1. Introduction
Breast cancer is the most common cancer across the
world and despite increased awareness and screening, it is still
one of the leading causes of mortality in females (1). There is an obvious need for improved
breast cancer therapeutics. In comparison to chemotherapy and
radiation therapy for cancer treatment, immunotherapy is still in
its infancy, but is a rapidly expanding repertoire of anticancer
therapies. Immunotherapies help the patient's own immune system to
fight tumour cells (2). There are
several different types of cancer immunotherapies, including, but
not limited to, checkpoint inhibitors, cytokines, immunomodulators,
cancer vaccines, and monoclonal antibodies (3).
Recent evidence has suggested a link between certain
immune system receptors and cancer development. Toll-like receptors
(TLRs) are a family of pattern recognition receptors (PRRs) that
play important roles in innate immunity (4). TLRs recognize cognate ligands
including pathogen-associated molecular patterns (PAMPs) and
damage-associated molecular patterns (DAMPs). Recognition of PAMPs
or DAMPs triggers signalling cascades that culminate in the
expression of proinflammatory cytokines and/or type I interferon
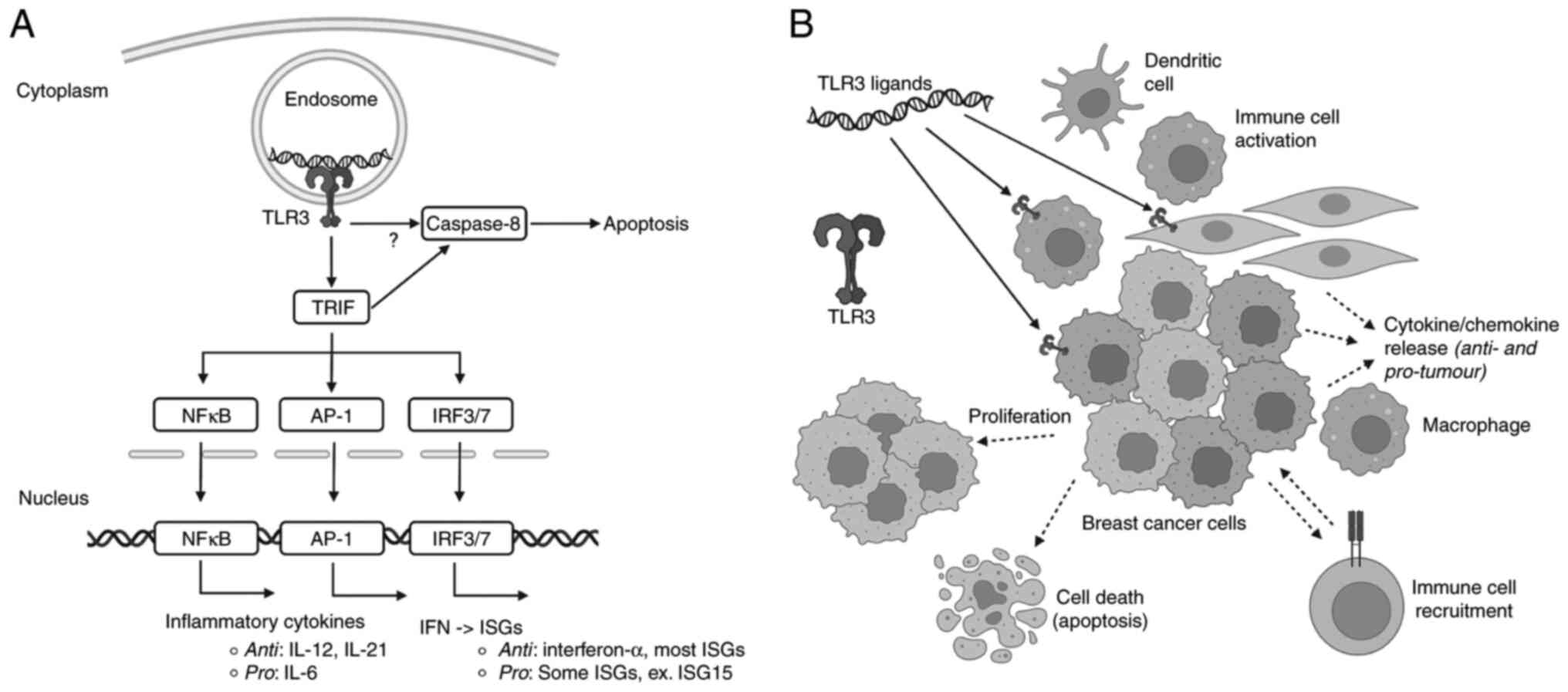
(IFN) pathways, Fig. 1 (4). TLRs can be expressed on a variety of
immune cells, such as dendritic cells and macrophages, and
non-immune cells, such as fibroblasts and epithelial cells
(5). Certain cancer cells have
been found to have dysregulated expression of TLRs compared to
normal cells, suggesting the potential application of TLR ligands
as cancer therapies (6). Despite
their important roles in innate immunity, and the established link
between immunity and cancer establishment and progression, there
are only three TLR-agonists that have been approved for anti-cancer
usages (7). The role of TLRs in
cancer has been previously reviewed for multiple types of cancer
(8).
TLR3, the focus of this review, binds to nucleic
acids, specifically double stranded (ds)RNA (9). Binding to long dsRNA in the endosome
leads to dimerization and activation of TLR3, recruiting the
adaptor protein a toll/interleukin (IL)-1) receptor
(TIR)-domain-containing adapter-inducing interferon-β (TRIF; also
known as TICAM-1) protein. TRIF initiates downstream pathways which
lead to activation of transcription factors including
interferon-regulatory factors (IRF3/7), activator protein 1 (AP-1),
and Nuclear Factor kappa B (NF-kB), culminating in type I
IFN/IFN-stimulated genes, and inflammatory cytokines, Fig. 1 (4). TLR3 is characterized by extracellular
leucine-rich repeats (LRRs), a transmembrane domain, and a
cytoplasmic tail with a TIR domain (9). TLR3 is highly expressed in endosomes
of antigen-presenting cells (APCs), epithelial cells, and other
cells with expression varying depending on the tissue and cell-type
(10). TLR3 is also expressed on
endosomal membranes of myeloid dendritic cells, endothelial cells,
keratinocytes, and some cancer cells (11,12).
TLR3 activation can occur in cancerous cells or surrounding cells
within the tumour microenvironment, including immune cells, and
activation can have a variety of outcomes, Fig. 1. While there is variability in
responses, general anticancer effects of treatment with
TLR3-activators include production of anticancer cytokines, such as
IFN-α, IL-12, IL-21, and the induction of apoptosis, possibly
through intrinsic and extrinsic pathways (9,10,12-14)
The cytokines produced through TLR3 stimulation induce the
activation of tumour-suppressive macrophages and neutrophils
(15).
On the other hand, TLR3 activation can promote
tumour recurrence, metastasis, and cell proliferation through the
production of pro-tumour cytokines, such as IL-6, and machinery
involved in other hallmarks of cancer, such as production of
hypoxia-include factor 1α to improve resistance to hypoxia, and
secretion of vascular endothelia growth factor to support
angiogenesis (16-17). To further highlight the duality of
this receptor, pro- and antitumour effects have been seen within
the same cell line depending on the delivery mode of the
TLR3-ligand, in two breast cancer cell lines surface stimulation
has resulted in protumoural effects whereas cytoplasmic stimulation
has been antitumoural (16). In
human lung cancer cells, TLR3 activation leads to the production of
cytokines that enhance migration, and in human melanoma cells
TLR-agonist mediated effects were improved when one of the induced
cytokines was blocked, demonstrating the complexity of the induced
proteins from TLR3 activation (18,19).
In breast cancer, TLR3 expression can be increased or decreased
compared to normal cells, and the function of TLR3 in various
cancerous cells differs. TLR3 activation can induce apoptosis in
some tumour cells, while inducing tumour cell proliferation in
other cells (10). In a large
association study, it was found that two single-nucleotide
polymorphisms were susceptibility variants within TLR3, and these
variants were associated with larger tumour size (20).
There are currently no TLR3-based therapies approved
for any cancers, however the preclinical data suggest that these
therapies may prove to be novel treatments or may enhance existing
therapies (7). As TLR3 activation
can hinder or promote cancer development, despite appearing
paradoxical, there is the potential for TLR-agonist or -antagonists
as breast cancer therapies (21).
In this review we will summarize the current TLR3-dependent breast
cancer immunotherapies, Table I,
and associated clinical trials, Table
II, to provide perspective on the TLR3-dependent therapy
pipeline and to help guide further research into breast cancer
therapeutics (22-34).
 | Table IA summary of TLR3 agonist and
antagonist therapies and their proposed mechanisms. |
Table I
A summary of TLR3 agonist and
antagonist therapies and their proposed mechanisms.
| Therapy (Poly
(I:C)) | Composition | Mechanism |
|---|
| Polyinosinic:
polycytidylic acid | Synthetic dsRNA,
TLR3 agonist | - Activation of NK
cells |
| | | - Induction of type
I interferons |
| | | - Cytotoxicity |
| | | - Dendritic cell
maturation |
| Poly ICLC
(Hiltonol) | Stabilized with
poly-lysine, TLR3 agonist | - Activation and
infiltration of CTLs and NK cells |
| Poly
IC12U (Rintatolimod, IPH 3102, Ampligen) | Modified Poly (I:C)
with cytidine replaced by uridine, TLR3 agonist | - Activation of
CTLs and NK cells |
| | | - Converts M2-type
macrophages to M1-type macrophage |
| | | - Induction of type
I interferons |
| | | - Cytotoxicity |
|
Polyadenylic-polyuridylic acid
(Poly(A:U) | Synthetic dsRNA,
TLR3 agonist | - Induction of type
I interferons |
| | | - Cytotoxicity |
| C10
(Phenylmethimazole) | TLR3 inhibitor | - Blocks IL-6 |
 | Table IIA summary of clinical trials using
TLR3 ligands. |
Table II
A summary of clinical trials using
TLR3 ligands.
| A, Poly ICLC
(Hiltonol) |
|---|
| Clinical trial
number | Combination
treatments | Phase | Status |
|---|
| NCT00986609 | - MUC-1 peptide
vaccine | Early phase 1 | Completed |
| NCT02643303 | -
Durvalumaba | Phase 1 | Completed |
| | -
Tremelimumaba | Phase 2 | |
| NCT02826434 | - PVX-410
vaccine | Phase 1 | Active (not
recruiting) |
| | -
Durvalumaba | | |
| NCT03362060 | -
Pembrolizumaba | Phase 1 | Active (not
recruiting) |
| | - PVX-410
vaccine | | |
| | - Montanide
(adjuvant) | | |
| NCT05098210 | - Neoantigen
peptide vaccine | Phase 1 | Recruiting |
| | -
Nivolumaba | | |
| NCT03789097 | -
Pembrolizumaba | Phase 1 | Recruiting |
| | - Flt3 ligand
vaccine | Phase 2 | |
| | - Radiation | | |
| NCT05098210 | - Neoantigen
peptide vaccine | Phase 1 | Recruiting |
| | -
Nivolumaba | | |
| NCT03606967 | -
Carboplatinb | Phase 2 | Recruiting |
| | -
Durvalumaba | | |
| | - Gemcitabine
hydrochlorideb | | |
| | -
Nab-paclitaxelb | | |
| | - Personalized
synthetic long peptide vaccine | | |
| | -
Tremelimumaba | | |
| NCT03606967 | -
Nab-paclitaxelb | Phase 2 | Recruiting |
| | - Durvalumab | | |
| | -
Tremelimumaba | | |
| | - Neoantigen
vaccine | | |
| NCT04116320 | - Echopulse
device | Phase 1 | Recruiting |
| | - Standard of care
PD-1 therapya | | |
| NCT01532960 | - 9 peptides from
Her-1/neu, CEA, CTA | Phase 1 | Terminated
(Futility for immune responses to the vaccine, component of study
drug was in short supply) |
| | - Peptide-TET | | |
| NCT02427581 | - Personalized
synthetic long peptide vaccine | Phase 1 | Withdrawn (drugs
not available) |
| NCT04616248 | - CDX01140
(anti-CD40 agonist mAb)a | Phase 1 | Withdrawn
(implementation issues) |
| | - Radiation
therapy | | |
| | - Recombinant Flt3
liganda | | |
| NCT02427581 | - Personalized
synthetic long peptide vaccine | Phase 1 | Withdrawn (drugs
not available) |
| NCT02661100 | -
Pembrolizumaba | Phase 1 | Withdrawn (drug
unavailable) |
| | -
CDX1401a | Phase 2 | |
| NCT01984892 | | Phase 2 | Terminated (low
enrollment) |
| B, Poly
IC12U (Rintatolimod, IPH 3102, Ampligen) |
| Clinical trial
number | Combination
treatments | Phase | Status |
| NCT01355393 | - HER-2/neu peptide
vaccine - Sargramostima | Phase 1 | Completed |
| | | Phase 2 | |
| NCT03599453 | - Chemokine
modulation therapy | Early phase 1 | Active (not
recruiting) |
| | - Celecoxib | | |
| | - Recombinant
interferon alfa-2b | | |
| | -
Pembrolizumab | | |
| NCT04081389 | - Celecoxib | Phase 1 | Suspended
(analyzing data) |
| | -
Cyclophosphamideb | | |
| | - Doxorubicin
hydrochlorideb | | |
| | -
Paclitaxelb | | |
| | - Recombinant
Interferon Alfa-2b | | |
| C, Poly (A:U) |
| Clinical trial
number | Combination
treatments | Phase | Status |
| NCT01355393 | - HER-2/neu peptide
vaccine | Phase 1 | Completed |
| | -
Sargramostima | Phase 2 | |
| NCT03599453 | - Chemokine
modulation therapy | Early phase 1 | Active (not
recruiting) |
| | - Celecoxib | | |
| | - Recombinant
interferon alfa-2b | | |
| | -
Pembrolizumab | | |
| NCT04081389 | - Celecoxib | Phase 1 | Suspended
(analyzing data) |
| | -
Cyclophosphamideb | | |
| | - Doxorubicin
hydrochlorideb | | |
| | -
Paclitaxelb | | |
| | - Recombinant
Interferon alpha-2b | | |
2. TLR3 agonist therapies
TLR3 has the capability, when activated, to induce
apoptosis and recruit immune cells to attack tumour cells,
therefore TLR3 agonists could have anticancer capabilities
(21). A main consideration is the
administration of the therapy. With systemic administration of TLR3
agonists, there is potential for chronic inflammation and concerns
regarding therapeutic doses reaching the tumour cells (34). Multiple TLR3 agonists have been
explored as potential cancer treatments and cancer vaccine
adjuvants, Table I, many of which
have reached phase 1 and 2 clinical trials, Table II (35). TLR3 agonists are strong
immunomodulators that activate adaptive and innate immune responses
and help promote the recruitment of cluster of differentiation
(CD)8+ T lymphocytes, natural killer (NK) cells, and
dendritic cell maturation, Table I
(36). They also promote the
production of anti-tumour Th1 (type 1 T helper) cytokines (28). TLR3 agonists have been explored as
monotherapies or combination therapies with existing treatments,
with many in vitro studies elucidating monotherapies, and
most clinical trials focused on combination therapies with standard
chemotherapeutics or other immunotherapies (37-39).
Combination therapies have the potential to increase efficacy of
the standard dosage or maintain efficacy while reducing the dosage
of conventional treatments, decreasing negative side effects
(39).
Poly (I:C)
The largest body of dsRNA research in breast cancer
relies on polyinosinic:polycytidylic acid (poly (I:C)) and poly
(I:C) derivatives. Poly (I:C) is a synthetic dsRNA that has no
sequence variation and has a high affinity for mammalian TLR3
compared to other dsRNA molecules (40). In many cells, including in breast
cancer, poly (I:C) binds and activates pathways which lead to the
activation of transcription factors (e.g., NF-kB, IRF3/7, AP-1)
that promote the production of proinflammatory cytokines, type I
IFNs, as well as costimulatory molecules (41). Not only can poly (I:C) activate
innate immune responses, it can additionally help in the activation
of long-lasting T cell immunity (41). By directly or indirectly recruiting
leukocytes to the tumour microenvironment, the activation of TLR3
can aid in tumour lysis and induction of apoptosis by NK cells as
well as cytotoxic T cells (41).
Breast cancer cell apoptosis can be induced by poly (I:C) and can
be achieved in a TLR3-dependent fashion (12). Poly (I:C) can also activate myeloid
dendritic cells using similar pathways that involve TRIF and type I
IFNs, which promotes NK cells to attack major histocompatibility
complex (MHC) class I negative tumours (12).
The activation of TLR3 with poly (I:C) leads to
downstream pathways that produce STAT1 (signal transducer and
activator of transcription 1) phosphorylation, production of
TRIF-dependent IFN-β, NF-κB activation, and cytokines that are
pro-apoptotic, Fig. 1 (21). In mice, poly (I:C) complexed with
polyethylenimine was delivered via intratumoural injection into 4T1
tumours; the treatment was effective at reducing tumour size
(42). Nanoparticles have been
employed in many poly (I:C) studies to increase delivery and
efficacy in breast cancer cells, some examples include, but are not
limited to, mesoporous silica, mannosylated poly lactic-co-glycolic
acid, magnetic dendrimers, liposome-silica hybrids (43-46).
While poly (I:C) is common in in vitro studies, it has
issues with stability and toxicity in clinical trials, and as such
most therapies involve modified versions of the dsRNA, such as Poly
IC12U (also known as Rintatolimod, Ampligen or IPH 3102)
or the poly ICLC (also known as Hiltonol) (47,48).
Poly IC12U is a poly (I:C) derivative whereby an
unpaired uracil/guanine introduction results in mismatched dsRNA
that shows reduced toxicity; poly ICLC is a poly (I:C) derivative
stabilized with the poly-l-lysine and carboxymethylcellulose
(47,48). Poly (I:C)-based therapies are
frequently tested as adjuvants or combination therapies, currently
there is only one clinical therapy with poly ICLC as a standalone
treatment, Table II.
Poly (I:C) and derivatives:
combination therapies
The combination therapy of poly (I:C) with
chemotherapeutics has shown synergistic effects on cytotoxicity and
inhibitory tumour growth effects (49). This increase in efficacy may help
decrease some of the side effects that come with higher doses of
chemotherapy treatments (49).
Several studies have shown synergy between poly (I:C) and
doxorubicin in breast cancer cells, a combination of poly (I:C) and
doxorubicin delivered with iron oxide nanoparticle, mesoporous
silica nanoparticle, and magnetic dendrimer nanoparticle induced
higher levels of apoptosis; in the case of the iron oxide
nanoparticle tumour apoptosis was caused through direct killing,
dendritic cell-initiate and cytotoxic T cell-mediated responses
(43-54).
Poly (I:C) can synergistically improve the efficacy of the
chemotherapy gemcitabine in breast cancer mouse models (51). While there are a multitude of
preliminary studies on these combinations there are fewer clinic
trials into these combinations, as seen by bolded co-interventions
in Table II, and a greater effort
into combination poly (I:C)/derivatives and immunotherapies.
Additionally, poly (I:C) improved the efficacy of
other drugs not traditionally used for cancer treatments, such as
retinoic acid and ferumoxytol (41,52).
Poly (I:C) combination therapies allow for potential improvement
upon existing therapies, decreasing doses to improve patient
experience, and to repurpose non-traditional drugs into cancer
therapies. There are limited published results from the clinical
trials in Table II. The results
from (NCT02643303) have been reported and the combination treatment
of intratumoural tremelimumab and poly ICLC combined with systemic
durvalumab demonstrated clinical responsiveness and induced an
immune response mediated by increased CD8+ T cells (and
increased cytotoxicity, activation, and proliferation),
CD20+ B cells, mature dendritic cells, macrophages, and
CD56+ NK cells (53).
Poly (I:C) and derivatives: cancer
vaccine adjuvants
Poly (I:C), as a clear inducer of the innate immune
response, is an adjuvant candidate. To this end there are several
clinical trials that have explored poly (I:C) derivatives in
combination with PVX-410 (Oncopep Inc. human leukocyte antigen
A2-restricted multi-peptide cancer vaccine), MUC-1 (mucin 1), Her-2
(human epidermal growth factor receptor 2), Flt3L (Fms-like
tyrosine kinase 3 ligand), and neoantigen peptide vaccines,
Table II. In a phase 1 trial
(NCT02826434), although full results have not yet been published,
the combination of the PVX-410 vaccine with durvalumab and poly
ICLC was tolerated and induced antigen-specific T cell expansion
and activation, this response persisted for six months in some
patients (54). A novel triple
negative breast cancer (TNBC) vaccine has been developed to
stimulate antigen-specific T cell responses using a multi-peptide
anti-cancer vaccine. CD4+ T cells are activated which in turn leads
to dendritic cell generated immune responses (55). A peptide vaccine, with a tetanus
helper, was tested as a combination therapy with Poly ICLC in a
clinical setting on individuals with various stages of breast
cancer (55). While an increased
immune response was observed in response to several of the peptides
in the vaccine, response levels were lower than expected in
comparison to similar peptide vaccines tested on breast cancer
patients. An increase in T cell responses was observed in some of
the patients, however these results were lower than expected.
Although the results of this combination therapy were not as
significant as had been hoped, this study proved that the
combination of Poly-ICLC with multi-peptide cancer vaccines is safe
for use in a clinical setting (55). In another model of TNBC poly (I:C)
significantly enhanced the benefits of an anti-PD-1 therapy,
prolonging metastasis-free survival (56). A MUC-1 glycopeptide including a
T-cell epitope from polio virus was combined with poly (I:C) and
induced approximately 6 times as much IgG antibody compared to
those without poly (I:C) in mice (57). This antibody response was able to
respond to aberrantly glycosylated MUC-1 on MCF-7 breast cancer
cells (57).
Poly (A:U)
Polyadenylic:polyuridylic acid (poly (A:U)) is
another synthetic, non-variable sequence dsRNA. Poly (A:U) has been
tested for adjuvant activity in breast cancer (58). A randomized clinical trial of 194
breast cancer patients showed that adjuvant treatment with dsRNA
was found to be associated with a reduced risk of breast cancer
metastatic relapse in patients with TLR3-positive cancers (58) Data on this therapeutic efficacy
shows that dsRNA can mediate its therapeutic effects on tumour
cells that express TLR3(58).
Since certain breast cancer cells express TLR3, this dsRNA adjuvant
treatment can lead to the recruitment of immune cells to the
tumours, ultimately leading to apoptosis of tumour cells.
Poly (A:U) is recognized by only TLR3 whereas the
poly (I:C) agonist is recognized by TLR3 in the endosome, and
cytosolic receptors such as RIG-I and MDA-5 (melanoma
differentiation-associated protein 5) (19). Poly (I:C) has been found to enhance
antigen-specific CD8+ T cell responses and helps in antigen
presentation and antigen cross-presentation by dendritic cells
(57). Effector CD8+ T cells and
NK cells also help increase the IFN-γ release (19). In mice, it was shown that poly
(A:U) treatment promoted Th1-immune responses as well as enhanced
antibody production (19). Poly
(A:U), was not able to trigger a potent immunostimulatory effect on
its own, but if it was combined with a vaccine or chemotherapy, the
treatment was able to trigger a T-cell dependent and TRIF-dependent
response (19). A randomized trial
completed in 1980 administered Poly (A:U) as a combination therapy
with conventional treatment (surgery alone or surgery plus cobalt
therapy) on patients with operable breast cancer. Survival time was
found to be significantly higher in the combination therapy group
compared to those who received conventional therapy alone, and
incidence of relapse, particularly in patients with lymph node
disease, was lower in the combination group (59).
3. TLR antagonists
Most research conducted with TLR3, and breast cancer
have found positive effects against tumour cells, however, there is
some evidence that TLR3 expression can promote carcinogenesis and
resistance to antitumor drugs. Studies on breast cancer have found
a supportive role of TLR3 in their metastasis and increased tumour
growth (10). There are some cells
in tumour masses that can aid in the stimulation of tumour growth
and development (60). For
example, dendritic cells, macrophages and myeloid-derived
suppressor cells (MDSCs) may have a role in tumour neoangiogenesis
by inhibiting some immune responses against the tumour (60).
C10 Phenylmethimazole
Many of the immunotherapies studied have promoted
TLR3 activation, some inhibit the signalling pathways. In breast
cancer an agonist that is in the earlier stages of exploration is
the TLR3 inhibitor phenylmethimazole (C10), delivered in
combination with tamoxifen (33).
In breast cancer cells, MCF-7 (expressing oestrogen, progesterone,
and glucocorticoid receptors), the combination of C10 and tamoxifen
resulted in an enhanced anticancer response than either treatment
administered alone (33). As TLR3
activation can enhance tumour cell growth and proliferation, TLR3
inhibitors can potentially allow mediation of the immune responses
within the tumour microenvironment. C10 can block the production of
1L-6, a cytokine that has a known role in tumour growth and helps
to drive STAT3, an oncogene which also helps promote cancer
development and metastasis (33).
The combination of C10 with tamoxifen enhanced tamoxifen's
cytotoxic potential by over 50% compared to tamoxifen alone and
decreased cellular migration (33). A more potent derivative of C10 has
also been developed, COB-141 and both compounds were able to
further inhibit IL-6 secretion in a TNBC cell line,
MDA-MB-231(61). COB-141
furthermore reduced IL-6 secretion in two additional TNBC cell
lines, MDA-MB-468 and Hs578T, and in all three TNBC cell lines
there was a decrease in NF-kB DNA binding, but interestingly was
not found to limit metabolic activity, as was seen in MCF7 with
C10(61). This highlights
differences in cells from different cancer types, and the need to
consider different mechanisms and efficacy across cancer subtypes
(61).
4. Conclusion
Immunotherapies are a promising, novel method for
the treatment of breast cancers. TLRs, specifically TLR3, are a
type of receptor that is expressed on a variety of different cells,
including tumour cells. The activation of TLR3 has been shown to
have both carcinogenic and anticancer properties and therefore
should be further researched as potential cancer treatment options.
The subtype of cancer needs to be considered, as many TLR3-based
therapies have not been broadly tested against subtypes, and
likelihood of success is tied to the expression of TLR3 in the
patient-specific tumour. In most breast cancer studies,
TLR3-agonists have reported anticancer effects, however, as there
is evidence of TLR-antagonists limiting breast cancer cell
metabolism, there is a need for more research (61). In particular, the use of 2D
monoculture breast cancer cell lines fail to explore the role of
the tumour microenvironment. Creation of more relevant models, such
as co-culture of tumour cells and immune cells, could provide more
relevant information into the development of TLR-based therapies,
and could help further clarify the paradoxical pro- and anti-cancer
responses of TLR3 stimulation. Immunostimulatory nucleic acids,
such as the majority of TLR agonists, require an effective delivery
system to improve stability and efficacy, current research leads to
nanocarriers to serve this purpose. Up to this point, research
conducted on TLR3 agonist therapies in cancer treatment has made it
evident that the use of combination therapies compared to
monotherapies will most likely be more effective in treating breast
cancers.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by Wilfrid Laurier
University (grant no. 240564).
Availability of data and materials
Not applicable.
Authors' contributions
CB conceptualized and wrote the first draft of the
review. NA contributed to the second draft and final edits of the
review. SJP provided mentorship and contributed editorial feedback
to the review. Data authentication is not applicable. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication.
Not applicable.
Authors' information
ORCID iDs: Carly Butkowsky, 0000-0001-8049-022X;
Natalie Aldor, 0000-0002-7053-3705; Sarah J. Poynter,
0000-0002-7063-7504.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sancho-Garnier H and Colonna M: Breast
cancer epidemiology. Presse Med. 48:1076–1084. 2019.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
2
|
Waldman AD, Fritz JM and Lenardo MJ: A
guide to cancer immunotherapy: From T cell basic science to
clinical practice. Nat Rev Immunol. 20:651–668. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Oiseth SJ and Aziz MS: Cancer
immunotherapy: A brief review of the history, possibilities, and
challenges ahead. J Cancer Metastitis Treat. 3:250–261. 2017.
|
|
4
|
Le Naour J, Galluzzi L, Zitvogel L,
Kroemer G and Vacchelli E: Trial watch: TLR3 agonists in cancer
therapy. Oncoimmunology. 9(1771143)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kawasaki T and Kawai T: Toll-like receptor
signaling pathways. Frontiers Immunol. 5(461)2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Iwasaki A and Medzhitov R: Toll-like
receptor control of the adaptive immune responses. Nat Immunol.
5:987–995. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Vacchelli E, Eggermont A, Sautès-Fridman
C, Galon J, Zitvogel L, Kroemer G and Galluzzi L: Trial Watch:
Toll-like receptor agonists for cancer therapy. Oncoimmunology.
2(e25238)2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chen X, Zhang Y and Fu Y: The critical
role of Toll-like receptor-mediated signaling in cancer
immunotherapy. Med Drug Disc. 14(100122)2022.
|
|
9
|
Bianchi F, Pretto S, Tagliabue E, Balsari
A and Sfondrini L: Exploiting poly(I:C) to induce cancer cell
apoptosis. Cancer Biol Ther. 18:747–756. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Muresan XM, Bouchal J, Culig Z and Souček
K: Toll-like receptor 3 in solid cancer and therapy resistance.
Cancers (Basel). 12(3227)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Matsumoto M, Takeda Y, Tatematsu M and
Seya T: Toll-Like receptor 3 signal in dendritic cells benefits
cancer immunotherapy. Front Immunol. 8(1897)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yu M and Levine SJ: Toll-like receptor 3,
RIG-I-like receptors and the NLRP3 inflammasome: Key modulators of
innate immune responses to double-stranded RNA viruses. Cytokine
Growth Factor Rev. 22:63–72. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Holm CK, Petersen CC, Hvid M, Petersen L,
Paludan SR, Deleuran B and Hokland M: TLR3 ligand polyinosinic:
Polycytidylic acid induces IL-17A and IL-21 synthesis in human Th
cells. J Immunol. 183:4422–4431. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Weber A, Kirejczyk Z, Besch R, Potthoff S,
Leverkus M and Hacker G: Proapoptotic signalling through Toll-like
receptor-3 involves TRIF-dependent activation of caspase-8 and is
under the control of inhibitor of apoptosis proteins in melanoma
cells. Cell Death Differ. 17:942–951. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shime H, Matsumoto M and Seya T:
Double-stranded RNA promotes CTL-independent tumor cytolysis
mediated by CD11b+Ly6G+ intratumor myeloid cells through the
TICAM-1 signaling pathway. Cell Death Differ. 24:385–396.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Bondhopadhyay B, Moirangthem A and Basu A:
Innate adjuvant receptor Toll-like receptor 3 can promote breast
cancer through cell surface. Tumor Biol. 36:1261–1271.
2015.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Paone A, Galli R, Gabellini C, Lukashev D,
Starace D, Gorlach A, De Cesaris P, Ziparo E, Del Bufalo D,
Sitkovsky MV, et al: Toll-like receptor 3 regulates angiogenesis
and apoptosis in prostate cancer cell lines through
hypoxia-inducible factor 1 alpha. Neoplasia. 12:539–549.
2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhan Z, Xie X, Cao H, Zhou X, Zhang XD,
Fan H and Liu Z: Autophagy facilitates TLR4-and TLR3-triggered
migration and invasion of lung cancer cells through the promotion
of TRAF6 ubiquitination. Autophagy. 10:257–268. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zhao J, Zhang Z, Xue Y, Wang G, Cheng Y,
Pan Y, Zhao S and Hou Y: Anti-tumor macrophages activated by
ferumoxytol combined or surface-functionalized with the TLR3
agonist poly (I: C) promote melanoma regression. Theranostics.
8:6307–6321. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Fan L, Zhou P, Hong Q, Chen AX, Liu GY, Yu
KD and Shao ZM: Toll-like receptor 3 acts as a suppressor gene in
breast cancer initiation and progression: A two-stage association
study and functional investigation. Oncoimmunology.
8(e1593801)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Salaun B, Coste I, Rissoan MC, Lebecque SJ
and Renno T: TLR3 can directly trigger apoptosis in human cancer
cells. J Immunol. 176:4894–4901. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wulff S, Pries R and Wollenberg B:
Cytokine release of human NK cells solely triggered with Poly I: C.
Cell Immunol. 263:135–137. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Harashima N, Minami T, Uemura H and Harada
M: Transfection of poly (I: C) can induce reactive oxygen
species-triggered apoptosis and interferon-β-mediated growth arrest
in human renal cell carcinoma cells via innate adjuvant receptors
and the 2-5A system. Mol Cancer. 13(217)2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chew V, Tow C, Huang C, Bard-Chapeau E,
Copeland NG, Jenkins NA, Weber A, Lim KH, Toh HC, Heikenwalder M,
et al: Toll-like receptor 3 expressing tumor parenchyma and
infiltrating natural killer cells in hepatocellular carcinoma
patients. J Natl Cancer Inst. 104:1796–1807. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wischke C, Zimmermann J, Wessinger B,
Schendler A, Borchert HH, Peters JH, Peters JH, Nesselhut T and
Lorenzen DR: Poly (I:C) coated PLGA microparticles induce dendritic
cell maturation. Int J Pharm. 365:61–68. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhu X, Fallert-Junecko BA, Fujita M, Ueda
R, Kohanbash G, Kastenhuber ER, McDonald HA, Liu Y, Kalinski P,
Reinhart TA, et al: Poly-ICLC promotes the infiltration of effector
T cells into intracranial gliomas via induction of CXCL10 in
IFN-alpha and IFN-gamma dependent manners. Cancer Immunol
Immunother. 59:1401–1409. 2010.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kyi C, Roudko V, Sabado R, Saenger Y,
Loging W, Mandeli J, Thin TH, Lehrer D, Donovan M, Posner M, et al:
Therapeutic immune modulation against solid cancers with
intratumoral poly-ICLC: A pilot trial. Clin Cancer Res.
24:4937–4948. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Navabi H, Jasani B, Reece A, Clayton A,
Tabi Z, Donninger C, Mason M and Adams M: A clinical grade poly I:
C-analogue (Ampligen) promotes optimal DC maturation and Th1-type T
cell responses of healthy donors and cancer patients in vitro.
Vaccine. 27:107–115. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Brodsky I, Strayer DR, Krueger LJ and
Carter WA: Clinical studies with ampligen (mismatched
double-stranded RNA). J Biol Response Mod. 4:669–675.
1985.PubMed/NCBI
|
|
30
|
Martins KA, Bavari S and Salazar AM:
Vaccine adjuvant uses of poly-IC and derivatives. Expert Rev
Vaccines. 14:447–459. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hubbell HR: Synergistic antiproliferative
effect of human interferons in combination with mismatched
double-stranded RNA on human tumor cells. Int J Cancer. 37:359–365.
1986.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gatti G, Nunez NG, Nocera DA, Dejager L,
Libert C, Giraudo C and Maccioni M: Direct effect of ds RNA
mimetics on cancer cells induces endogenous IFN-β production
capable of improving dendritic cell function. Eur J Immunol.
43:1849–1861. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Schwartz AL, Dickerson E, Dagia N, Malgor
R and McCall KD: TLR signaling inhibitor, phenylmethimazole, in
combination with tamoxifen inhibits human breast cancer cell
viability and migration. Oncotarget. 8:113295–113302.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sharma S, Zhu L, Davoodi M, Harris-White
M, Lee JM, St John M, Salgia R and Dubinett S: TLR3 agonists and
proinflammatory antitumor activities. Expert Opin Ther Targets.
17:481–483. 2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gosu V, Basith S, Kwon OP and Choi S:
Therapeutic applications of nucleic acids and their analogues in
Toll-like receptor signaling. Molecules. 17:13503–13529.
2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Owen AM, Fults JB, Patil NK, Hernandez A
and Bohannon JK: TLR agonists as mediators of trained immunity:
Mechanistic insight and immunotherapeutic potential to combat
infection. Front Immunol. 11(622614)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Pahlavanneshan S, Sayadmanesh A,
Ebrahimiyan H and Basiri M: Toll-Like receptor-based strategies for
cancer immunotherapy. J Immunol Res. 2021(9912188)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kaczanowska S, Joseph AM and Davila E: TLR
agonists: Our best frenemy in cancer immunotherapy. J Leukoc Biol.
93:847–863. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ding L, Ren J, Zhang D, Li Y, Huang X, Ji
J, Hu Q, Wang H, Ni Y and Hou Y: The TLR3 agonist inhibit drug
efflux and sequentially consolidates low-dose cisplatin-based
chemoimmunotherapy while reducing side effects. Mol Cancer Ther.
16:1068–1079. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Matsuo A, Oshiumi H, Tsujita T, Mitani H,
Kasai H, Yoshimizu M, Matsumoto M and Seya T: Teleost TLR22
recognizes RNA duplex to induce IFN and protect cells from
birnaviruses. J Immunol. 181:3474–3485. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bernardo AR, Cosgaya JM, Aranda A and
Jiménez-Lara AM: Synergy between RA and TLR3 promotes type I
IFN-dependent apoptosis through upregulation of TRAIL pathway in
breast cancer cells. Cell Death Dis. 4(e479)2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Aznar MA, Planelles L, Perez-Olivares M,
Molina C, Garasa S, Etxeberría I, Perez G, Rodriguez I, Bolaños E,
Lopez-Casas P, et al: Immunotherapeutic effects of intratumoral
nanoplexed poly I:C. J Immunother Cancer. 7(116)2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ultimo A, Giménez C, Bartovsky P, Aznar E,
Sancenón F, Marcos MD, Amorós P, Bernardo AR, Martínez-Máñez R,
Jiménez-Lara AM and Murguía JR: Targeting innate immunity with
dsRNA-Conjugated mesoporous silica nanoparticles promotes antitumor
effects on breast cancer cells. Chemistry. 22:1582–1586.
2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Khodadust R, Alpsoy A, Ünsoy G and GÜndÜz
U: Poly (I: C)-and doxorubicin-loaded magnetic dendrimeric
nanoparticles affect the apoptosis-related gene expressions in
MCF-7 cells. Turk J Biol. 44:133–144. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Sheikhzadeh S, Delirezh N and Hobbenaghi
R: Mannosylated polylactic-co-glycolic acid (MN-PLGA) nanoparticles
induce potent anti-tumor immunity in murine model of breast cancer.
Biomed Pharmacother. 142(111962)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Colapicchioni V, Palchetti S, Pozzi D,
Marini ES, Riccioli A, Ziparo E, Papi M, Amenitsch H and Caracciolo
G: Killing cancer cells using nanotechnology: Novel poly (I: C)
loaded liposome-silica hybrid nanoparticles. J Mater Chem B.
3:7408–7416. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Sultan H, Salazar AM and Celis E:
Poly-ICLC, a multi-functional immune modulator for treating cancer.
Semin Immunol. 49(101414)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Jasani B, Navabi H and Adams M: Ampligen:
A potential toll-like 3 receptor adjuvant for immunotherapy of
cancer. Vaccine. 27:3401–3404. 2009.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Keshavarz A, Pourbagheri-Sigaroodi A,
Zafari P, Bagheri N, Ghaffari SH and Bashash D: Toll-like receptors
(TLRs) in cancer; with an extensive focus on TLR agonists and
antagonists. IUBMB Life. 73:10–25. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Mu QG, Lin G, Jeon M, Wang H, Chang FC,
Revia RA, Yu J and Zhang M: Iron oxide nanoparticle targeted
chemo-immunotherapy for triple negative breast cancer. Mat Today
(Kidlington). 50:149–169. 2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Le UM, Yanasarn N, Lohr CV, Fischer KA and
Cui Z: Tumor chemo-immunotherapy using gemcitabine and a synthetic
dsRNA. Cancer Biol Ther. 7:440–447. 2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Slingluff C, Mauldin I, Gaughan E, Dillon
P, Opyrchal M, Puzanov I, Kruse M, Gastman B, Friedlander P, Marron
T, et al: 337 Intratumoral immune therapy for recurrent breast
cancer with polyiclc, and tremelimumab combined with systemic
durvalumab. J Immunother Cancer 9: A1–A1054, 2021.
|
|
53
|
Isakoff SJ, Adams S, Soliman HH, Tung N,
Barry WT, Hu J, Trippa L, Deering R, Parker J, Park H, et al:
Abstract P3-09-15: A phase 1b study of PVX-410 (PVX) vaccine plus
durvalumab (DUR) as adjuvant therapy in HLA-A2+ early stage triple
negative breast cancer (eTNBC) to assess safety and immune
response. Cancer Res. 80: (suppl 4)(P3-06-15)2020.
|
|
54
|
Dillon PM, Petroni GR, Smolkin ME, Brenin
DR, Chianese-Bullock KA, Smith KT, Olson WC, Fanous IS, Nail CJ,
Brenin CM, et al: A pilot study of the immunogenicity of a
9-peptide breast cancer vaccine plus poly-ICLC in early stage
breast cancer. J Immunother Cancer. 5(92)2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Brockwell NK, Owen KL, Zanker D, Spurling
A, Rautela J, Duivenvoorden HM, Baschuk N, Caramia F, Loi S, Darcy
PK, et al: Neoadjuvant interferons: Critical for effective
PD-1-based immunotherapy in TNBC. Cancer Immunol Res. 5:871–884.
2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Glaffig M, Stergiou N, Schmitt E and Kunz
H: Immunogenicity of a fully synthetic MUC1 glycopeptide antitumor
vaccine enhanced by poly(I:C) as a TLR3-Activating adjuvant.
ChemMedChem. 12:722–727. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Salaun B, Zitvogel L, Asselin-Paturel C,
Morel Y, Chemin K, Dubois C, Massacrier C, Conforti R, Chenard MP,
Sabourin JC, et al: TLR3 as a biomarker for the therapeutic
efficacy of double-stranded RNA in breast cancer. Cancer Res.
71:1607–1614. 2011.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Conforti R, Ma Y, Morel Y, Paturel C,
Terme M, Viaud S, Ryffel B, Ferrantini M, Uppaluri R, Schreiber R,
et al: Opposing effects of toll-like receptor (TLR3) signaling in
tumors can be therapeutically uncoupled to optimize the anticancer
efficacy of TLR3 ligands optimizing poly (A: U) anticancer therapy.
Cancer Res. 70:490–500. 2010.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Lacour J, Lacour F, Spira A, Michelson M,
Petit JY, Delage G, Sarrazin D, Contesso G and Viguier J: Adjuvant
treatment with polyadenylic-polyuridylic acid in operable breast
cancer: Updated results of a randomised trial. Br Med J (Clin Res
Ed). 288:589–592. 1984.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Venkatesh A, Nandigam H, Muccioli M, Singh
M, Loftus T, Lewis D, Pate M and Benencia F: Regulation of
inflammatory factors by double-stranded RNA receptors in breast
cancer cells. Immunobiology. 223:466–476. 2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Noori MS, O'Brien JD, Champa ZJ, Deosarkar
SP, Lanier OL, Qi C, Burdick MM, Schwartz FL, Bergmeier SC, McCall
KD and Goetz DJ: Phenylmethimazole and a thiazole derivative of
phenylmethimazole inhibit IL-6 expression by triple negative breast
cancer cells. Eur J Pharmacol. 803:130–137. 2017.PubMed/NCBI View Article : Google Scholar
|