly coarse
cytoplasmic granules. Tumor cell chromatin exhibited a
salt-and-pepper or dispersed arrangement. Nuclear atypia (arrow)
was present but mitotic figures were rare (magnification, x400).
(C) ICC for expression of Ki-67 antigen (magnification, x400). (D)
Tumor cells form trabecular, glandular or mixed structures. The
tumor capsule/pseudocapsule was relatively well preserved, with
partial lymphovascular invasion. Rosette-like structures were
occasionally visible and mitotic figures were rare (hematoxylin and
eosin; magnification, x400). (E) IHC for expression of gastrin
(magnification, x400). (F) IHC for expression of Ki-67 antigen
(magnification, x400). Pap, Papanicolou; ICC, immunocytochemistry;
IHC, immunohistochemistry. |
Discussion
We found no remarkable differences in clinical
characteristics between Cases 1 and 2; that is, neither tumor
revealed ZES or MEN1 findings. Differences between Cases 1 and 2
were observed not only at the serum gastrin level but also in the
following cytopathological findings. Among cytological findings,
the Ki-67 index, the nuclear cleavage level, the major axis of
tumor cell nuclei, and the major axis of neurosecretory granules in
Case 1 were lower than in Case 2. Among histopathological findings,
focal margin invasion by tumor extension and lymphovascular
invasion were observed in Case 2 but not in Case 1 (Table I).
With regard to the development and differentiation
of gastrinoma cells, Imamura et al (11) have reported that duodenal gastrinomas
do not seem to behave in as malignant a fashion as sporadic
pancreatic gastrinomas. However, this report does, not validate
this difference. On the basis of the Ki-67 index as a potential
prognostic marker, we also applied the WHO Ki-67 labeling scheme
for grading on cytological samples. The two cases in this study
indicated that cytologic samples from duodenal gastrinomas can be
accurately graded on the basis of the WHO Ki-67 labeling scheme,
indicating that Ki-67 scoring of cytology preparations is an
alternative approach for establishing the pancreatic NET (PanNET)
grade, as shown by Farrell et al (8).
As stated by Adsay (12) in an editorial in the American
Journal of Surgical Pathology, the question is no longer
whether to count Ki-67 in gastrointestinal and pancreatobiliary
tract ΝΕΤs; rather, it is how to perform this count and how to
improve the diagnostic and prognostic value of this proliferation
marker (12). However, the new WHO
classification states, that mitotic figures are not usually seen in
gastrinomas and that nonfunctioning pancreatic neuroendocrine tumor
(NF-PanNET) grading on the basis of cytological specimens is not
well established (1). The data
presented here revealed that mitotic figures are not contributory
for the evaluation of potential malignancy; rather, the Ki-67 index
is really contributive.
According to Chatzipantelis et al (9), with regard to cytopathological
findings, nuclear pleomorphism/multinucleation and the presence of
nucleoli are also reliable for predicting malignant PanNETs.
Therefore, we also considered nuclear pleomorphism as a criterion
suggestive of potential malignancy. Our findings revealed a
positive relationship between nuclear pleomorphism and the Ki-67
index.
In recent years, numerous suggestions have been made
to predict the biological behavior of these tumors. The most recent
WHO classification includes several clinicopathologic criteria:
Tumor size, metastasis likelihood, vascular invasion, necrosis,
status of regional lymph nodes, tumor grade, mitotic rate, and
Ki-67 index (1).
In conclusion, this study demonstrates the role of
proliferative activity (Ki-67) in cytology specimens as a reliable
predictive factor for duodenal gastrinomas. In addition, specific
cytologic features such as nuclear cleavage and nuclear major axis
can contribute to the prediction of these tumors. However, because
of the limited number of cases here (two), large series will be
needed to confirm our findings.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
The present study was designed and organized by HN,
KM, MK, HM, CY and TKK. Clinical data were corrected by HN and CY.
Immunostaining was evaluated by HN. HN, CY and TKK contributed to
data analysis, interpretation and drafting manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate.
Ethical approval for the study was obtained from the
Ethics Committee of Amagasaki Chuo Hospital and the two patients
provided informed consent.
Patient consent for publication
Written informed consent was obtained from the two
patients for publication of the clinical data and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lloyd RV, Osamura RY, Klöppel G and Rosai
J: WHO classification of tumours of endocrine organs 4th edition,
Lyon, France: IARC Press: pp 229-232, 2017.
|
|
2
|
Zollinger RM and Ellison EH: Primary
peptic ulcerations of the jejunum associated with islet cell tumors
of the pancreas. 1955. CA Cancer J Clin. 39:231–247.
1989.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rosentraeger MJ, Garbrecht N, Anlauf M,
Raffel A, Knoefel WT, Wiedenmann B and Klöppel G: Syndromic versus
non-syndromic sporadic gastrin-producing neuroendocrine tumors of
the duodenum: Comparison of pathological features and biological
behavior. Virchows Arch. 468:277–287. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Roy PK, Venzon DJ, Shojamanesh H,
Abou-Saif A, Peghini P, Doppman JL, Gibril F and Jensen RT:
Zollinger-Ellison syndrome. Clinical presentation in 261 patients.
Medicine (Baltimore). 79:379–411. 2000.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang WD, Liu DR, Wang P, Zhao JG, Wang ZF
and Chen LI: Clinical treatment of gastrinoma: A case report and
review of the literature. Oncol Lett. 11:3433–3437. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Anlauf M, Garbrecht N, Henopp T, Schmitt
A, Schlenger R, Raffel A, Krausch M, Gimm O, Eisenberger CF,
Knoefel WT, et al: Sporadic versus hereditary gastrinomas of the
duodenum and pancreas: Distinct clinic-pathological and
epidemiogical features. World J Gastrenteterol. 12:5440–5446.
2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Norton JA, Fraker DL, Alexander HR, Venzon
DJ, Doppman JL, Serrano J, Goebel SU, Peghini PL, Roy PK, Gibril F
and Jensen RT: Surgery to cure the Zollinger-Ellison syndrome. N
Engl J Med. 341:635–644. 1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Farrell JM, Pang JC, Kim GE and Tabatabai
ZL: Pancreatic neuroendocrine tumors: Accurate grading with Ki-67
index on fine-needle aspiration specimens using the WHO 2010/ENETS
criteria. Cancer Cytopathol. 122:770–778. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chatzipantelis P, Konstantinou P,
Kaklamanos M, Apostolou G and Salla C: The role of cytomorphology
and proliferative activity in predicting biological behavior of
pancreatic neuroendocrine tumors: A study by endoscopic
ultrasound-guided fine-needle aspiration cytology. Cancer.
117:211–216. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Klöppel G and Anlauf M:
Gastrinoma-morphological aspects. Wien Klin Wochenschr.
119:579–584. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Imamura M, Kanda M, Takahashi K, Shimada
Y, Miyahara T, Wagata T, Hashimoto M, Tobe T and Soga J:
Clinicopathological characteristics of duodenal microgastrinomas.
World J Surg. 16:703–709. 1992.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Adsay V: Ki67 labeling index in
neuroendocrine tumors of the gastrointetinal and pancreatobiliary
tract: To count or not to count is not the question, but rather how
to count. Am J Surg Pathol. 36:1743–1746. 2012.PubMed/NCBI View Article : Google Scholar
|

















