Introduction
Hepatocellular carcinoma (HCC) was the third most
lethal cancer and the sixth most frequently diagnosed cancer
globally in 2020, with an estimated 830,000 deaths and 906,000 new
cases (1). The number of new liver
cancer cases continues to increase and an occurrence of ≥1 million
is estimated by 2025(2).
Unresectable HCC is defined when a patient is not a
candidate for resection or ablation. These patients are classified
as stage B or C according to the Barcelona Clinical Liver Cancer
(BCLC) staging system, updated version 2022(3), which is widely used to classify liver
cancer for treatment. Patients with BCLC stage B or intermediate
stage HCC typically undergo transarterial chemoembolization (TACE)
as the first choice of treatment, and patients with BCLC stage C or
advanced stage HCC (for example, patients with a portal invasion or
extrahepatic spread) typically undergo systemic therapies as the
first choice of treatment (4).
Lenvatinib (Lenvima®; Eisai Co., Ltd.)
exhibits antitumor and angiogenesis inhibitory effects on the basis
of the dual inhibition of the vascular endothelial growth factor
(VEGF) and fibroblast growth factor pathways (5). In a comparison of lenvatinib and
sorafenib treatments, the median overall survival (OS) for
lenvatinib showed a non-inferiority to sorafenib. Furthermore,
lenvatinib therapy significantly prolonged the progression-free
survival (PFS) time and the time to progression compared with
sorafenib therapy. The objective response (OR) rates categorized by
the modified Response Evaluation Criteria in Solid Tumors (mRECIST)
were 24.1 vs. 9.2% for lenvatinib and sorafenib, respectively
(6).
In clinical practice, patients administered
lenvatinib therapy often experience dose modification due to a
number of situations, including adverse events (AEs), deterioration
of hepatic reserve function and a decline in Eastern Cooperation
Oncology Group performance status (ECOG PS). Naturally, dose
reductions diminish the therapeutic effects of the drug (7-9).
The relative dose intensity (RDI) is the percentage amount out of
the dose intensity delivered compared with the reference standard
dose intensity for a regimen of chemotherapy including tyrosine
kinase inhibitors. Treatment with a higher RDI may enhance the
treatment outcomes due to the higher plasma concentration of the
drug (10). In a lenvatinib study,
patients administered an 8-week RDI of ≥75% had significantly
better response rates (68 vs. 20%) and a more prolonged PFS time
compared with those administered an 8-week RDI of <75% (11).
TACE is recommended by a number of clinical practice
guidelines worldwide for patients with intermediate-stage HCC:
American Association for the Study of Liver Disease (AASLD),
European Association for the Study of the Liver (EASL), Japan
Society of Hepatology (JSH) (4,12,13).
Based on randomized controlled trials comparing the prognosis of
patients with multiple HCCs treated with TACE or symptomatic
therapy, TACE has been recommended as the treatment of choice for
these patients (14). High tumor
recurrence rates are commonly found in clinical practice; thus,
this treatment is typically repeated a number of times and may
cause a decline in the hepatic reserve, leading to poor patient
prognoses (15). Pre-treatment
with sorafenib prior to TACE has been shown to result in a
significantly longer interval between procedures, resulting in less
hepatic deterioration (16). TACE
increases tumor hypoxia, which activates hypoxic inducible
factor-1α, promotes the upregulated expression of proangiogenic
factors, such as VEGF and platelet-derived growth factor, and
results in the promotion of tumor angiogenesis (17-19).
The addition of antiangiogenic medication to TACE may reduce tumor
size and vascular density; thus, this treatment may prolong the
survival time compared with using TACE alone (20). According to the results of the
LAUNCH trial, which was a randomized clinical trial, the addition
of lenvatinib to TACE (LEN-TACE) exhibited an improved coordinated
antitumor effect, and thus LEN-TACE had improved clinical outcomes
compared with lenvatinib treatment alone in patients with advanced
HCC. The results revealed a statistically significant improvement
in the median PFS time in the LEN-TACE group compared with the
lenvatinib alone (LEN) group (10.6 vs. 6.4 months, respectively),
and a higher OR rate according to mRECIST (54.1 vs. 25.0%,
respectively) (21).
To date and to the best of our knowledge, there have
been no reports examining how the efficacy of a combination of
lenvatinib with TACE varies with the RDI of lenvatinib. Therefore,
the present study examined whether there is a difference in the
combined effect of TACE with a high or low 8-week RDI of
lenvatinib.
Materials and methods
Patient characteristics
The present study retrospectively reviewed the
medical records of 66 patients with unresectable HCC who were
treated with lenvatinib at Kansai Medical University (Hirakata,
Japan). Patients were included if they were aged ≥20 years,
diagnosed with unresectable HCC or BCLC stage B or C, had a
Child-Pugh grade A or B, and had no prior history of treatment with
lenvatinib. Patients were excluded if they had decompensated liver
function or poor hepatic reserve, a history of other previous or
current advanced cancers as comorbidities, a short of observation
period (<8 weeks), incomplete medical records or missing data,
an inability to undergo imaging with contrast media, lenvatinib
therapy combined with treatments other than TACE. Eligible patients
were those who initiated lenvatinib therapy between April 2018 and
September 2020. Among these patients, the etiology was considered
to be hepatitis B virus (HBV) if the test for the HBV surface
antigen was positive, the etiology was considered to be hepatitis C
virus (HCV) if the test for anti-HCV antibodies was positive and
the etiology was considered to be non-B-non-C (NBNC) hepatitis if
the tests for both HBV surface antigen and HCV antibodies were
negative. To access hepatic reserves, the Child-Pugh and modified
albumin-bilirubin (ALBI) grades were determined as previously
reported (22-24).
HCC was diagnosed using contrast-enhanced computed tomography (CT)
or magnetic resonance imaging (MRI) (4,12,13).
For atypical imaging results, histopathology was performed to
confirm the diagnosis.
Treatment protocol and RDI
Lenvatinib was orally administered to patients with
unresectable HCC, and both the body weight and hepatic reserves of
the patient were considered for determining the dosage of
lenvatinib. Lenvatinib was administered at a starting dose of 8 or
12 mg once daily for those ≤60 kg or >60 kg, respectively
(25). The starting dose was 8 mg
for patients with Child-Pugh grade B. Dose reduction was
administered at the consideration of the attending physician based
on the recent guideline of dosage modification for patients with
drug-related toxicities (26).
Unacceptable toxicity, grade ≥3 according to the Common Terminology
Criteria for AEs (CTCAE) (27)
definition, or the progression of disease were considered for the
discontinuation of lenvatinib. The RDI was determined as previously
reported (10,11,28),
by dividing the dose delivered by the reference dose of the
regimen. Combined immunotherapy is a standard treatment for
unresectable HCC (4). In Japan,
combined immunotherapy was approved in 2020; therefore, for
patients who received treatment before that period were treated
with lenvatinib or sorafenib as the first-line therapies. In the
present study, there were 17 (58.6%) and 29 (78.3%) patients who
received lenvatinib as a first-line therapy in the high- and
low-RDI groups, respectively. Furthermore, there were 12 (41.3%)
and 8 (21.6%) patients who received sorafenib as a first-line
therapy and then switched to lenvatinib in the high- and low-RDI
groups, respectively. However, only data from the period during
lenvatinib therapy were analysed in the present study. After
discontinuation of lenvatinib therapy, several treatments were
continued as second- and third-line treatments, with combined
immunotherapy being the main post-lenvatinib treatment for 8
(27.6%) and 7 (18.9%) patients in the high- and low-RDI groups,
respectively (Table SI). After
starting lenvatinib treatment, the attending physician considered
the suitability of performing TACE based on the imaging evaluations
of each patient. The median timing of TACE after lenvatinib
treatment was 180.5 days (range, 64-365) among 6 patients in the
high-RDI group and 38 days (range, 6-512) in 7 patients in the
low-RDI group (P=0.1741). A total of three variations of TACE
procedures were used based on the chemotherapy administered:
Conventional (c)TACE, which uses an epirubicin (Kyowa Hakko Bio
Co., Ltd.)-lipiodol (Guerbet Japan Co., Ltd.) suspension; the
IA-call® procedure, which uses a cisplatin fine powder
formulation (IA-call®; Nippon Kayaku, Co., Ltd.); and
drug-eluting beads (DEB)-TACE, which uses epirubicin accompanied by
DEBs (DC bead™, Boston Scientific Corporation). After the cytotoxic
agents were completely intra-arterially injected, an embolic agent,
gelatin sponge particles (Gelpart®; Nippon Kayaku Co.,
Ltd), was administered until the complete cessation of blood supply
to the nodules. The details of each patient are presented in
Table SII. Administration of
lenvatinib was discontinued for a minimum of 2 days prior and after
TACE. Lenvatinib was then readministered at the same dose as
previously given before discontinuation, after determining that the
status and liver biochemical results of the patient were
adequate.
Evaluation criteria for AEs and
treatment response
The CTCAE (version 5.0) was applied to assess any
AEs (27). The outcome of the
treatment response was evaluated according to the RECIST 1.1 and
mRECIST guidelines using CT or MRI with the triphasic scanning
technique (29,30). Tumors were evaluated once within 8
weeks of lenvatinib initiation and then every 8-12 weeks
thereafter. In addition, for patients who had additional TACE
treatment, imaging evaluations were performed within 4 weeks
following TACE.
Statistical analysis
Continuous variables are presented as the median
(range) and as n (%) for categorical variables. Between-group
comparisons of continuous variables were analyzed using the
Mann-Whitney U test, and between-group comparisons of categorical
variables were analyzed using Fisher's exact test or the
χ2 test (if the criteria were matched). Changes in liver
function before and after lenvatinib treatment in each group were
analyzed using the Wilcoxon signed-rank test. Kaplan-Meier analysis
was used to estimate the OS, which were analyzed using the log-rank
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
A total of 66 patients with advanced HCC were
included in the present study, the baseline characteristics of
which are shown in Table I. The
median age of the patients was 72.5 years (range, 28-89 years) and
83.3% of the patients were male. In addition, the median body
weight of the patients was 60.3 kg (range, 34-104 kg). The etiology
of liver disease was classified as NBNC hepatitis (40.9%), HBV
hepatitis (31.8%) or HCV hepatitis (16.7%), and HBV and HCV
co-infection (10.6%). According to the hepatic reserve function,
the median albumin level was 3.4 g/dl (range, 2.1-4.5 g/dl), the
median total bilirubin level was 0.9 g/dl (range, 0.4-3.2 g/dl) and
the median prothrombin time (PT) was 85.5% (range, 28.6-114.2%).
With regards to the tumor markers, the median α-fetoprotein (AFP)
level was 14.0 ng/ml (range, 2-530,000 ng/ml), and the median
des-γ-carboxyprothrombin (DCP) level was 84.0 mAU/ml (range,
11-300,000 mAU/ml). Among the 66 patients, 19 had ascites (28.8%),
20 were classified as Child-Pugh grade B (30.3%), 40 were
classified as ALBI grade 2b (60.6%), 45 were classified as BCLC
stage B (68.2%), 11 had macroscopic portal vein invasion (16.7%)
and 14 had extrahepatic spread (21.2%).
 | Table IPatient baseline characteristics. |
Table I
Patient baseline characteristics.
| Baseline
characteristics | Value |
|---|
| Demographic
data | |
|
Median age,
years (range) | 72.5 (28-89) |
|
Male sex, n
(%) | 55 (83.3) |
|
Median
weight, kg (range) | 60.3 (34-104) |
| Clinical
characteristics, n (%) | |
|
Etiology | |
|
HBV | 21 (31.8) |
|
HCV | 11 (16.7) |
|
HBV
and HCV | 7 (10.6) |
|
NBNC | 27 (40.9) |
|
Ascites | 19 (28.8) |
|
Child-Pugh | |
|
A | 46 (69.7) |
|
B | 20 (30.3) |
|
ALBI
grade | |
|
1 | 9 (13.6) |
|
2a | 17 (25.8) |
|
2b | 40 (60.6) |
|
BCLC
stage | |
|
B | 45 (68.2) |
|
C | 21 (31.8) |
|
Macrovascular
invasion | 11 (16.7) |
|
Extrahepatic
spread | 14 (21.2) |
| Biochemical
characteristics | |
|
Median
albumin, g/dl (range) | 3.4 (2.1-4.5) |
|
Median total
bilirubin, g/dl (range) | 0.9 (0.4-3.2) |
|
Median
prothrombin time, % (range) | 85.5
(28.6-114.2) |
|
Median AFP,
ng/ml (range) | 14.0
(2-530,000) |
|
Median DCP,
mAU/ml (range) | 84.0
(11-300,000) |
AEs associated with lenvatinib therapy
in the high- and low-RDI groups
The AEs associated with lenvatinib therapy are
presented in Table SIII. In the
8-week RDI ≥60% group, 65.5 and 20.7% of patients had any-grade and
grade ≥3 AEs, respectively. The incidences of any AE grade of
fatigue, thrombocytopenia, hand-foot-skin reaction, elevated
aspartate aminotransferase, diarrhea, hypertension, proteinuria,
and hyperbilirubinemia during the observation period were 34.5,
20.7, 10.3, 6.9, 10.3, 6.9, 3.4 and 3.4%, respectively. Of the
grade ≥3 AEs, fatigue was observed frequently in this group of
patients. Furthermore, in the 8-week RDI <60% group, 78.4 and
35.1% of patients had any-grade and grade ≥3 AEs, respectively. The
incidences of any AE grade of fatigue, thrombocytopenia,
hand-foot-skin reaction, elevated aspartate aminotransferase,
diarrhea, decrease appetite, hypertension, proteinuria, and
hyperbilirubinemia during the observation period were 37.8, 16.2,
16.2, 13.5, 2.7, 10.8, 5.4, 8.1 and 8.1%, respectively. Of the
grade ≥3 AEs, elevated aspartate aminotransferase was observed
frequently in this group of patients. Of the 66 included patients,
56 (84.8%) had dose reductions of lenvatinib or were withdrawn from
lenvatinib therapy due to encountering AEs.
A high RDI of lenvatinib over an
8-week period is associated with a favorable radiological response
and a prolonged OS
The median RDI at 8 weeks when including all 66
patients was 55.3%. Among the 66 patients, 29 had an RDI of ≥60%
for the first 8 weeks and were designated the high-RDI (RDI ≥60%)
group, and 37 had an RDI of <60% for the first 8 weeks and were
designated the low-RDI (RDI <60%) group. Table II shows a comparison of the
patient backgrounds of these two groups of patients. There were
statistically significant differences in the albumin level
(P=0.0100), total bilirubin level (P=0.0108), AFP level (P=0.0346)
and ALBI grade (P=0.0031) between the two groups.
 | Table IIPatient background comparison of the
8-week RDI ≥60% and <60% groups. |
Table II
Patient background comparison of the
8-week RDI ≥60% and <60% groups.
| Baseline
characteristics | RDI <60%
(n=37) | RDI ≥60%
(n=29) | P-value |
|---|
| Demographic
data | | | |
|
Median age,
years (range) | 73 (28-87) | 71 (36-89) | 0.9639 |
|
Male sex, n
(%) | 29 (78.4) | 26 (89.7) | 0.3255 |
|
Median
weight, kg (range) | 61.0
(34.0-104.0) | 57.2
(48.6-95.0) | 0.4894 |
| Clinical
characteristics, n (%) | | | |
|
Etiology | | | 0.3853 |
|
HBV | 11 (29.7) | 10 (34.5) | |
|
HCV | 7 (18.9) | 4 (13.8) | |
|
HBV
and HCV | 2 (5.4) | 5 (17.2) | |
|
NBNC | 17 (46.0) | 10 (34.5) | |
|
Ascites | 13 (35.1) | 6 (20.7) | 0.2752 |
|
Child-Pugh | | | 0.1797 |
|
A | 23 (62.2) | 23 (79.3) | |
|
B | 14 (37.8) | 6 (20.7) | |
|
ALBI
grade | | | 0.0031 |
|
1 | 2 (5.4) | 7 (24.2) | |
|
2a | 6 (16.2) | 11 (37.9) | |
|
2b | 29 (78.4) | 11 (37.9) | |
|
BCLC
stage | | | 0.9037 |
|
B | 25 (67.6) | 20 (69.0) | |
|
C | 12 (32.4) | 9 (31.0) | |
|
Macrovascular
invasion | 5 (13.5) | 6 (20.7) | 0.5153 |
|
Extrahepatic
spread | 9 (24.3) | 5 (17.2) | 0.5555 |
| Biochemical
characteristics | | | |
|
Median
albumin, g/dl (range) | 3.3 (2.1-4.5) | 3.7 (2.8-4.5) | 0.0100 |
|
Median total
bilirubin, g/dl (range) | 1.1 (0.4-3.2) | 0.8 (0.4-1.6) | 0.0108 |
|
Median
prothrombin time, % (range) | 85.4
(28.6-114.2) | 85.7
(62.3-110.6) | 0.4156 |
|
Median AFP,
ng/ml (range) | 16.7
(2-530,000) | 6.4 (2-1,757) | 0.0346 |
|
Median DCP,
mAU/ml (range) | 185
(12-300,000) | 43 (11-10,184) | 0.0881 |
The best tumor responses following treatment in the
low and high 8-week RDI groups according to the RECIST and mRECIST
guidelines were compared, as shown in Table III. According to the RECIST
guidelines, 34.5 and 18.9% of patients in the high- and low-RDI
groups had an OR rate, respectively, which was not statistically
significant (P=0.1691). In the high-RDI group, the patients had a
higher disease control rate compared with the low-RDI group, which
was statistically significant (62.1 vs. 35.1%, respectively;
P=0.0464). According to the mRECIST guidelines, 65.5 and 27.0% of
the patients in the high- and low-RDI groups had an OR rate,
respectively, and the difference was statistically significant
(P=0.0026). In the high-RDI group, the patients had a higher
disease control rate compared with the low-RDI group, which was
statistically significant (69.0 vs. 43.2%, respectively;
P=0.0482).
 | Table IIIComparison of the best tumor
responses following treatment in the 8-week RDI ≥60% (n=29) and
<60% (n=37) groups. |
Table III
Comparison of the best tumor
responses following treatment in the 8-week RDI ≥60% (n=29) and
<60% (n=37) groups.
| | RECIST | | mRECIST | |
|---|
| Variable | RDI <60%, n
(%) | RDI ≥60%, n
(%) | P-value | RDI <60%, n
(%) | RDI ≥60%, n
(%) | P-value |
|---|
| Objective
response | 7 (18.9) | 10 (34.5) | 0.1691 | 10 (27.0) | 19 (65.5) | 0.0026 |
|
Complete
response | 2 (5.4) | 4 (13.8) | 0.3924 | 2 (5.4) | 8 (27.6) | 0.0171 |
|
Partial
response | 5 (13.5) | 6 (20.7) | 0.5153 | 8 (21.6) | 11 (37.9) | 0.1774 |
| Stable disease | 6 (16.2) | 8 (27.6) | 0.3647 | 6 (16.2) | 1 (3.5) | 0.1244 |
| Disease control
rate | 13 (35.1) | 18 (62.1) | 0.0464 | 16 (43.2) | 20 (69.0) | 0.0482 |
| Progressive
disease | 16 (43.2) | 8 (27.6) | 0.2097 | 13 (35.1) | 6 (20.7) | 0.2752 |
| No evaluation | 8 (21.6) | 3 (10.3) | 0.3225 | 8 (21.6) | 3 (10.4) | 0.3225 |
Comparison of liver function and tumor
markers before and after lenvatinib administration
When comparing liver function markers before and
after 8 weeks of lenvatinib treatment among patients in the
high-RDI group, no significant changes were found in the albumin
level, total bilirubin level, PT, DCP level, Child-Pugh score or
ALBI score, but a significantly decreased AFP level was observed
(P=0.0090; Table SIVA). In the
low-RDI group, there were no significant changes in the AFP and DCP
levels, but the albumin level was significantly decreased
(P=0.0023; Table SIVB). These
results suggested that in the high-RDI group, 8 weeks of lenvatinib
treatment provided an antitumor effect demonstrated by the
significant decline in AFP level, no deterioration of hepatic
reserves and more specifically, no significant decrease in albumin
levels. However, in the low-RDI group, 8 weeks of lenvatinib
therapy resulted in a decrease in albumin level with an inadequate
antitumor effect, which was demonstrated by no significant change
in the levels of both tumor markers.
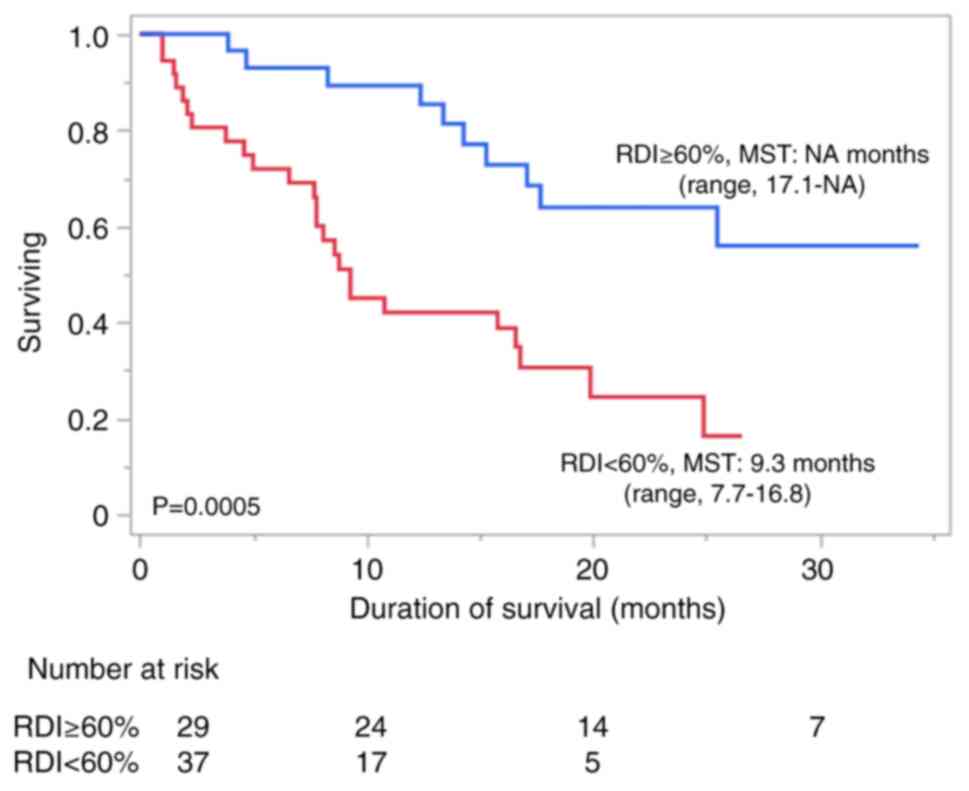
A median OS time of 16.8 months (range, 10.8-25.5
months) was observed for all patients. The high-RDI group had an OS
time of ≥17.1 months [range, 17.1 - not applicable (NA) months],
which was significantly longer than the OS time of the low-RDI
group (9.3 months; range, 7.7-16.8 months) (P=0.0005; Fig. 1).
Effect of TACE on the radiological
response and OS of the high- and low-RDI groups
The radiological tumor response and impairment of
liver function following LEN-TACE treatment were investigated to
predict the efficacy of the addition of TACE. There were 6 patients
in the high-RDI group who underwent LEN-TACE treatment. As shown in
Table IVA, in the high-RDI group,
the addition of TACE did not have a significant antitumor effect
according to evaluations using either RECIST or mRECIST. In
addition, as shown in Table SVA,
when comparing the changes in liver function before and after 4
weeks of TACE treatment, no significant changes in the albumin
level, total bilirubin level, AFP level, DCP level, Child-Pugh
score or ALBI score were observed, but a statistically significant
change in PT was found (P=0.0469). These findings suggested that
the addition of TACE did not show a good radiological response in
the high-RDI group, although it did not decrease the hepatic
reserve.
There were 7 patients in the low-RDI group who
underwent LEN-TACE treatment. As shown in Table SVB, when comparing the changes in
liver function before and after 4 weeks of TACE treatment, there
were no significant changes in the total bilirubin level, PT, AFP
level, DCP level, Child-Pugh score or ALBI score, but the change in
albumin level was statistically significant (P=0.0391). The best
tumor responses, as determined using the RECIST and mRECIST
guidelines, in the LEN-TACE and LEN subgroups of the low-RDI group
were compared, as shown in Table
IVB. According to the RECIST guidelines, 14.3 and 20.0% of
patients in the LEN-TACE and LEN groups had an OR, respectively,
which was not significantly different (P=0.7282), and the disease
control rate was 42.9 and 33.3% in the LEN-TACE and LEN groups,
respectively, which was not a statistically significant difference
(P=0.6780). According to the mRECIST guidelines, the OR of patients
in the LEN-TACE and LEN groups was 57.1 and 20.0%, respectively,
which was not significantly different (P=0.0688); however, the
disease control rate was 100 and 30% in the LEN-TACE and LEN
groups, respectively, which was a statistically significant
difference (P=0.0011). Conversely, 43.3% of patients in the LEN
group had progressive disease, whilst there were no patients with
progressive disease in the LEN-TACE group, which was a
statistically significant difference (P=0.0378).
 | Table IVComparison of the best tumor
responses following LEN-TACE treatment in the 8-week RDI ≥60% (A)
and <60% (B) groups. |
Table IV
Comparison of the best tumor
responses following LEN-TACE treatment in the 8-week RDI ≥60% (A)
and <60% (B) groups.
| A, 8-week RDI ≥60%
group |
|---|
| | RECIST | | mRECIST | |
|---|
| Variable | LEN-TACE (n=6) | LEN (n=23) | P-value | LEN-TACE (n=6) | LEN (n=23) | P-value |
|---|
| OR, n (%) | 3 (50.0) | 7 (30.4) | 0.6328 | 5 (83.3) | 14 (60.9) | 0.6328 |
|
CR, n
(%) | 2 (33.3) | 2 (8.7) | 0.1798 | 1 (16.7) | 7 (30.4) | 0.6472 |
|
PR, n
(%) | 1 (16.7) | 5 (21.7) | 0.7847 | 4 (66.7) | 7 (30.4) | 0.1638 |
| SD, n (%) | 1 (16.7) | 7 (30.4) | 0.6472 | 0 (0.0) | 1 (4.4) | 0.6032 |
| Disease control
rate, n (%) | 4 (66.7) | 14 (60.9) | 0.7944 | 5 (83.3) | 15 (65.2) | 0.6328 |
| PD, n (%) | 2 (33.3) | 6 (26.1) | 0.7236 | 1 (16.7) | 5 (21.7) | 0.7847 |
| NE, n (%) | 0 (0.0) | 3 (13.0) | 0.3502 | 0 (0.0) | 3 (13.0) | 0.3502 |
| B, 8-week RDI
<60% group |
| | RECIST | | mRECIST | |
| Variable | LEN-TACE (n=7) | LEN, n=30 | P-value | LEN-TACE (n=7) | LEN (n=30) | P-value |
| OR, n (%) | 1 (14.3) | 6 (20.0) | 0.7282 | 4 (57.1) | 6 (20.0) | 0.0688 |
|
CR, n
(%) | 1 (14.3) | 1 (3.3) | 0.3468 | 1 (14.3) | 1 (3.3) | 0.3468 |
|
PR, n
(%) | 0 (0.0) | 5 (16.7) | 0.5599 | 3 (42.9) | 5 (16.7) | 0.1563 |
| SD, n (%) | 2 (28.6) | 4 (13.3) | 0.3155 | 3 (42.9) | 3 (10.0) | 0.0679 |
| Disease control
rate, n (%) | 3 (42.9) | 10 (33.3) | 0.6780 | 7 (100.0) | 9 (30.0) | 0.0011 |
| PD, n (%) | 4 (57.1) | 12 (40.0) | 0.4373 | 0 (0.0) | 13 (43.3) | 0.0378 |
| NE, n (%) | 0 (0.0) | 8 (26.7) | 0.3079 | 0 (0.0) | 8 (26.7) | 0.3079 |
In the high- and low-RDI groups, the patients were
divided into two subgroups stratified by treatment, which TACE were
added or not (LEN-TACE or LEN groups) and the median OS times were
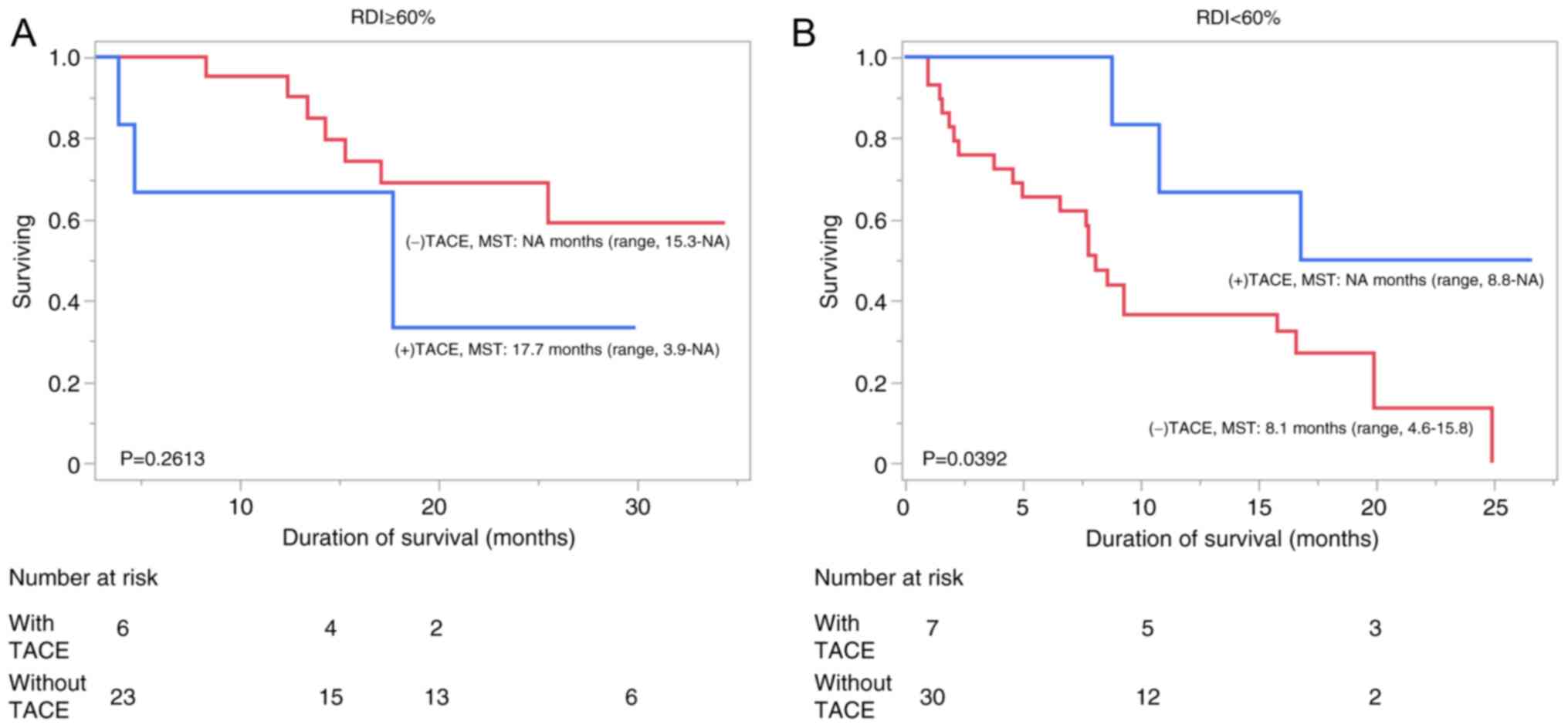
investigated. In the high-RDI group, the median OS time was 17.7
months (range, 3.9-NA months) and at least 15.3 months (range,
15.3-NA) in patients with and without TACE, respectively, which was
not a significant difference in OS time (P=0.2613; Fig. 2A). However, in the low-RDI group
the median OS time was at least 8.8 months (range, 8.8-NA months)
and 8.1 months (range, 4.6-15.8 months) in patients with and
without TACE, respectively, which was a significant difference in
OS time (P=0.0392; Fig. 2B).
Discussion
Randomized trials that combined lenvatinib treatment
with TACE have demonstrated the add-on effects of TACE (16,21).
The results of the present study demonstrated the benefits of
add-on TACE in the low-RDI subgroup, which had significantly
prolonged overall survival with add-on TACE compared to no
additional TACE, suggesting the efficacy of subsequent TACE in
patients who received an insufficient dose of lenvatinib. However,
the present study also demonstrated that there were no improvements
in the tumor response in the high-RDI group with or without the
addition of TACE, according to imaging results and both the RECIST
and mRECIST guidelines. This may explain why the OS time was not
significantly prolonged with the addition of TACE in this group.
Therefore, when considering TACE for patients with a high RDI, it
is important to ensure that TACE results in tumor response such as
a complete response (CR). Conversely, in the low-RDI group, even if
a CR was not achieved with TACE, early addition of TACE was shown
to improve the therapeutic effect, when compared with the continued
treatment of lenvatinib alone. As previously reported, patients who
were able to maintain a high RDI had improved ALBI and Child-Pugh
scores (11,28,31).
Therefore, in patients with a good hepatic reserve, it may be
effective to continue lenvatinib treatment alone for as long as
possible and to add TACE at the point where a CR can be expected.
Whereas, in patients with a poor hepatic reserve, it is difficult
to maintain a high RDI and therefore a different strategy is
required. Specifically, the addition of TACE early after the
initiation of lenvatinib followed by a continuation of lenvatinib
treatment may prolong the OS time, compared with continuing
lenvatinib alone, even if the dose of lenvatinib is reduced.
TACE is known to induce the expression of angiogenic
factors involved in tumor metastasis by creating an ischemic
environment in the tumor (17-19,32,33).
Lenvatinib has a potent anti-angiogenesis effect that inhibits
these angiogenic factors (34),
thus it may suppress tumor growth after treatment with TACE
(35,36). However, lenvatinib may promote
tumor vascular normalization, improve embolization agent delivery,
optimize the embolic effect, reduce the permeability of tumor
vessel and interstitial pressure, optimize intra-tumoral delivery
of systemic anticancer agents and increase response rates (37,38).
The inhibitory effect of lenvatinib on angiogenesis and tumor
growth after TACE may be achieved even at low doses, as was
observed in the low-RDI group of the present study, which had a
longer OS time after the addition of TACE. Therefore, when is the
optimal time to add TACE when lenvatinib is administered to
patients with impaired liver function? There are notable findings
from an animal study and two clinical studies that may help answer
this question. In an experiment where mice were subcutaneously
implanted with mouse HCC cells, tumor vasculature was examined
after only 4 days of treatment with lenvatinib or sorafenib, and
lenvatinib had significantly decreased the microvascular density
and normalized the tumor vasculature compared with sorafenib
(39). These outcomes indicated
that lenvatinib induced the normalization of HCC tumor vasculature
earlier and more effectively than sorafenib, thus improving the
intratumor microenvironment. In a phase Ib/II clinical study of
lenvatinib therapy combined with letrozole in patients with
advanced estrogen receptor+/HER2+ breast
cancer, a decrease in microvessel density and an increase in the
vascular normalization index, which are surrogate markers for the
VEGFR pathway, were observed in tumor tissues within 2 weeks of
lenvatinib administration (40).
These results indicated that the VEGFR pathway in tumor tissues was
inhibited within 2 weeks of lenvatinib administration, resulting in
reduced neovascularization and normalization of blood vessels
through the mobilization of pericytes around leaking tumor vessels.
This was demonstrated via the marked decrease in the expression of
CD31+ in the immunohistochemistry staining results of
the lenvatinib treatment group. A recently published case report
regarding the short-term administration of lenvatinib in 2 patients
with unresectable HCC, reported the administration of 12 mg/day for
7 days or 8 mg/day for 4 days. The results of high-resolution
digital subtraction angiography after the lenvatinib treatment
showed normalization of the tumor vasculature, as evidenced by the
tumor staining becoming more refined and newly formed tiny tumor
vessels being observed in both cases. Furthermore, perfusion 4D-CT
during hepatic arteriography showed reduced arterial blood flow to
the tumor after only 4 or 7 days of lenvatinib administration
(41). Based on these reports,
lenvatinib appears to induce a relatively early normalization of
the tumor vasculature, and the addition of TACE a few days to a
week after the start of lenvatinib treatment, followed by continued
lenvatinib administration, appears to be an effective treatment
strategy.
The present study does however have several
limitations. First, this retrospective cohort study was not
randomized. Second, the sample size may have been too small to
detect statistically significant differences in certain treatment
outcomes. Specifically, the small sample size may be why the median
OS time was not significantly different between the LEN-TACE and
LEN subgroups of the high-RDI group. A previous study reported that
the addition of TACE to lenvatinib notably improved the CR rate and
ORR compared with LEN alone in the high-RDI group (42). Likewise, the Asia-Pacific Primary
Liver Cancer Expert consensus recommended superselective
conventional TACE with curative intent as the first choice of
treatment in the eligible patients (43). In the subgroup analysis of the
high-RDI group in the present study, none of the patients who
underwent TACE received superselective cTACE strategy (Table SII). This may be another reason
why the results in the present study were not statistically
significant. However, this preliminary report on strategies for
limiting the number of patients receiving TACE according to RDI may
still be useful.
In summary, consistent with previous reports, in the
present study, patients who were able to maintain a high RDI of
lenvatinib had an improved prognosis, and these patients also had
improved ALBI and Child-Pugh scores. There was a significant
difference in the radiological response and OS time with TACE
combination therapy in the low-RDI group, while there was no
difference in the radiological response and OS time with TACE
combination therapy in the high-RDI group. From those results, we
suggest that early TACE must be considered as an effective therapy,
instead of continuing with lenvatinib treatment alone in patients
receiving an insufficient dose of lenvatinib. Therefore, to
maximize the add-on effect of TACE, an appropriate time of TACE
addition should be considered for each group of patients.
Supplementary Material
Post-lenvatinib therapy.
The details of TACE techniques.
AEs related to lenvatinib
therapy.
Changes in liver function before and
after lenvatinib treatment among the 8-week RDI ≥60% (A) and
<60% (B) group patients.
Changes in liver function before and
after LEN-TACE treatment among the 8-week RDI ≥60% (A) and <60%
(B) group patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
PP was responsible for the collection of data from
medical records, analysis, drafting the manuscript, review and
editing. TY and HK were responsible for conception and design,
patient enrollment, the collection of data from medical records,
analysis and drafting the manuscript. PP and TY confirmed the
authenticity of all the raw data. KA, KY, HM, and KM were
responsible for patient enrollment, the collection of data from
medical records, analysis, review and editing of the manuscript.
SS, MK, and MN were responsible for the review and editing of the
manuscript, supervision and project administration. SS was
responsible for conception and design. MK, and MN were responsible
for patient enrollment and the collection of data from medical
records. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
Declaration of Helsinki and was approved by the Ethics Committee of
Kansai Medical University Medical Center (Hirakata, Japan; approval
no. 2022307). The requirement for informed written consent was
waived due to the retrospective nature of this study. However, we
maintained the opt-out policy mentioned on our hospital's webpage,
whereby eligible participants were free to opt out of the
study.
Patient consent for publication
Informed consent for publication was obtained in the
form of an opt-out feature on our website, with the approval of the
Ethics Committee of Kansai Medical University Medical Center
(Hirakata, Japan).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Llovet JM, Kelley RK, Villanueva A, Singal
AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J and
Finn RS: Hepatocellular carcinoma. Nat Rev Dis Primers.
7(6)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Singal AG, Llovet JM, Yarchoan M, Mehta N,
Heimbach JK, Dawson LA, Jou JH, Kulik LM, Agopian VG, Marrero JA,
et al: AASLD Practice Guidance on prevention, diagnosis, and
treatment of hepatocellular carcinoma. Hepatology. 78:1922–1965.
2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Boss DS, Glen H, Beijnen JH, Keesen M,
Morrison R, Tait B, Copalu W, Mazur A, Wanders J, O'Brien JP, et
al: A phase I study of E7080, a multitargeted tyrosine kinase
inhibitor, in patients with advanced solid tumours. Br J Cancer.
106:1598–1604. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ikeda M, Okusaka T, Mitsunaga S, Ueno H,
Tamai T, Suzuki T, Hayato S, Kadowaki T, Okita K and Kumada H:
Safety and Pharmacokinetics of Lenvatinib in Patients with Advanced
Hepatocellular Carcinoma. Clin Cancer Res. 22:1385–1394.
2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ikeda K, Kudo M, Kawazoe S, Osaki Y, Ikeda
M, Okusaka T, Tamai T, Suzuki T, Hisai T, Hayato S, et al: Phase 2
study of lenvatinib in patients with advanced hepatocellular
carcinoma. J Gastroenterol. 52:512–519. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kuzuya T, Ishigami M, Ito T, Ishizu Y,
Honda T, Ishikawa T, Hirooka Y and Fujishiro M: Clinical
characteristics and outcomes of candidates for second-line therapy,
including regorafenib and ramucirumab, for advanced hepatocellular
carcinoma after sorafenib treatment. Hepatol Res. 49:1054–1065.
2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Havrilesky LJ, Reiner M, Morrow PK, Watson
H and Crawford J: A review of relative dose intensity and survival
in patients with metastatic solid tumors. Crit Rev Oncol Hematol.
93:203–210. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Takahashi A, Moriguchi M, Seko Y, Ishikawa
H, Yo T, Kimura H, Fujii H, Shima T, Mitsumoto Y, Ishiba H, et al:
Impact of relative dose intensity of early-phase lenvatinib
treatment on therapeutic response in hepatocellular carcinoma.
Anticancer Res. 39:5149–5156. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
European Association for the Study of the
Liver. Electronic address: simpleeasloffice@easloffice.eu;
European Association for the Study of the Liver. EASL Clinical
Practice Guidelines: Management of hepatocellular carcinoma. J
Hepatol. 69:182–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kudo M, Kawamura Y, Hasegawa K, Tateishi
R, Kariyama K, Shiina S, Toyoda H, Imai Y, Hiraoka A, Ikeda M, et
al: Management of Hepatocellular Carcinoma in Japan: JSH Consensus
Statements and Recommendations 2021 Update. Liver Cancer.
10:181–223. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Llovet JM, Real MI, Montaña X, Planas R,
Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al:
Arterial embolisation or chemoembolisation versus symptomatic
treatment in patients with unresectable hepatocellular carcinoma: A
randomised controlled trial. Lancet. 359:1734–1739. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hiraoka A, Kumada T, Kudo M, Hirooka M,
Koizumi Y, Hiasa Y, Tajiri K, Toyoda H, Tada T, Ochi H, et al:
Hepatic function during repeated TACE procedures and prognosis
after introducing sorafenib in patients with unresectable
hepatocellular carcinoma: Multicenter Analysis. Dig Dis.
35:602–610. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kudo M, Ueshima K, Ikeda M, Torimura T,
Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, et al:
Randomised, multicentre prospective trial of transarterial
chemoembolisation (TACE) plus sorafenib as compared with TACE alone
in patients with hepatocellular carcinoma: TACTICS trial. Gut.
69:1492–1501. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li X, Feng GS, Zheng CS, Zhuo CK and Liu
X: Expression of plasma vascular endothelial growth factor in
patients with hepatocellular carcinoma and effect of transcatheter
arterial chemoembolization therapy on plasma vascular endothelial
growth factor level. World J Gastroenterol. 10:2878–2882.
2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Carmeliet P and Jain RK: Angiogenesis in
cancer and other diseases. Nature. 407:249–257. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Wang B, Xu H, Gao ZQ, Ning HF, Sun YQ and
Cao GW: Increased expression of vascular endothelial growth factor
in hepatocellular carcinoma after transcatheter arterial
chemoembolization. Acta Radiol. 49:523–529. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rizzo A, Ricci AD and Brandi G:
Trans-Arterial chemoembolization plus systemic treatments for
hepatocellular carcinoma: An Update. J Pers Med.
12(1788)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Peng Z, Fan W, Zhu B, Wang G, Sun J, Xiao
C, Huang F, Tang R, Cheng Y, Huang Z, et al: Lenvatinib combined
with transarterial chemoembolization as first-line treatment for
advanced hepatocellular carcinoma: A phase III, Randomized clinical
trial (LAUNCH). J Clin Oncol. 41:117–127. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Johnson PJ, Berhane S, Kagebayashi C,
Satomura S, Teng M, Reeves HL, O'Beirne J, Fox R, Skowronska A,
Palmer D, et al: Assessment of liver function in patients with
hepatocellular carcinoma: A new evidence-based approach-the ALBI
grade. J Clin Oncol. 33:550–558. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hiraoka A, Michitaka K, Kumada T, Izumi N,
Kadoya M, Kokudo N, Kubo S, Matsuyama Y, Nakashima O, Sakamoto M,
et al: Validation and potential of albumin-bilirubin grade and
prognostication in a nationwide survey of 46,681 hepatocellular
carcinoma patients in Japan: The need for a more detailed
evaluation of hepatic function. Liver Cancer. 6:325–336.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hiraoka A, Kumada T, Tsuji K, Takaguchi K,
Itobayashi E, Kariyama K, Ochi H, Tajiri K, Hirooka M, Shimada N,
et al: Validation of Modified ALBI grade for more detailed
assessment of hepatic function in hepatocellular carcinoma
patients: A multicenter analysis. Liver Cancer. 8:121–129.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Okusaka T, Ikeda K, Kudo M, Finn R, Qin S,
Han KH, Cheng AL, Piscaglia F, Kobayashi M, Sung M, et al: Safety
and efficacy of lenvatinib by starting dose based on body weight in
patients with unresectable hepatocellular carcinoma in REFLECT. J
Gastroenterol. 56:570–580. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gordan JD, Kennedy EB, Abou-Alfa GK, Beg
MS, Brower ST, Gade TP, Goff L, Gupta S, Guy J, Harris WP, et al:
Systemic therapy for advanced hepatocellular carcinoma: ASCO
Guideline. J Clin Oncol. 38:4317–4345. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
U.S. Department of Health and Human
Services: Common Terminology Criteria for Adverse Events (CTCAE).
Version 5.0. National Cancer Institute, Bethesda, MD, 2017.
|
|
28
|
Kirino S, Tsuchiya K, Kurosaki M, Kaneko
S, Inada K, Yamashita K, Osawa L, Hayakawa Y, Sekiguchi S, Okada M,
et al: Relative dose intensity over the first four weeks of
lenvatinib therapy is a factor of favorable response and overall
survival in patients with unresectable hepatocellular carcinoma.
PLoS One. 15(e0231828)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lencioni R and Llovet JM: Modified RECIST
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Schwartz LH, Seymour L, Litiere S, Ford R,
Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, et
al: RECIST 1.1 - Standardisation and disease-specific adaptations:
Perspectives from the RECIST Working Group. Eur J Cancer.
62:138–145. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sasaki R, Fukushima M, Haraguchi M, Miuma
S, Miyaaki H, Hidaka M, Eguchi S, Matsuo S, Tajima K, Matsuzaki T,
et al: Response to lenvatinib is associated with optimal
relativedose intensity in hepatocellular carcinoma: Experience in
clinical settings. Cancers (Basel). 11(1769)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Shim JH, Park JW, Kim JH, An M, Kong SY,
Nam BH, Choi JI, Kim HB, Lee WJ and Kim CM: Association between
increment of serum VEGF level and prognosis after transcatheter
arterial chemoembolization in hepatocellular carcinoma patients.
Cancer Sci. 99:2037–2044. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Yamamoto Y, Matsui J, Matsushima T,
Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A,
Hoshi SS, et al: Lenvatinib, an angiogenesis inhibitor targeting
VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft
models associated with microvessel density and pericyte coverage.
Vasc Cell. 6(18)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jiang H, Meng Q, Tan H, Pan S, Sun B, Xu R
and Sun X: Antiangiogenic therapy enhances the efficacy of
transcatheter arterial embolization for hepatocellular carcinomas.
Int J Cancer. 121:416–424. 2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kawamura Y, Kobayashi M, Shindoh J,
Kobayashi Y, Okubo S, Tominaga L, Kajiwara A, Kasuya K, Iritani S,
Fujiyama S, et al: Lenvatinib-Transarterial chemoembolization
sequential therapy as an effective treatment at progression during
lenvatinib therapy for advanced hepatocellular carcinoma. Liver
Cancer. 9:756–770. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fu Z, Li X, Zhong J, Chen X, Cao K, Ding
N, Liu L, Zhang X, Zhai J and Qu Z: Lenvatinib in combination with
transarterial chemoembolization for treatment of unresectable
hepatocellular carcinoma (uHCC): A retrospective controlled study.
Hepatol Int. 15:663–675. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Jain RK: Normalization of tumor
vasculature: An emerging concept in antiangiogenic therapy.
Science. 307:58–62. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kano MR, Komuta Y, Iwata C, Oka M, Shirai
YT, Morishita Y, Ouchi Y, Kataoka K and Miyazono K: Comparison of
the effects of the kinase inhibitors imatinib, sorafenib, and
transforming growth factor-beta receptor inhibitor on extravasation
of nanoparticles from neovasculature. Cancer Sci. 100:173–180.
2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Une N, Takano-Kasuya M, Kitamura N, Ohta
M, Inose T, Kato C, Nishimura R, Tada H, Miyagi S, Ishida T, et al:
The anti-angiogenic agent lenvatinib induces tumor vessel
normalization and enhances radiosensitivity in hepatocellular
tumors. Med Oncol. 38(60)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lim JSJ, Wong ALA, Ow SGW, Ngoi NYL, Chan
GHJ, Ang YLE, Chong WQ, Lim SE, Lim YW, Lee M, et al: Phase Ib/II
dose expansion study of lenvatinib combined with letrozole in
postmenopausal women with hormone receptor-positive breast cancer.
Clin Cancer Res. 28:2248–2256. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Tachiiri T, Nishiofuku H, Maeda S, Sato T,
Toyoda S, Matsumoto T, Chanoki Y, Minamiguchi K, Taiji R, Kunichika
H, et al: Vascular normalization caused by short-term lenvatinib
could enhance transarterial chemoembolization in hepatocellular
carcinoma. Curr Oncol. 30:4779–4786. 2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kudo M, Ueshima K, Saeki I, Ishikawa T,
Inaba Y, Morimoto N, Aikata H, Tanabe N, Wada Y, Kondo Y, et al: A
phase 2, prospective, multicenter, single-arm trial of
transarterial chemoembolization therapy in combination strategy
with lenvatinib in patients with unresectable intermediate-stage
hepatocellular carcinoma: TACTICS-L Trial. Liver Cancer. 13:99–112.
2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kudo M, Han KH, Ye SL, Zhou J, Huang YH,
Lin SM, Wang CK, Ikeda M, Chan SL, Choo SP, et al: A changing
paradigm for the treatment of intermediate-stage hepatocellular
carcinoma: Asia-Pacific primary liver cancer expert consensus
statements. Liver Cancer. 9:245–260. 2020.PubMed/NCBI View Article : Google Scholar
|