Introduction
Breast cancer remains the leading type of cancer
globally according to the 2023 Global Cancer Statistics from the
American Cancer Society, accounting for 31% of female cancers
(1). Accurate diagnosis of breast
lesions and use of appropriate surgical approach are important to
improving breast cancer prognosis. Breast cancer screening is an
effective method of decreasing breast cancer-associated mortality.
There are numerous methods of breast cancer screening, such as
mammography, ultrasonography (US) and magnetic resonance imaging
(2). Mammography is recommended
for patients aged >40 years and is not sensitive for patients
with high breast density (3,4).
Digital breast tomosynthesis improves breast lesion visibility and
cancer detection rates in both dense and fatty breasts but is not
widely used in clinical practice (5). US is an important tool for breast
cancer screening that can better detect mammographically occult
breast cancer in patients with high mammary parenchymal density
(6). In patients with
predominantly fatty breasts, the sensitivity of mammography and US
are 82.2 and 71.1%, respectively, while in patients with
heterogeneously dense breasts they are 23.7 and 57.0%, respectively
(7). Magnetic resonance imaging
(MRI) is less commonly used for screening due to its high cost and
lengthy time consumption (8).
The Breast Imaging Reporting and Data System
(BI-RADS) (9) of the American
College of Radiology is a widely used breast lesion imaging
evaluation system for standardizing risk evaluation of breast
lesions. Several diagnostic imaging features of the risk of breast
cancer are incorporated into the BI-RADS lexicon, such as fuzzy
boundary, irregular shape, calcification and vascularity (10,11).
Moreover, age is widely accepted as an independent prognostic
factor for breast cancer and is associated with breast density,
hormone levels and breast cancer subtypes (12-15).
Moreover, the peak age of breast cancer diagnosis in China (45-59
years) is significantly lower than that in Western countries (60-70
years) (16,17). Some studies have discovered that
the incidence of breast cancer increases with age for lesions with
identical BI-RADS category (17-19).
To the best of our knowledge, however, the diagnostic value of age
has not been integrated into the BI-RADS lexicon. The clinical
criteria for BI-RADS subcategory 4A and category 3 are different,
with BI-RADS 4A recommending biopsy and BI-RADS 3 recommending
short-term follow-up; thus, reclassifying a lesion from BI-RADS 4A
to 3 has clinical implications, indicating a change in clinical
management. Age can affect BI-RADS categorization, primarily
because the risk of breast lesions and breast density vary with
age. However, the specific age cut-off for adjusting BI-RADS 4A to
BI-RADS 3 based on risk probability remains unknown. To the best of
our knowledge, the effect of age on characteristic US features of
breast cancer has not been reported recently.
The present study focused on breast lesions
classified as BI-RADS category 4, which have a high level of
uncertainty (2-95%) regarding their diagnosis as breast cancer
during biopsy (18). The present
study aimed to construct a model that integrates age with BI-RADS
lexicon to improve the diagnostic accuracy of US in breast
cancer.
Patients and methods
Patients
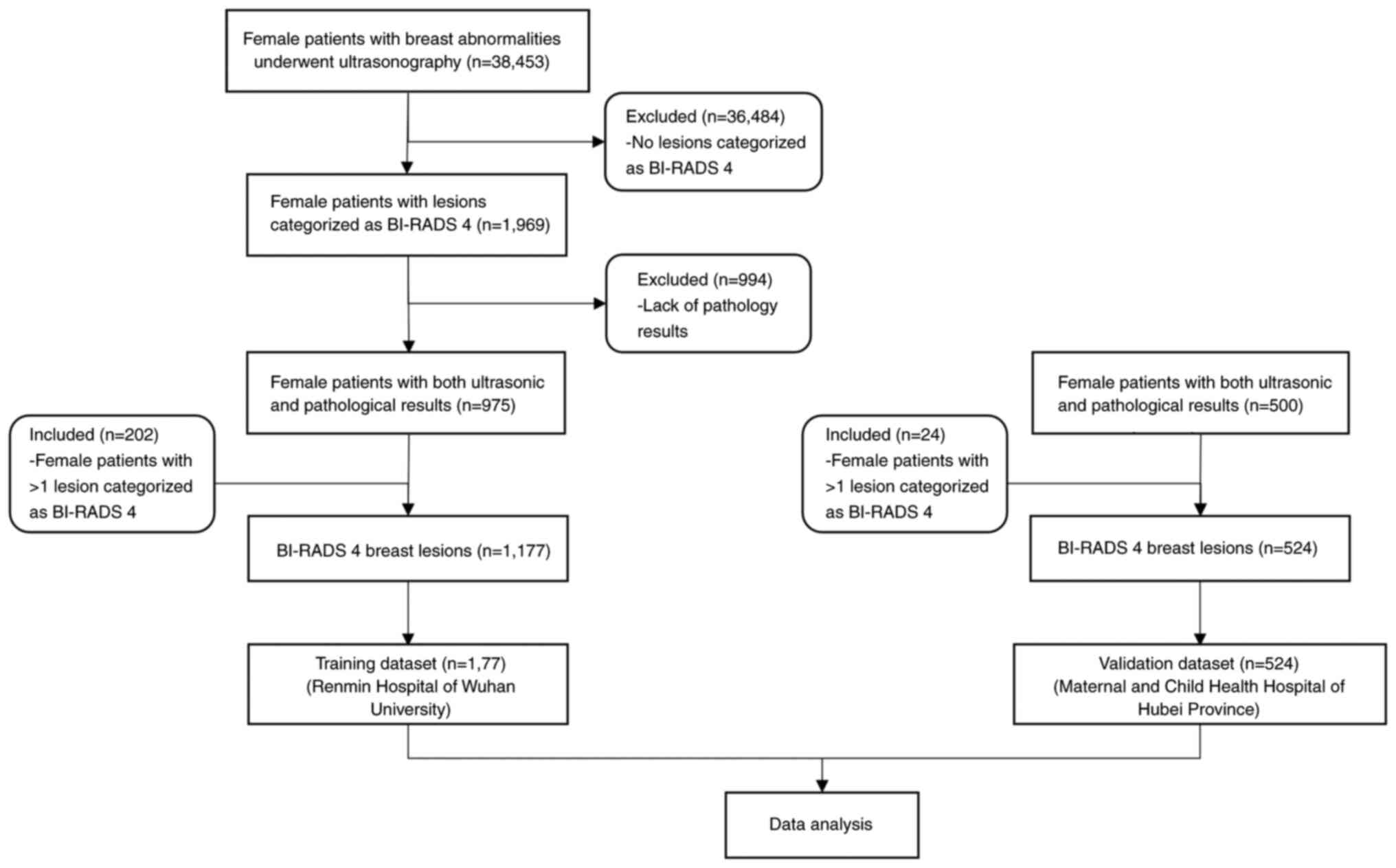
According to the BI-RADS guidelines (Table I), breast lesion biopsy was
performed for patients diagnosed with BI-RADS category ≥4 lesion by
US (19). In this retrospective
study, patients were screened for BI-RADS classification by breast
US, and results were collected from the existing clinical records
of the US department. From November 2018 to November 2020, a total
of 38,453 patients underwent breast US examination, of whom 9,116
were diagnosed with BI-RADS category 2 lesion, 16,642 with BI-RADS
category 3 lesion, 1,969 with BI-RADS category 4 lesion, 136 with
BI-RADS category 5 lesion and 10,590 with other diseases, such as
mastitis and simple breast hyperplasia. In the training cohort, 975
female patients with US BI-RADS category 4 breast lesions from the
Renmin Hospital of Wuhan University (Wuhan, China) were analyzed. A
total of 202 patients were diagnosed with two BI-RADS category 4
lesions. Therefore, 1,177 lesions were included in the training
cohort. The validation cohort included 500 female patients with 524
BI-RADS category 4 lesions from the Maternal and Child Health
Hospital of Hubei Province (Wuhan, China). The training cohort had
an age range of 15-85 years, while the validation cohort a had an
age range of 19-79 years.
 | Table IUltrasonic Breast Imaging Reporting
and Data System categorization and recommended action. |
Table I
Ultrasonic Breast Imaging Reporting
and Data System categorization and recommended action.
| Category | Description | Probability of
malignancy, % | Recommendation |
|---|
| 0 | Incomplete
assessment | Not applicable | Additional
evaluation |
| 1 | Normal breast | 0 | Not applicable |
| 2 | Benign lesions | 0 | Not applicable |
| 3 | Highly probable
benign lesions | <2 | Regular
follow-up |
| 4a | Low malignant
potential lesions | 2-10 | Biopsy |
| 4b | Intermediate
malignant potential lesions | 10-50 | |
| 4c | High malignant
potential lesions | 50-95 | |
| 5 | Highly probable
malignant lesions | >95 | Appropriate
treatment |
The inclusion criteria were as follows: i) Patients
were aged 15-85 years, ii) lesions were diagnosed as BI-RADS
category 4 by US and iii) lesions were assessed via pathology and
US. If a patient had >1 BI-RADS category 4 lesion, each lesion
was considered separately.
The exclusion criteria were as follows: i) Patients
with abnormal breast anatomy or breast implants, ii) patients
without pathological examination, iii) patients with a history of
breast cancer or recent chemotherapy, radiotherapy or surgery for
breast cancer and iv) time between US and biopsy was >2 months
(Fig. 1).
US
ESOTE MEGAS GPX FD570A (Esaote SpA), a US diagnostic
instrument with probe frequencies of 5-13 MHz was used to examine
the patients. The patients were placed in a supine or side-lying
position with both arms lifted and abducted to fully expose the
breast.
The diagnostic criteria were based on the guidelines
of the American College of Radiology BI-RADS (9) for the diagnosis of benign and
malignant lesions. US was performed independently by two
experienced breast sonographers, who were medical doctors with
specialized knowledge of breast US. Results that did not conform to
the diagnostic criteria were interpreted by a senior doctor.
Data on the characteristic US features of breast
lesions, including shape, boundary, calcification and blood flow
signal were collected to further determine the influence of age on
these features. Basic information, including BI-RADS category and
patient age, was obtained from medical records for model
development and validation.
Pathological results
Breast lesion biopsy was performed by surgeons.
Patient was positioned either supine or laterally on the
examination table. The designated area was disinfected and draped
with a sterile cover. Optimal needle trajectory and depth were
determined prior to the procedure. A disposable core needle biopsy
(CNB) needle was prepared, and local anesthesia was administered
using 1% lidocaine. The lesion was accurately localized within the
needle's path under ultrasound guidance. Upon confirming the
correct localization, the CNB safety mechanism was engaged, and the
biopsy needle was activated to procure the tissue sample.
Hemostasis was achieved by applying pressure to the biopsy site.
The collected specimen was subsequently fixed in a 10% neutral
buffered formalin solution and forwarded to the Department of
Pathology at each hospital. All pathological results were obtained
from the existing clinical records and verified by at least two
experienced pathologists. The pathological samples diagnosed
strictly according to the WHO Classification of Tumors 5th Edition
(20) standard procedures.
Pathological diagnosis is the gold standard for breast cancer
diagnosis.
Pathological and US examinations were performed
separately. The pathologists were not aware of the results obtained
by the sonographers, and vice versa, to avoid subjective bias.
Statistical analysis
All statistical analyses were performed using R
version 4.2.1 (mirrors.tuna.tsinghua.edu.cn/CRAN/src/base/R-4/). Data
are presented as the mean ± SD. The prevalence of breast cancer in
patients with BI-RADS category 4 breast lesions in each age group
was determined. For dichotomous variables, Pearson χ2
test and Fisher's exact test of association was performed. A
logistic regression model was used for multivariate analysis.
Collinearity of age and BI-RADS was evaluated.
Based on differences in age-specific incidence rate
of breast cancer among female Chinese patients (16,21,22),
which peaks between the ages of 45 and 59 years, and variations in
menopausal status, patients were categorized into three age groups:
Group 1, <45 years; group 2, 45-60 years and group 3, >60
years. The positive predictive values (PPVs) of US features in
different age groups and each BI-RADS subcategory (4A, 4B and 4C)
were calculated. The age-associated PPVs of each BI-RADS
subcategory were compared using the χ2 test.
Three models, M1, M2, and M3, were developed. Their
covariates were as follows: M1, age; M2, BI-RADS score and M3,
BI-RADS score and age. Logistic regression was used to predict
prognostic ability of the integrated model (M3). Data of patients
from the Renmin Hospital of Wuhan University were used in the
training cohort and data of patients from the Maternal and Child
Health Hospital of Hubei Province were used in the external
validation cohort. By plotting the receiver operating
characteristics (ROC) curves, diagnostic accuracies were expressed
as the area under the curve (AUC). A linear regression curve was
plotted with age as the horizontal coordinate and the percentage of
malignant tumors to total tumors in each age group as the vertical
coordinate. R2 values were calculated to measure how
well the statistical model predicted an outcome. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient information
The basic information of the patients in the
training and validation cohorts is summarized in Table II. Significant differences were
exhibited in age, malignancy distribution and BI-RADS 4
subcategories distribution between cohorts. The training cohort had
an age range of 15-85 years, with a mean age of 48±12 years, while
the validation cohort age range from 19 to 79 years, with a mean
age of 39±11 years. The training cohort had 718 benign (61.0%) and
459 malignant (39.0%) cases, whereas the validation cohort
presented with 444 benign (84.7%) and 80 malignant (15.3%) cases.
Regarding BI-RADS 4 subcategories, the training cohort reported 794
cases (67.5%) as 4A, 197 (16.7%) as 4B, and 186 (15.8%) as 4C. By
contrast, the validation cohort had a higher proportion of 4A cases
at 435 (83.0%), with fewer 4B and 4C cases at 68 (13.0%) and 21
(4.0%), respectively.
 | Table IIComparison of basic information
between the training cohort and the validation cohort. |
Table II
Comparison of basic information
between the training cohort and the validation cohort.
| Characteristic | Training cohort
(n=1,177) | Validation cohort
(n=524) | P-value |
|---|
| Mean age (range),
years | 48±12 (15-85) | 39±11 (19-79) | <0.001 |
| Pathology (%) | | | <0.001 |
|
Benign | 718 (61.0) | 444 (84.7%) | |
|
Malignant | 459 (39.0%) | 80 (15.3%) | |
| Breast Imaging
Reporting and Data | | | |
| System
category | | | <0.001 |
|
4A | 794 (67.5%) | 435 (83.0%) | |
|
4B | 197 (16.7%) | 68 (13.0%) | |
|
4C | 186 (15.8%) | 21 (4.0%) | |
Effect of age on PPV of the BI-RADS
lesion categories
The influence of age on the PPV of BI-RADS lesion
categories was determined. The proportion of malignant lesions
varied significantly between the three age groups in all three
BI-RADS subcategory 4 lesions (Table
III). There was a significant positive association between
proportion of malignant lesions and increasing age in training
cohort (4A, 10.7 vs. 17.6 vs. 47.7; 4B, 54.4 vs. 78.8 vs. 80.6 and
4C, 89.5 vs. 95.6 vs. 98.2 for groups 1-3, respectively). The
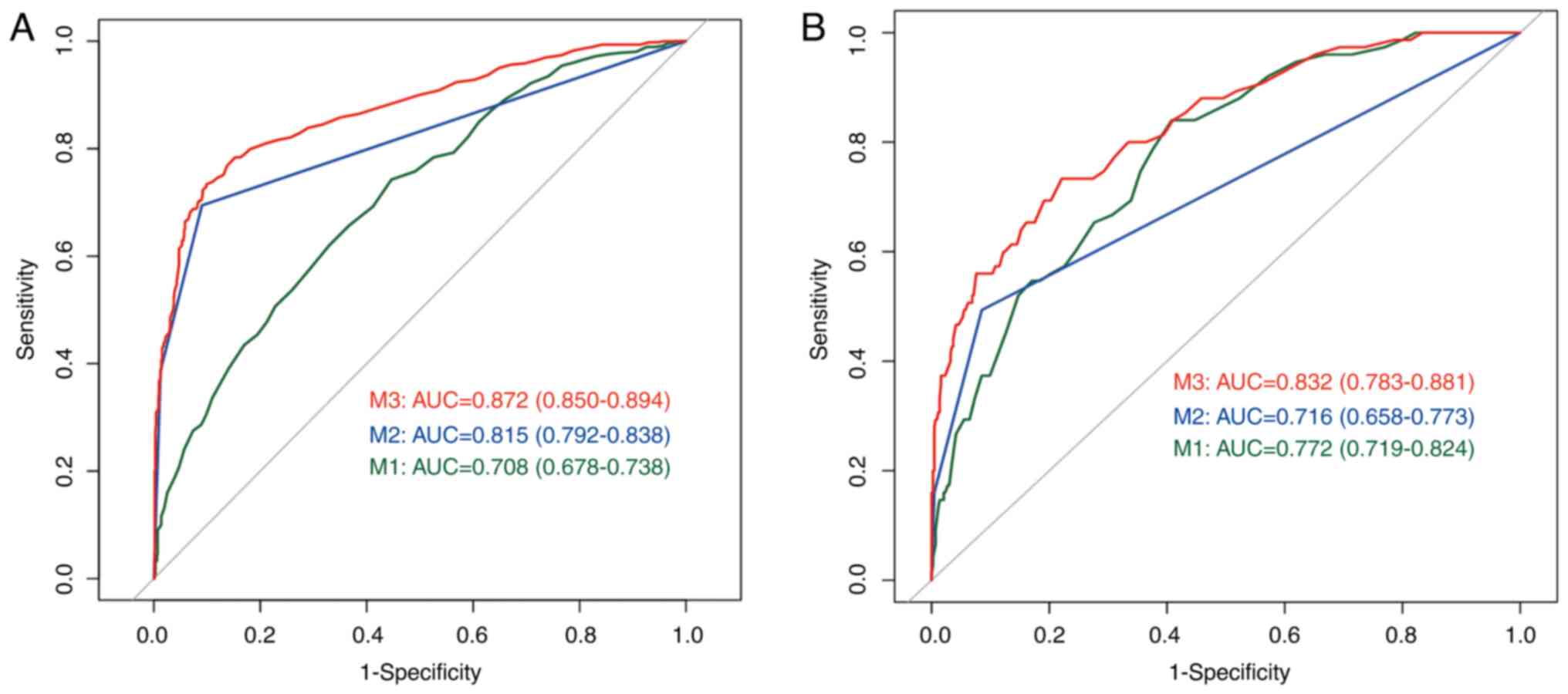
performances of models based on the ROC curves are illustrated in
Fig. 2. The ROC curves of data in
the training and validation cohorts showed similar results. The
integrated model (M3) showed the best predictive ability in both
the training (AUC=0.872, 95% CI: 0.850-0.894) and validation
cohorts (AUC=0.832, 95% CI: 0.783-0.881). For the ROC curve in the
training cohort, the age model (M1) showed the worst performance
with an AUC of 0.708 (95% CI: 0.678-0.738); BI-RADS score model
(M2) performed better with an AUC of 0.815 (95% CI: 0.792-0.838).
For the ROC curve in the validation cohorts, M2 (AUC=0.716, 95% CI:
0.658-0.773) performed worse than M1 (AUC=0.772, 95% CI:
0.719-0.824).
 | Table IIIAssociation between age and BI-RADS
category 4 lesions. |
Table III
Association between age and BI-RADS
category 4 lesions.
| A, BI-RADS 4A |
|---|
| | Training
cohort | Validation
cohort |
|---|
| Age group | Benign (%) | Malignant (%) | P-value | Benign (%) | Malignant (%) | P-value |
|---|
| 1 | 310 (89.9) | 35 (10.1) | <0.001 | 307 (94.2) | 19 (5.8) | <0.001 |
| 2 | 299 (82.4) | 64 (17.6) | | 80 (85.1) | 14 (14.9) | |
| 3 | 45 (52.3) | 41 (47.7) | | 12 (66.7) | 6 (33.3) | |
| B, BI-RADS 4B |
| 1 | 26 (45.6) | 31 (54.4) | 0.002 | 27 (75.0) | 9 (25.0) | 0.106 |
| 2 | 22 (21.2) | 82 (78.8) | | 8 (42.1) | 11 (57.9) | |
| 3 | 7 (19.4) | 29 (80.6) | | 2 (28.6) | 5 (71.4) | |
| C, BI-RADS 4C |
| 1 | 4 (10.5) | 34 (89.5) | 0.082 | 2 (16.7) | 10 (83.3) | 0.062 |
| 2 | 4 (4.4) | 87 (95.6) | | 0 (0.0) | 8 (100.0) | |
| 3 | 1 (1.8) | 56 (98.2) | | 0 (0.0) | 4 (100.0) | |
Analysis of the US
characteristics
In the training cohort, significant statistical
differences were observed in calcification, fuzzy boundary,
irregular shape and blood flow signal between benign and malignant
lesions, which are commonly considered indications of malignancy
and frequently described in breast cancer (23-26)
(Table IV). However, there was no
significant difference in posterior echo attenuation.
 | Table IVUltrasonographic characteristics of
patients. |
Table IV
Ultrasonographic characteristics of
patients.
| Characteristic | Benign (%) | Malignancy (%) | Total | P-value |
|---|
| Calcification | | | | 0.003 |
|
Absent | 347 (65.7) | 181 (34.3) | 528 | |
|
Present | 371 (57.2) | 278 (42.8) | 649 | |
| Fuzzy boundary | | | | <0.001 |
|
Absent | 466 (72.9) | 173 (27.1) | 639 | |
|
Present | 252 (46.8) | 286 (53.2) | 538 | |
| Irregular
shape | | | | <0.001 |
|
Absent | 400 (84.9) | 71 (15.1) | 471 | |
|
Present | 318 (45.0) | 388 (55.0) | 706 | |
| Blood flow signal
in the tumor | | | | <0.001 |
|
Absent | 596 (72.0) | 232 (28.0) | 828 | |
|
Present | 122 (35.0) | 227 (65.0) | 349 | |
| Posterior echo
attenuation | | | | 0.084 |
|
Absent | 657 (60.4) | 431 (39.6) | 1,088 | |
|
Present | 62 (69.7) | 27 (30.3) | 89 | |
Although suspicious imaging descriptors are helpful
in predicting breast cancer, their accuracy may be affected by age
(14). Young patients with many
suspicious imaging descriptors sometimes have false-positive
results (27). By contrast, older
patients may have malignant tumors with less suspicious imaging
descriptors (Fig. 3).
The present study explored the association between
age groups and the PPVs of the suspicious US image features in the
different BI-RADS 4 lesion subcategories (Table V). Irregular shape was associated
with the highest PPV for diagnosing malignant breast lesions in all
BI-RADS 4 lesion subcategories. Age-related PPVs of fuzzy
boundaries varied significantly between the three age groups in the
BI-RADS 4A (65.7 vs. 46.9 vs. 28.6 for groups 1-3, respectively)
and 4B subcategory (86.7 vs. 67.1 vs. 51.7, for groups 1-3,
respectively). The prevalence of fuzzy boundary decreased with age.
No other feature was significantly associated with age or BI-RADS
subcategories.
 | Table VPPVs of ultrasonographic features of
BI-RADS 4 subcategories by age group. |
Table V
PPVs of ultrasonographic features of
BI-RADS 4 subcategories by age group.
| A, BI-RADS 4A |
|---|
| Age Group | Feature | PPV (%) | 1-PPV (%) |
|---|
| 1 | Calcification | 15 (42.9) | 20 (57.1) |
| | Fuzzy boundary | 23 (65.7) | 12 (34.3) |
| | Irregular
shape | 26 (74.3) | 9 (25.7) |
| | Blood flow
signal | 15 (42.9) | 20 (57.1) |
| 2 | Calcification | 31 (48.4) | 33 (51.6) |
| | Fuzzy boundary | 30 (46.9) | 34 (53.1) |
| | Irregular
shape | 46 (71.9) | 18 (28.1) |
| | Blood flow
signal | 22 (34.4) | 42 (65.6) |
| 3 | Calcification | 19 (45.2) | 22 (54.8) |
| | Fuzzy boundary | 12 (28.6) | 29 (71.4) |
| | Irregular
shape | 32 (76.2) | 10 (23.8) |
| | Blood flow
signal | 13 (31.0) | 28 (69.0) |
| B, BI-RADS 4B |
| 1 | Calcification | 19 (60.0) | 12 (40.0) |
| | Fuzzy boundary | 26 (86.7) | 4 (13.3) |
| | Irregular
shape | 28 (93.3) | 2 (6.7) |
| | Blood flow
signal | 20 (66.7) | 10 (33.3) |
| 2 | Calcification | 54 (65.9) | 28 (34.1) |
| | Fuzzy boundary | 55 (67.1) | 27 (32.9) |
| | Irregular
shape | 71 (86.6) | 11 (13.4) |
| | Blood flow
signal | 38 (46.3) | 44 (53.7) |
| 3 | Calcification | 15 (51.7) | 14 (48.3) |
| | Fuzzy boundary | 15 (51.7) | 14 (48.3) |
| | Irregular
shape | 23 (79.3) | 6 (20.7) |
| | Blood flow
signal | 16 (55.2) | 13 (44.8) |
| C, BI-RADS 4C |
| 1 | Calcification | 24 (70.6) | 10 (29.4) |
| | Fuzzy boundary | 25 (73.5) | 9 (26.5) |
| | Irregular
shape | 30 (88.2) | 4 (11.8) |
| | Blood flow
signal | 24 (70.6) | 10 (29.4) |
| 2 | Calcification | 66 (75.9) | 21 (24.1) |
| | Fuzzy boundary | 63 (72.4) | 24 (27.6) |
| | Irregular
shape | 81 (93.1) | 6 (6.9) |
| | Blood flow
signal | 48 (55.2) | 39 (44.8) |
| 3 | Calcification | 36 (64.3) | 20 (35.7) |
| | Fuzzy boundary | 37 (66.1) | 19 (33.9) |
| | Irregular
shape | 51 (91.1) | 5 (8.9) |
| | Blood flow
signal | 31 (55.4) | 25 (44.6) |
Moreover, collinearity test was used to examine
whether there was an interaction between age and BI-RADS lesion
category in the diagnosis of benign and malignant breast lesions.
The results showed that age, BI-RADS and imaging features were
independent (all variance inflation factor <10; Table SI).
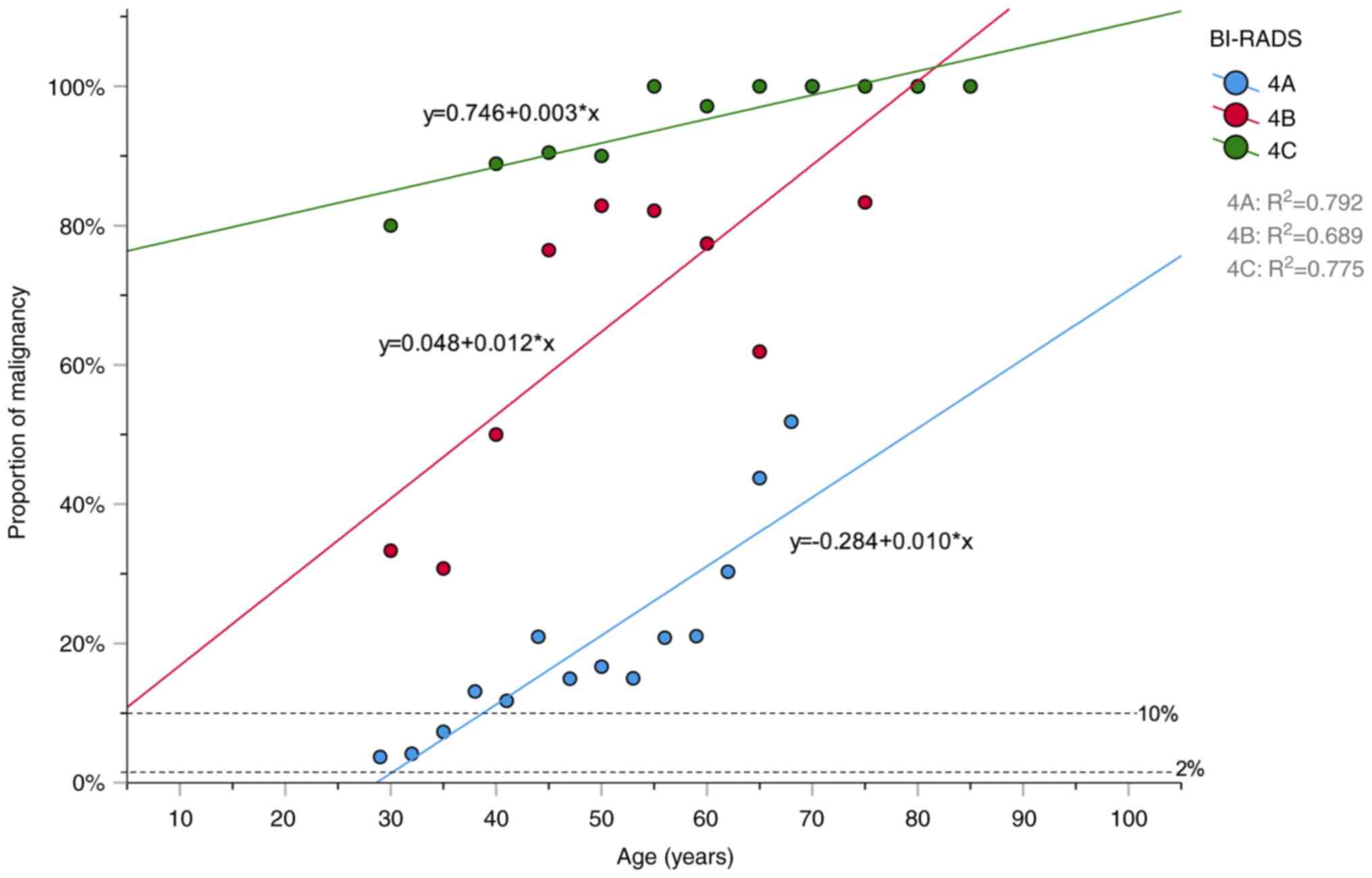
Linear regression model for age and
proportion of malignancy
Based on the BI-RADS guidelines (9), the reference ranges for the
malignancy probabilities of BI-RADS 4A, 4B, and 4C malignancies are
2-10, 10-50 and 50-95%, respectively. The distribution of benign
and malignant tumors with age was normal (Fig. S1). The distribution of the
proportion of malignancy with age using a linear regression model
is depicted in Fig. 4. R2 values
for BI-RADS 4A, 4B, and 4C were 0.792, 0.689 and 0.775,
respectively. For patients with BI-RADS subcategory 4A lesions, the
likelihood of malignancy was lower than the lower limit of the
reference range (2%) for patients aged <30 years. This suggests
that short-term follow-up could be performed instead of biopsy.
Discussion
The present study categorized patients into age
groups to examine the impact of age on the PPV of BI-RADS for
breast US. Findings across training and validation cohorts reveal
that breast cancer incidence varied by age even within the same
BI-RADS score, with older patients more likely to receive a breast
cancer diagnosis than younger patients. This indicates that
incorporating age into the BI-RADS criteria could refine diagnostic
accuracy.
A model combining age with BI-RADS score was
developed to evaluate whether this approach enhances breast cancer
diagnosis compared with age or BI-RADS scores alone. AUCs
demonstrated that both factors independently predicted breast
cancer, but the combined model exhibited superior predictive
accuracy in both cohorts, highlighting the benefit of this
integrative method for more precise diagnosis.
Moreover, the present study proposes adjusting the
BI-RADS 4A category with an age cutoff as patients aged <30
years have a lower likelihood of breast cancer than the reference
range lower limit (2%). This suggests reclassifying BI-RADS 4A
lesions for patients aged <30 years to category 3, potentially
avoiding unnecessary biopsies and minimizing harm (19). This suggests that age influences
breast cancer risk assessment within BI-RADS categories and
supports a more customized diagnostic approach.
To clarify the underlying mechanisms by which age
affects BI-RADS classification, the present study analyzed the
incidence of malignancy-related US features in the different age
groups. In US diagnosis of breast lesions, malignant signs include
fuzzy boundary, irregular shape, calcification and blood flow
signal (12). The present study
assessed age-related PPVs of these US features in BI-RADS category
4 lesions and found that a fuzzy boundary was the only significant
age-associated imaging feature. However, as not all suspicious
malignant features were analyzed in the present study (such as
echo, dilated duct and axillary adenopathy), fuzzy boundaries may
not be the only age-related US feature for breast cancer.
Previous research (27-30)
has indicated that the likelihood of malignancy in breast lesions,
as assessed by BI-RADS score, increases with age. Fu et al
(27) and Hu et al
(28) demonstrated that age
influences the predictive value of BI-RADS categories 3-5 in breast
US, using similar age groupings to the present study. Notably, Fu
et al found no significant difference (P=0.1853) in the
predictive value for category 4C lesions, consistent with the
present study. The differences between the present and
aforementioned study are the broader focus and the lack of
age-specific analysis of BI-RADS category 4 lesions in the
aforementioned study. Noonpradej et al (29), assessing patients with BI-RADS
category 4 lesions, identified a clear positive link between age
and predictive value but did not investigate the connection between
US features and age. The present study suggested that fuzzy
boundaries in US images may be an age-associated characteristic of
breast cancer, warranting further investigation. Xie et al
(30) developed a nomogram
integrating clinical, MRI, US and mammography data to reclassify
patients with BI-RADS 4A lesions to category 3. Although the
present model was solely based on US, its accuracy (AUC=0.872) was
not inferior to that shown by the comprehensive model (AUC=0.859)
in the aforementioned study, especially considering that most
outpatients undergo only US examinations, rather than mammography
and MRI, due to economic and practical considerations. Therefore,
the present model has broader applicability.
The present study analyzed 1,475 patients between
the ages of 15 and 85 years, demonstrating the broad applicability
of integrating age with BI-RADS for breast cancer diagnosis.
Despite age differences between the training and validation
cohorts, the findings suggested that combining age with BI-RADS
improves breast cancer diagnostic accuracy, suggesting the
scientific rigor of the present findings. The larger, age-diverse
training cohort resulted in a robust model capable of accurately
classifying breast lesions across a wide age range, accounting for
age-associated variabilities in lesion characteristics. The
efficacy of the model in the relatively younger validation cohort
underscored its suitability for the age group most affected by the
clinical question of downgrading BI-RADS 4A lesions. The use of US,
a common diagnostic tool, further supports the potential of the
model to enhance breast cancer screening protocols. There are some
limitations to the present study. Firstly, as a retrospective
study, a larger lesion sample is necessary to validate the present
findings, particularly for establishing a definitive cut-off age.
The generalizability of the study may be limited because
ultrasound, typically used as an adjunct to mammography rather than
a standalone tool in Western countries (31,32),
shows significant operator dependency and lacks evidence on its
impact in reducing breast cancer mortality, making it less
applicable to practices relying solely on its diagnostic
efficacy.
Future work should focus on validating the present
findings through prospective studies, assessing the real-world
effectiveness of integrating age with BI-RADS. Larger sample sizes
and multi-center studies are required to enhance the
generalizability and reliability of the present findings. Enhancing
diagnostic reliability involves standardized scanning protocols,
rigorous operator training, quality control programs, advanced
technologies such as 3D ultrasonography and elastography,
artificial intelligence and machine learning for improved image
analysis and cross-verification with multiple imaging modalitie
(4,33,34).
Additionally, longitudinal studies are key for understanding the
long-term benefits of this approach in patient outcomes, especially
its effectiveness in decreasing over-diagnosis in younger
patients.
The present study has notable clinical application.
Integrating age with BI-RADS facilitates age-specific risk
assessment, allowing for more tailored breast cancer screening
guidelines and improved diagnostic precision. This approach
recognizes that different age groups exhibit varying risks and
characteristics of breast cancer, necessitating adjustments in
screening protocols to enhance effectiveness. For example, younger
patients, who typically have denser breast tissue, might benefit
from adjusted screening methods. By combining age with BI-RADS
categories, clinicians can offer a nuanced understanding of breast
lesions, leading to personalized management plans and decreasing
overtreatment and associated anxiety in younger patients.
In conclusion, the present study investigated
clinical and imaging risk features associated with breast cancer
diagnosis in a large population. The present study analyzed
predictive risk factors of age-specific US images and the role of
age in diagnosing BI-RADS 4 cases. The present results may improve
diagnostic accuracy, facilitating clinicians in assessing breast
cancer risk based on age and US reports, choosing appropriate
treatments, controlling disease progression and improving patient
survival rate and quality of life.
Supplementary Material
Distribution of benign and malignant
tumors by age. The age distribution of the benign and malignant
tumors was normal. With increasing age, ratio of benign to
malignant tumors decreases.
Collinearity analysis between age, BI
RADS and ultrasonic image features.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported in part by the National
Natural Science Foundation of China (grant no. 81502665).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
JD and MW confirm the authenticity of all the raw
data. JD, MS and MWanalyzed and interpreted data. NL and YJ
interpreted the ultrasound reports. YZ, FY, WL and SS conceived and
designed the study. All authors wrote the manuscript. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was performed in line with the
principles of the Declaration of Helsinki. Ethical approval was
obtained from the ethics committee of Renmin Hospital of Wuhan
University (approval no. WDRY2021-KS025) and Maternal and Child
Health Hospital of Hubei Province (approval no. 2024ICE-LW035). As
this was a retrospective study, the ethics committees of both
institutions waived the requirement for informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48.
2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tagliafico AS, Piana M, Schenone D, Lai R,
Massone AM and Houssami N: Overview of radiomics in breast cancer
diagnosis and prognostication. Breast. 49:74–80. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bevers TB, Helvie M, Bonaccio E, Calhoun
KE, DalyMB FarrarWB, Garber JE, Gray R, Greenberg CC, Greenup R, et
al: Breast cancer screening and diagnosis, version 3.2018, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
16:1362–1389. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Salmanoglu E, Klinger K, Bhimani C,
Sevrukov A and Thakur ML: Advanced approaches to imaging primary
breast cancer: An update. Clin Transl Imaging. 7:381–404. 2019.
|
|
5
|
Chong A, Weinstein SP, McDonald ES and
Conant EF: Digital breast tomosynthesis: Concepts and clinical
practice. Radiology. 292:1–14. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hou XY, Niu HY, Huang XL and Gao Y:
Correlation of breast ultrasound classifications with breast cancer
in Chinese women. Ultrasound Med Biol. 42:2616–2621.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Devolli-Disha E, Manxhuka-Kërliu S, Ymeri
H and Kutllovci A: Comparative accuracy of mammography and
ultrasound in women with breast symptoms according to age and
breast density. Bosn J Basic Med Sci. 9:131–136. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kuhl CK: Abbreviated magnetic resonance
imaging (MRI) for breast cancer screening: Rationale, concept, and
transfer to clinical practice. Annu Rev Med. 70:501–519.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Magny SJ, Shikhman R and Keppke AL: Breast
imaging reporting and data system. In: StatPearls [Internet].
StatPearls Publishing, Treasure Island, FL, 2023.
|
|
10
|
Lazarus E, Mainiero MB, Schepps B,
Koelliker SL and Livingston LS: BI-RADS lexicon for US and
mammography: Interobserver variability and positive predictive
value. Radiology. 239:385–391. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
He P, Cui LG, Chen W and Yang RL:
Subcategorization of ultrasonographic BI-RADS category 4:
Assessment of diagnostic accuracy in diagnosing breast lesions and
influence of clinical factors on positive predictive value.
Ultrasound Med Biol. 45:1253–1258. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hsu W, Zhou X, Petruse A, Chau N,
Lee-Felker S, Hoyt A, Wenger N, Elashoff D and Naeim A: Role of
clinical and imaging risk factors in predicting breast cancer
diagnosis among BI-RADS 4 cases. Clin Breast Cancer. 19:e142–e151.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kang YJ, Ahn SK, Kim SJ, Oh H, Han J and
Ko E: Relationship between mammographic density and age in the
United Arab Emirates population. J Oncol.
2019(7351350)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
McGuire A, Brown JAL, Malone C, McLaughlin
R and Kerin MJ: Effects of age on the detection and management of
breast cancer. Cancers (Basel). 7:908–929. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Clendenen TV, Ge W, Koenig KL, Afanasyeva
Y, Agnoli C, Brinton LA, Darvishian F, Dorgan JF, Eliassen AH, Falk
RT, et al: Breast cancer risk prediction in women aged 35-50 years:
Impact of including sex hormone concentrations in the gail model.
Breast Cancer Research. 21(42)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
DeSantis C, Ma J, Bryan L and Jemal A:
Breast cancer statistics, 2013. CA Cancer J Clin. 64:52–62.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Leong SP, Shen ZZ, Liu TJ, Agarwal G,
Tajima T, Paik NS, Sandelin K, Derossis A, Cody H, Foulkes WD, et
al: Is breast cancer the same disease in Asian and Western
countries? World J Surg. 34:2308–2324. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Spinelli Varella MA, Teixeira da Cruz J,
Rauber A, Varella IS, Fleck JF and Moreira LF: Role of BI-RADS
ultrasound subcategories 4A to 4C in predicting breast cancer. Clin
Breast Cancer. 18:e507–e511. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Raza S, Chikarmane SA, Neilsen SS, Zorn LM
and Birdwell RL: BI-RADS 3, 4, and 5 lesions: Value of US in
managementfollow-up and outcome. Radiology. 248:773–781.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cheung A (ed): WHO classification of
tumours 5th edition. World Health Organization, Geneva, 2018.
|
|
21
|
Lei S, Zheng R, Zhang S, Chen R, Wang S,
Sun K, Zeng H, Wei W and He J: Breast cancer incidence and
mortality in women in China: Temporal trends and projections to
2030. Cancer Biol Med. 18:900–909. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li JW, Zhang K, Shi ZT, Zhang X, Xie J,
Liu JY and Chang C: Triple-negative invasive breast carcinoma: The
association between the sonographic appearances with
clinicopathological feature. Sci Rep. 8(9040)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tian L, Wang L, Qin Y and Cai J:
Systematic review and meta-analysis of the malignant ultrasound
features of triple-negative breast cancer. J Ultrasound Med.
39:2013–2025. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wojcinski S, Soliman AA, Schmidt J,
Makowski L, Degenhardt F and Hillemanns P: Sonographic features of
triple-negative and non-triple-negative breast cancer. J Ultrasound
Med. 31:1531–1541. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang K, Zou Z, Shen H, Huang G and Yang S:
Calcification, posterior acoustic, and blood flow: Ultrasonic
characteristics of triple-negative breast cancer. J Healthc Eng.
2022(9336185)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fu CY, Hsu HH, Yu JC, Hsu GC, Hsu KF, Chan
DC, Ku CH, Lu TC and Chu CH: Influence of age on PPV of sonographic
BI-RADS categories 3, 4, and 5. Ultraschall Med. 32 (Suppl
1):S8–S13. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hu Y, Yang Y, Gu R, Jin L, Shen S, Liu F,
Wang H, Mei J, Jiang X, Liu Q and Su F: Does patient age affect the
PPV3 of ACR BI-RADS ultrasound categories 4 and 5 in the diagnostic
setting? Eur Radiol. 28:2492–2498. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Noonpradej S, Wangkulangkul P,
Woodtichartpreecha P and Laohawiriyakamol S: Prediction for breast
cancer in BI-RADS category 4 lesion categorized by age and breast
composition of women in Songklanagarind hospital. Asian Pac J
Cancer Prev. 22:531–536. 2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xie Y, Zhu Y, Chai W, Zong S, Xu S, Zhan W
and Zhang X: Downgrade BI-RADS 4A patients using nomogram based on
breast magnetic resonance imaging, ultrasound, and mammography.
Front Oncol. 12(807402)2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Engmann NJ, Golmakani MK, Miglioretti DL,
Sprague BL and Kerlikowske K: Breast Cancer Surveillance
Consortium. Population-attributable risk proportion of clinical
risk factors for breast cancer. JAMA Oncol. 3:1228–1236.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Corsetti V, Houssami N, Ferrari A,
Ghirardi M, Bellarosa S, Angelini O, Bani C, Sardo P, Remida G,
Galligioni E and Ciatto S: Breast screening with ultrasound in
women with mammography-negative dense breasts: Evidence on
incremental cancer detection and false positives, and associated
cost. Eur J Cancer. 44:539–544. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Le EPV, Wang Y, Huang Y, Hickman S and
Gilbert FJ: Artificial intelligence in breast imaging. Clin Radiol.
74:357–366. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lei YM, Yin M, Yu MH, Yu J, Zeng SE, Lv
WZ, Li J, Ye HR, Cui XW and Dietrich CF: Artificial intelligence in
medical imaging of the breast. Front Oncol.
11(600557)2021.PubMed/NCBI View Article : Google Scholar
|