|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Llovet JM, Kelley RK, Villanueva A, Singal
AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J and
Finn RS: Hepatocellular carcinoma. Nat Rev Dis Primers.
7(6)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Singal AG, Llovet JM, Yarchoan M, Mehta N,
Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA,
et al: AASLD Practice Guidance on prevention, diagnosis, and
treatment of hepatocellular carcinoma. Hepatology. 78:1922–1965.
2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Boss DS, Glen H, Beijnen JH, Keesen M,
Morrison R, Tait B, Copalu W, Mazur A, Wanders J, O'Brien JP, et
al: A phase I study of E7080, a multitargeted tyrosine kinase
inhibitor, in patients with advanced solid tumours. Br J Cancer.
106:1598–1604. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ikeda M, Okusaka T, Mitsunaga S, Ueno H,
Tamai T, Suzuki T, Hayato S, Kadowaki T, Okita K and Kumada H:
Safety and Pharmacokinetics of Lenvatinib in Patients with Advanced
Hepatocellular Carcinoma. Clin Cancer Res. 22:1385–1394.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ikeda K, Kudo M, Kawazoe S, Osaki Y, Ikeda
M, Okusaka T, Tamai T, Suzuki T, Hisai T, Hayato S, et al: Phase 2
study of lenvatinib in patients with advanced hepatocellular
carcinoma. J Gastroenterol. 52:512–519. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kuzuya T, Ishigami M, Ito T, Ishizu Y,
Honda T, Ishikawa T, Hirooka Y and Fujishiro M: Clinical
characteristics and outcomes of candidates for second-line therapy,
including regorafenib and ramucirumab, for advanced hepatocellular
carcinoma after sorafenib treatment. Hepatol Res. 49:1054–1065.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Havrilesky LJ, Reiner M, Morrow PK, Watson
H and Crawford J: A review of relative dose intensity and survival
in patients with metastatic solid tumors. Crit Rev Oncol Hematol.
93:203–210. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
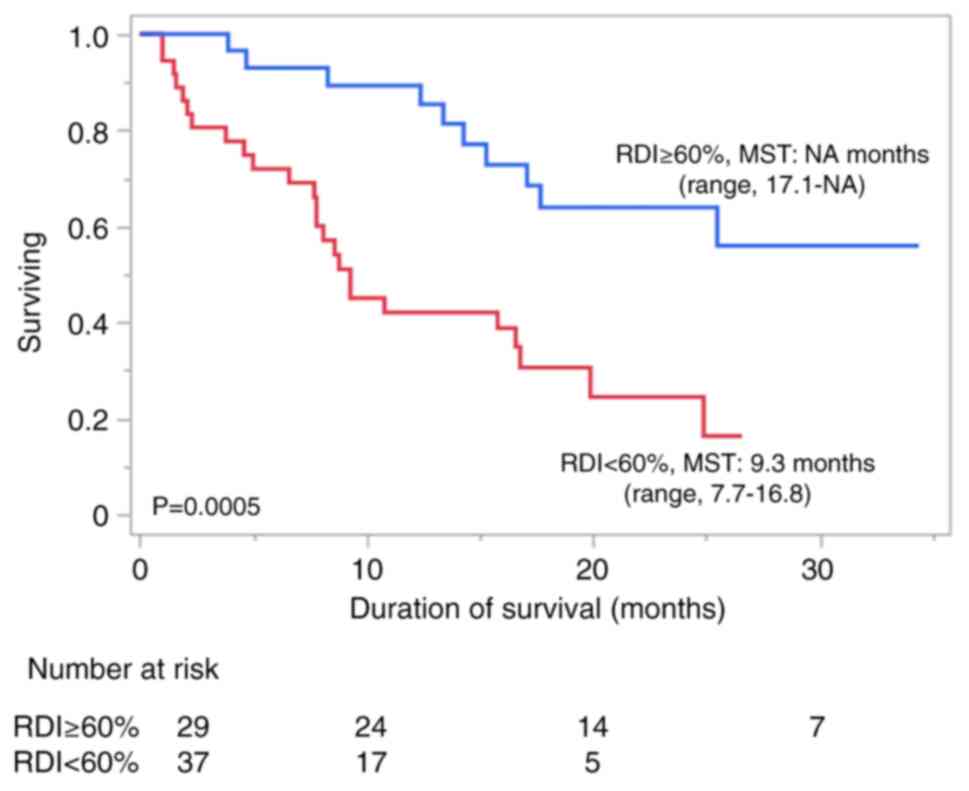
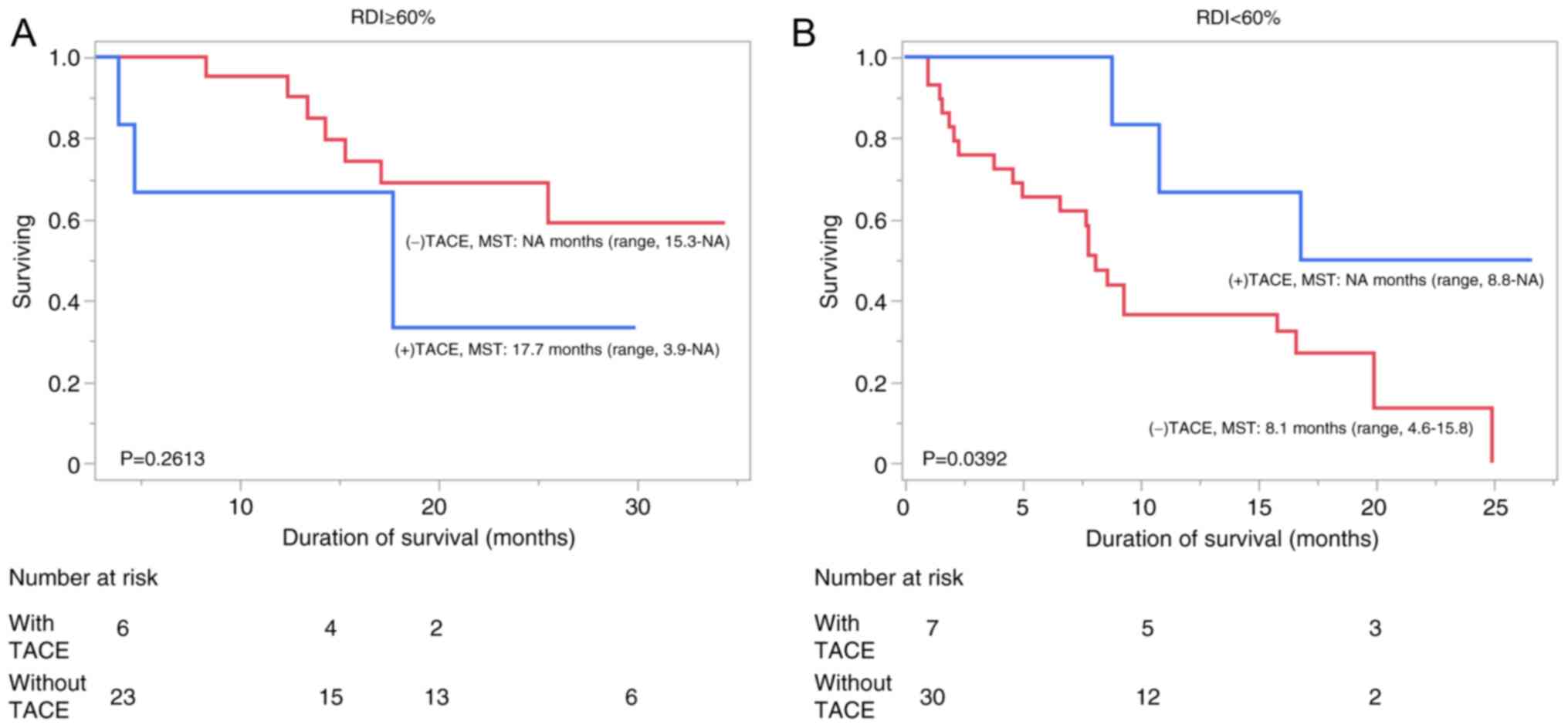
Takahashi A, Moriguchi M, Seko Y, Ishikawa
H, Yo T, Kimura H, Fujii H, Shima T, Mitsumoto Y, Ishiba H, et al:
Impact of relative dose intensity of early-phase lenvatinib
treatment on therapeutic response in hepatocellular carcinoma.
Anticancer Res. 39:5149–5156. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
European Association for the Study of the
Liver. Electronic address: simpleeasloffice@easloffice.eu;
European Association for the Study of the Liver. EASL Clinical
Practice Guidelines: Management of hepatocellular carcinoma. J
Hepatol. 69:182–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kudo M, Kawamura Y, Hasegawa K, Tateishi
R, Kariyama K, Shiina S, Toyoda H, Imai Y, Hiraoka A, Ikeda M, et
al: Management of Hepatocellular Carcinoma in Japan: JSH Consensus
Statements and Recommendations 2021 Update. Liver Cancer.
10:181–223. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Llovet JM, Real MI, Montaña X, Planas R,
Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al:
Arterial embolisation or chemoembolisation versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma: A
randomised controlled trial. Lancet. 359:1734–1739. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hiraoka A, Kumada T, Kudo M, Hirooka M,
Koizumi Y, Hiasa Y, Tajiri K, Toyoda H, Tada T, Ochi H, et al:
Hepatic function during repeated TACE procedures and prognosis
after introducing sorafenib in patients with unresectable
hepatocellular carcinoma: Multicenter Analysis. Dig Dis.
35:602–610. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kudo M, Ueshima K, Ikeda M, Torimura T,
Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, et al:
Randomised, multicentre prospective trial of transarterial
chemoembolisation (TACE) plus sorafenib as compared with TACE alone
in patients with hepatocellular carcinoma: TACTICS trial. Gut.
69:1492–1501. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li X, Feng GS, Zheng CS, Zhuo CK and Liu
X: Expression of plasma vascular endothelial growth factor in
patients with hepatocellular carcinoma and effect of transcatheter
arterial chemoembolization therapy on plasma vascular endothelial
growth factor level. World J Gastroenterol. 10:2878–2882.
2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ and
Cao GW: Increased expression of vascular endothelial growth factor
in hepatocellular carcinoma after transcatheter arterial
chemoembolization. Acta Radiol. 49:523–529. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rizzo A, Ricci AD and Brandi G:
Trans-Arterial chemoembolization plus systemic treatments for
hepatocellular carcinoma: An Update. J Pers Med.
12(1788)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao
C, Huang F, Tang R, Cheng Y, Huang Z, et al: Lenvatinib combined
with transarterial chemoembolization as first-line treatment for
advanced hepatocellular carcinoma: A phase III, Randomized clinical
trial (LAUNCH). J Clin Oncol. 41:117–127. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Johnson PJ, Berhane S, Kagebayashi C,
Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A,
Palmer D, et al: Assessment of liver function in patients with
hepatocellular carcinoma: A new evidence-based approach-the ALBI
grade. J Clin Oncol. 33:550–558. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hiraoka A, Michitaka K, Kumada T, Izumi N,
Kadoya M, Kokudo N, Kubo S, Matsuyama Y, Nakashima O, Sakamoto M,
et al: Validation and potential of albumin-bilirubin grade and
prognostication in a nationwide survey of 46,681 hepatocellular
carcinoma patients in Japan: The need for a more detailed
evaluation of hepatic function. Liver Cancer. 6:325–336.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hiraoka A, Kumada T, Tsuji K, Takaguchi K,
Itobayashi E, Kariyama K, Ochi H, Tajiri K, Hirooka M, Shimada N,
et al: Validation of Modified ALBI grade for more detailed
assessment of hepatic function in hepatocellular carcinoma
patients: A multicenter analysis. Liver Cancer. 8:121–129.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Okusaka T, Ikeda K, Kudo M, Finn R, Qin S,
Han KH, Cheng AL, Piscaglia F, Kobayashi M, Sung M, et al: Safety
and efficacy of lenvatinib by starting dose based on body weight in
patients with unresectable hepatocellular carcinoma in REFLECT. J
Gastroenterol. 56:570–580. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gordan JD, Kennedy EB, Abou-Alfa GK, Beg
MS, Brower ST, Gade TP, Goff L, Gupta S, Guy J, Harris WP, et al:
Systemic therapy for advanced hepatocellular carcinoma: ASCO
Guideline. J Clin Oncol. 38:4317–4345. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
U.S. Department of Health and Human
Services: Common Terminology Criteria for Adverse Events (CTCAE).
Version 5.0. National Cancer Institute, Bethesda, MD, 2017.
|
|
28
|
Kirino S, Tsuchiya K, Kurosaki M, Kaneko
S, Inada K, Yamashita K, Osawa L, Hayakawa Y, Sekiguchi S, Okada M,
et al: Relative dose intensity over the first four weeks of
lenvatinib therapy is a factor of favorable response and overall
survival in patients with unresectable hepatocellular carcinoma.
PLoS One. 15(e0231828)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Schwartz LH, Seymour L, Litiere S, Ford R,
Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, et
al: RECIST 1.1 - Standardisation and disease-specific adaptations:
Perspectives from the RECIST Working Group. Eur J Cancer.
62:138–145. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sasaki R, Fukushima M, Haraguchi M, Miuma
S, Miyaaki H, Hidaka M, Eguchi S, Matsuo S, Tajima K, Matsuzaki T,
et al: Response to lenvatinib is associated with optimal
relativedose intensity in hepatocellular carcinoma: Experience in
clinical settings. Cancers (Basel). 11(1769)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shim JH, Park JW, Kim JH, An M, Kong SY,
Nam BH, Choi JI, Kim HB, Lee WJ and Kim CM: Association between
increment of serum VEGF level and prognosis after transcatheter
arterial chemoembolization in hepatocellular carcinoma patients.
Cancer Sci. 99:2037–2044. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yamamoto Y, Matsui J, Matsushima T,
Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A,
Hoshi SS, et al: Lenvatinib, an angiogenesis inhibitor targeting
VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft
models associated with microvessel density and pericyte coverage.
Vasc Cell. 6(18)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jiang H, Meng Q, Tan H, Pan S, Sun B, Xu R
and Sun X: Antiangiogenic therapy enhances the efficacy of
transcatheter arterial embolization for hepatocellular carcinomas.
Int J Cancer. 121:416–424. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kawamura Y, Kobayashi M, Shindoh J,
Kobayashi Y, Okubo S, Tominaga L, Kajiwara A, Kasuya K, Iritani S,
Fujiyama S, et al: Lenvatinib-Transarterial chemoembolization
sequential therapy as an effective treatment at progression during
lenvatinib therapy for advanced hepatocellular carcinoma. Liver
Cancer. 9:756–770. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fu Z, Li X, Zhong J, Chen X, Cao K, Ding
N, Liu L, Zhang X, Zhai J and Qu Z: Lenvatinib in combination with
transarterial chemoembolization for treatment of unresectable
hepatocellular carcinoma (uHCC): A retrospective controlled study.
Hepatol Int. 15:663–675. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kano MR, Komuta Y, Iwata C, Oka M, Shirai
YT, Morishita Y, Ouchi Y, Kataoka K and Miyazono K: Comparison of
the effects of the kinase inhibitors imatinib, sorafenib, and
transforming growth factor-beta receptor inhibitor on extravasation
of nanoparticles from neovasculature. Cancer Sci. 100:173–180.
2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Une N, Takano-Kasuya M, Kitamura N, Ohta
M, Inose T, Kato C, Nishimura R, Tada H, Miyagi S, Ishida T, et al:
The anti-angiogenic agent lenvatinib induces tumor vessel
normalization and enhances radiosensitivity in hepatocellular
tumors. Med Oncol. 38(60)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lim JSJ, Wong ALA, Ow SGW, Ngoi NYL, Chan
GHJ, Ang YLE, Chong WQ, Lim SE, Lim YW, Lee M, et al: Phase Ib/II
dose expansion study of lenvatinib combined with letrozole in
postmenopausal women with hormone receptor-positive breast cancer.
Clin Cancer Res. 28:2248–2256. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tachiiri T, Nishiofuku H, Maeda S, Sato T,
Toyoda S, Matsumoto T, Chanoki Y, Minamiguchi K, Taiji R, Kunichika
H, et al: Vascular normalization caused by short-term lenvatinib
could enhance transarterial chemoembolization in hepatocellular
carcinoma. Curr Oncol. 30:4779–4786. 2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kudo M, Ueshima K, Saeki I, Ishikawa T,
Inaba Y, Morimoto N, Aikata H, Tanabe N, Wada Y, Kondo Y, et al: A
phase 2, prospective, multicenter, single-arm trial of
transarterial chemoembolization therapy in combination strategy
with lenvatinib in patients with unresectable intermediate-stage
hepatocellular carcinoma: TACTICS-L Trial. Liver Cancer. 13:99–112.
2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kudo M, Han KH, Ye SL, Zhou J, Huang YH,
Lin SM, Wang CK, Ikeda M, Chan SL, Choo SP, et al: A changing
paradigm for the treatment of intermediate-stage hepatocellular
carcinoma: Asia-Pacific primary liver cancer expert consensus
statements. Liver Cancer. 9:245–260. 2020.PubMed/NCBI View Article : Google Scholar
|