1. Introduction
Leukemia is defined by the unregulated proliferation
and accumulation of immature precursors of white blood cells in
bone marrow and peripheral blood. There are two types of leukemia:
Acute and chronic, with acute lymphoblastic leukemia (ALL) being
the most common category of acute leukemias. ALL requires immediate
treatment due to the rapid accumulation of lymphoblasts. Chronic
leukemia is characterized by excessive production of relatively
mature but abnormal white blood cells, and includes chronic
lymphocytic and myelogenous leukemia. Treatment in lymphocytic
leukemia treatment is administered only to symptomatic patients,
while in chronic myeloid leukemia (CML), therapy is offered in all
cases (1).
The purpose of the present study was to analyze and
describe how to manage the most common hematological malignancies,
especially leukemias and lymphomas, during pregnancy. Although
rare, occurring in ~1 in 75,000 to 100,000 pregnancies, the
concurrent management of leukemia and pregnancy requires a careful
balance to optimize outcomes for both the mother and the fetus. The
physiological changes in pregnancy can complicate the diagnosis of
leukemia, as symptoms such as fatigue, anemia and thrombocytopenia
overlap with normal gestational changes. The primary objective in
treating leukemia during pregnancy is to achieve the best possible
outcome for the mother while minimizing risks to the developing
fetus. Chemotherapy is the cornerstone of leukemia treatment and
can be administered during pregnancy, but the timing and choice of
chemotherapeutic agents must be carefully considered. During the
first trimester, the fetus is particularly vulnerable to
teratogenic effects, thus posing a significant risk when
administering chemotherapeutic agents. In the second and third
trimesters, the risk of congenital malformations decreases, yet
concerns about preterm labor, intrauterine growth restriction, and
long-term developmental issues remain. The likelihood of achieving
a future pregnancy is additionally described in women with
hematological malignancies if they are receiving medication. The
current study also analyzed the general characteristics of
leukemias and lymphomas related to the epidemiology,
pathophysiology, diagnosis, clinical appearance and treatment of
these diseases. Acute myeloid leukemia (AML) constitutes 90% of all
acute leukemias in adults. Intensive chemotherapy regimens and bone
marrow transplantation can succeed in high cure rates, but they are
rarely applicable, while recently targeted therapies were able to
improve survival (1,2).
2. Methods
For the present study, articles published from 2013
to 2024 were reviewed. Relevant articles were identified through
searches in the following databases: PubMed (https://pubmed.ncbi.nlm.nih.gov/), ScienceDirect
(https://www.sciencedirect.com/),
Cochrane Library (https://www.cochranelibrary.com/), Web of Science
(https://www.webofscience.com/wos/),
Embase (https://www.embase.com/) and Google
Scholar (https://scholar.google.com/). To
ensure comprehensive results, terms in Medical Subject Headings
(https://www.nlm.nih.gov/mesh/meshhome.html) such as
‘pregnancy’, ‘gestational’, ‘pregnant women’, ‘leukemia’,
‘lymphoma’, ‘metastasis’, ‘bone marrow’ as well as ‘chemotherapy’
were searched in the international electronic database search.
Boolean operators (AND & OR) were used to combine search terms
and obtain comprehensive results. Additionally, references cited in
the retrieved articles were examined to identify further relevant
studies.
Inclusion and exclusion criteria
The inclusion criteria for the present study
encompass prospective and retrospective studies aimed at
investigating the association between maternal anemia during
pregnancy and premature birth. The exclusion criteria were as
follows: i) Non-related studies and duplicates; ii) studies
involving preexisting leukemia or lymphoma; and iii) studies with
unavailable full text.
Study selection
Initially, two researchers conducted the search
process. Non-relevant articles were excluded by reviewing the title
and abstract at each stage of the search. Full-text articles were
then reviewed, and all studies pertaining to leukemia, lymphomas,
as well as bone marrow metastasis during pregnancy were selected.
The authors focused on review articles and meta-analyses, as these
publications are crucial in literature searches. Their ability to
synthesise extensive research, conserve time, deliver critical
evaluations, and present comprehensive insights into a subject
significantly aids in making informed and evidence-based decisions.
In addition, in instances of rare types of leukemia, such as hairy
cell leukemia, case reports were evaluated (Table I).
 | Table IStudies retrieved for conducting the
review. |
Table I
Studies retrieved for conducting the
review.
| First author,
year | Type of study | Summary | (Refs.) |
|---|
| Voulgaris et
al, 2011 | Review | Optimal patient
care necessitates collaboration among multidisciplinary teams. | (5) |
| Thomas, 2015 | Review | Although
chemotherapy carries certain risks during the first trimester, it
is acknowledged that it can be safely administered during the
second and third trimesters. | (6) |
| Zhu et al,
2021 | Review | In cases of acute
leukemia diagnosed during the first trimester or late stage of
pregnancy (>30 weeks), opting for elective termination or
induced delivery before initiating chemotherapy may lead to
improved maternal and fetal outcomes. | (7) |
| Ali et al,
2015 | Practice
guideline | For women diagnosed
with AML during pregnancy, prompt treatment is crucial. Diagnosis
in the first trimester often leads to poor pregnancy outcomes, with
significant risks of spontaneous pregnancy loss. The decision of
elective termination should be carefully discussed with the
patient, weighing the reasons for and against. If AML is diagnosed
beyond 32 weeks of gestation, delivering the fetus before starting
chemotherapy may be considered. Between 24 and 32 weeks, the
decision regarding fetal exposure to chemotherapy vs. risks of
premature delivery must be balanced carefully. | (8) |
| Sanz et al,
2019 | Review | In patients
diagnosed APL, those with ATRA-sensitive variants should receive
treatment combining ATRA with anthracycline-based chemotherapy.
However, for patients with ATRA-resistant variants, the addition of
ATRA is less effective, and management should follow approaches
similar to those used for AML. | (13) |
| Santolaria et
al, 2020 | Systematic
review | In APL, the
likelihood of achieving CR and subsequent cure is generally very
high and comparable to non-pregnant patients. However, fetal
outcomes are closely tied to gestational age, with a notable
increase in abortion rates during early pregnancy stages. | (14) |
| Ticku et al,
2013 | Case report and
review | ALL may be treated
with HyperCVAD during the third trimester of pregnancy. | (19) |
| Vlijm-Kievit et
al, 2018 | Case reports | In acute
lymphoblastic leukemia, the placenta typically acts as a barrier
preventing the transfer of maternal leukemia cells to the fetus. In
rare instances where this barrier fails, allowing leukemia cells to
pass to the fetus, the infant's immune system may mount a response
to clear the leukemia cells. | (23) |
| Abruzzese et
al, 2020 | Review | If pregnancy is
suspected or confirmed in a female CML patient, it is generally
recommended to interrupt TKI treatment. For patients with a high
tumor burden (≤MR2), management should parallel that of newly
diagnosed or recent CML cases, regardless of whether they have been
on treatment for less than or more than 3 years. This approach
typically involves a combination strategy using IFN and TKI therapy
timed appropriately. | (31) |
| Soverini et
al, 2016 | Review | In the context of
CML, in subsequent MR reports, it is crucial for the pathologist or
molecular biologist to clearly state whether the sample is suitable
for MR evaluation and to specify whether the MR level indicates an
optimal response, a warning, or a failure. If the sample is
assessable, they should specify the exact MR level achieved. In
instances where there are discrepancies in MR results compared to
previous assessments, fluctuations in BCR-ABL1 transcript levels
without loss of MMR, or borderline findings, it is recommended that
the pathologist or molecular biologist advise on the necessity for
resampling and verification of results before making any clinical
decisions. | (32) |
| Luttwak et
al, 2021 | Review | Aggressive
lymphoproliferative diseases in pregnant patients have the
potential for cure. In recent years, new treatments have been
introduced that have not been studied in pregnant or breastfeeding
women. Rituximab, an anti-CD-20 monoclonal antibody, can be safely
given during the second and third trimesters of pregnancy. When
considering other treatments where the effects on the fetus are
unknown or could be harmful, a personalized approach should be
taken. This involves shared decision-making between the patient and
her healthcare team. | (38) |
| Daver et al,
2013 | Case report | A positive result
was achieved in a pregnant patient with HCL who underwent treatment
with a monoclonal antibody (rituximab) followed sequentially by a
purine analogue (cladribine). | (39) |
| Gurevich-Shapiro
and Avivi, 2019 | Review | If HL is diagnosed
in the first trimester and immediate treatment is not urgent,
therapy should be deferred until the second trimester. Bridging
therapy with vinblastine or corticosteroids can be considered
during the first trimester if necessary. ABVD is the preferred
chemotherapy regimen during pregnancy. It can be safely
administered during the second and third trimesters, once the
placenta is well-developed and fetal organo-genesis is mostly
complete. Radiation therapy is generally avoided throughout
pregnancy due to the potential risks to the fetus. In select cases
of localized supradiaphragmatic disease where radiation therapy
might be deemed necessary, it should be performed with careful
consideration and appropriate shielding techniques to minimize
fetal exposure. Treatment for newly diagnosed NHL during pregnancy
varies depending on the subtype and aggressiveness of the lymphoma.
Indolent lymphoma: For indolent NHL, a strategy of watchful waiting
until delivery can often be employed. In cases where treatment is
necessary during pregnancy, a short course of corticosteroids can
be used as bridging therapy to manage symptoms. Aggressive
lymphoma: R-CHOP is considered the standard regimen for aggressive
NHL. It can be safely administered beyond the first trimester of
pregnancy. CNS prophylaxis with high-dose methotrexate is
contraindicated before week 20 of pregnancy and is generally not
recommended during pregnancy due to potential fetal harm. Highly
aggressive lymphoma (e.g. Burkitt's lymphoma): Burkitt's lymphoma
requires immediate treatment with intensive chemotherapy regimens
that often include antimetabolites. In cases of highly aggressive
NHL, prompt termination of pregnancy may be recommended followed by
initiation of intensive chemotherapy. The decision to terminate
pregnancy and start chemotherapy is based on the urgency of
treatment and the potential risks to both the mother and the
fetus. | (47) |
| Ciccarone et
al, 2023 | Observational
study | Based on the
results obtained from hormonal measurements and follicle
echography, it appears that the adverse impact of ABVD on fertility
is temporary. However, by contrast, more intensive therapies may
have the potential to cause more significant and enduring harm to
fertility. | (49) |
| Câmara and Brandão,
2023 | Systematic review
and meta-analysis | Recent patents have
been published concerning the use of monoclonal antibodies in the
treatment of NHL. These antibodies are associated with adverse
effects such as neutropenia (grade 3-4) and thrombocytopenia. | (53) |
| Pirosa and
Peccatori, 2023 | Review | Hodgkin lymphoma
and pregnancy: ABVD regimen is considered safe if administered
after the 13th week of pregnancy. NHL and pregnancy: Indolent NHLs,
a watchful waiting approach is often considered. Indolent NHLs grow
slowly and may not require immediate treatment. Aggressive NHLs,
treatment approach depends on the timing of diagnosis relative to
pregnancy. If diagnosed in the first trimester, termination of
pregnancy might be considered due to potential risks of aggressive
treatment on fetal development. If diagnosed after the 13th week, a
standard R-CHOP regimen can be considered safe. R-CHOP is a common
regimen for aggressive NHL. New anti-lymphoma drugs and pregnancy:
Data on potential fetotoxicity (ability to cause harm to the fetus)
of newer anti-lymphoma drugs are limited. This suggests caution is
needed when considering these drugs during pregnancy, and decisions
would likely be made on a case-by-case basis weighing potential
risks to the fetus against benefits to the mother. | (55) |
| Evens et al,
2013 | Review | In lymphomas,
standard combination chemotherapy, which typically excludes
antimetabolite drugs, can be administered safely after the first
trimester of pregnancy. This timing (around 13 weeks gestation and
beyond) is chosen to minimize potential risks to fetal development
during the critical early stages. | (57) |
| Krashin and
Lishner, 2016 | Review | Several small-scale
studies indicate that the outcomes for infants born to mothers with
leukemia may not show significant differences compared to infants
born to mothers without health complications. The existing
literature on the long-term effects of chemotherapy for leukemia is
constrained and predominantly relies on retrospective data. A
comprehensive follow-up study, spanning an average of 18.7 years,
involving 84 children born to mothers with hematological
malignancies, including 29 with acute leukemia, documented normal
physical, neurological, and psychological development among these
children. The incidence of malignancy in this cohort was comparable
to that observed in the general population, and notably, 12 of
these children later became parents themselves. | (77) |
3. Acute myeloid leukemia
Pathophysiology of AML
The biology of AML is diverse, and is primarily
characterized by the abnormal proliferation and differentiation of
myeloid precursors within the bone marrow. AML is initiated by a
clonal process originating from transformed hematopoietic stem
cells, leading to clonal proliferation. Notably, this clonal
proliferation can occur in individuals without any apparent health
issues prior to the onset of leukemia (3).
Several factors distinguish age-related clonal
hematopoiesis from pre-AML, including the number of mutations per
sample, higher variant allele frequency, and mutations in specific
genes such as i) DNA methyltransferase 3 alpha (DNMT3A), which
encodes the enzyme DNA (cytosine-5)-methyltransferase 3 alpha,
which is involved in establishing and maintaining DNA methylation
patterns, playing a crucial role in gene expression regulation and
cellular differentiation, ii) Tet methylcytosine dioxygenase 2
(TET2), which encodes the TET2 protein, which is involved in the
process of DNA demethylation through the oxidation of
5-methylcytosine to 5-hydroxymethylcytosine, an important step in
epigenetic regulation, iii) serine and arginine rich splicing
factor 2 (SRSF2) which encodes a member of the serine/arginine (SR)
protein family, which is essential for pre-mRNA splicing (3). The SRSF2 protein plays a critical
role in the regulation of alternative splicing and the processing
of pre-mRNA, and iv) additional sex combs like 1, transcriptional
regulator (ASXL1), which is the gene that encodes the ASXL1
protein, is involved in chromatin remodeling and transcriptional
regulation. This protein is part of the polycomb repressive
complex, which plays a role in maintaining the transcriptional
repression of genes involved in development. These distinctions are
crucial for understanding the transition from clonal hematopoiesis
to AML and guiding clinical management strategies (3).
AML diagnosis traditionally involves
morphology and cytogenetics, identifying translocations and
recurrent cytogenetic abnormalities. Next generation sequencing
panels and fusion partner agnostic sequencing are increasingly used
for 4131428709AML diagnosis, providing information on mutations
with prognostic and therapeutic implications. Somatic mutations
play a crucial role in AML classification, diagnosis, prognosis and
treatment decisions. Genes such as nucleophosmin 1 (NPM1), Fms
related receptor tyrosine kinase 3 (FLT3) and CCAAT enhancer
binding protein alpha (CEBPA) impact diagnostic subtyping and
provide prognostic insights, especially in patients with a normal
karyotype. The 2022 European LeukemiaNet classification includes
mutations in 10 genes for adverse group assignment, and clinical
genomic sequencing aids in evaluating patients at risk for myeloid
malignancy (3). Assay sensitivity
and mutation type determine suitability for measuring measurable
residual disease (MRD), an area of active investigation (3).
Risk factors of AML
Inherited conditions such as Fanconi anemia and
Diamond-Blackfan anemia, along with genetic mutations in the
germline, namely runt-related transcription factor 1 (RUNX1), CEBPA
and GATA binding protein 2 (GATA2), increase the risk of AML.
Alterations in the RUNX1 gene are linked to both benign and
malignant blood disorders, with a notable impact on megakaryocyte
and myeloid lineages. Smoking, chemicals, prior chemotherapy,
radiotherapy and another myelodysplastic syndrome are associated
with AML (3,4).
General therapeutic approach for
AML
Chemotherapy (cytarabine and anthracycline) and bone
marrow transplantation are offered for young patients, while
targeted therapies are the main therapies used for the elderly
(3).
AML in pregnancy
The incidence of leukemia in pregnancy ranges from 1
in 75,000 to 1 in 100,000 pregnancies, with AML being the most
common subtype. Acute leukemias appear more often during the second
and third trimesters of pregnancy, while only 23% of them are
diagnosed in the first trimester. Their treatment should begin
immediately after diagnosis, and termination of pregnancy is mainly
recommended in the first trimester. Although the risk of
teratogenicity from chemotherapy is negligible in the second and
third trimesters, the severity of the disease and treatment
complications make the termination of pregnancy necessary (5).
Clinical presentation of AML in
pregnancy
Pregnancy often results in a delay in diagnosis.
Because the early symptoms are non-specific, the diagnosis is
mainly made during the second and third trimesters. Unspecific
symptoms such as fatigue, weakness, dyspnea and pallor are usually
physiological changes occurring during pregnancy. The physiological
changes associated with pregnancy, such as anemia of pregnancy,
leukocytosis or gestational thrombocytopenia, are typically present
in AML. Furthermore, recurrent infections and bleeding can reflect
bone marrow failure (6).
Diagnosis of AML in pregnancy
The diagnosis of AML is often complicated during
pregnancy. Laboratory findings such as neutropenia, anemia,
thrombocytopenia, thrombosis or coagulation disorders could appear.
The presence of blasts in peripheral blood results in a possible
diagnosis and is confirmed with bone marrow biopsy, while
immunophenotype of blasts and cytogenetics with genetic analysis
(FLT3, IRD, TKD and NPM1 mutations) are imperative for risk
stratification (5).
Management of AML in the first
trimester of pregnancy
Complications of AML include limitation of
endometrial development, abortion and perinatal death. Due to these
consequences, pregnancy termination is suggested when diagnosis of
AML is established in the first trimester (6).
Management of AML in the second and
third trimesters of pregnancy
Chemotherapy and pregnancy maintenance are
recommended between the 13 and 24th weeks, while between the 24 and
34th weeks the danger of prematurity overweighs that of
chemotherapy. When AML appears after the 32nd week, labor induction
is suggested, and chemotherapy is administered after labor. The
preferred chemotherapeutic schemes in the second and third
trimesters are anthracyclines. In pregnancy, doxorubicin is mainly
used, whereas idarubicin, as a lipophilic molecule, crosses the
placenta and is therefore not recommended. It has been shown that
anthracyclines are involved in fetal cardiotoxicity (7). Thus, regular fetal ultrasound during
treatment is suggested. Experience of cytarabine administration
during pregnancy is limited. However, cytarabine is involved in
fetal limb dysplasia, especially in the first trimester, and it is
responsible for pancytopenia, endometrial death, limitation of
endometrial development and neonatal death (8).
4. Acute promyelocytic leukemia
Pathophysiology of acute promyelocytic
leukemia (APL)
APL is characterized by the accumulation of immature
granulocytes and is caused by a chromosomal translocation of the
first intron of α-receptor of retinoic acid (RARα) on chromosome 17
and promyelocytic leukemia (PML) gene on chromosome 15. PML and
RARα fusion leads to the expression of a dysfunctional hybrid
protein, which inhibits the differentiation of promyelocytes into
mature granulocytes (8,9).
Risk factors of APL
Advanced age, chemical solvents, prior
myelodysplastic syndromes and germline mutations contribute to the
development of APL (9).
General therapeutic approach for
APL
Targeted therapy with all-trans retinoic acid
(ATRA), the prodromal form of vitamin A, in combination with
chemotherapy is the classic regimen termed ATRA and idarubicin
(AIDA), offering a cure in >90% of patients. Since 2013, a drug
combination of arsenic trioxide with ATRA can offer higher cure
rates and fewer relapses compared to AIDA, and consists of a
chemo-free therapeutic approach to the disease (10).
APL in pregnancy
APL is a rare comorbidity during pregnancy, with a
low prevalence of 1 in 75,000 to 1 in 100,000 pregnancies, the
majority of which are cases of AML (10,11).
Clinical presentation of APL in
pregnancy
Recurrent infections, fatigue and hemorrhagic risk
are associated with APL, while petechiae and coagulopathy are
common clinical manifestations (11).
Diagnosis of APL
Diagnosis is based on bone marrow biopsy and
detection of the characteristic PML/RARα chimeric oncoprotein.
Morphology is usually helpful with the presence of promyelocytes,
which are characterized by the presence of Auer rods in peripheral
blood samples (12).
Management of APL in the first
trimester of pregnancy
Treatment in pregnancy is administered following the
cooperation of specialized gynecologists-obstetricians,
hematologists and neonatologists. APL causes coagulation disorders
that can complicate gestation. For this reason, regular check-up is
mandatory. As for the therapeutic approach, it depends on the
trimester in which the disease is diagnosed. In the first
trimester, termination of pregnancy and prompt induction therapy
are advised. The standard induction chemotherapy is the ‘7+3’
regimen, which includes continuous infusion of high-dose cytarabine
for 7 days in combination with anthracycline infusion during the
first 3 days. These are teratogenic if administered during
organogenesis. In the first trimester, chemotherapy is recommended
in women who refuse terminating the pregnancy. ATRA should be
avoided in the first trimester because it increases the risk of
abortion, and enhances the possibilities of cardiovascular fetal
malformation and prematurity. Furthermore, it can lead to
microphthalmia, microcephaly, hearing loss and mental disturbances
in the newborn. The exclusive administration of chemotherapy
results in a lower response to therapy, smaller free-of-disease
intervals and a higher number of relapses. Daunorubicin is the
anthracycline of choice in the first trimester, since it may induce
less fetal toxicity, and ATRA can be added to treatment during the
second and third trimesters. Arsenic trioxide is teratogenic and
should be avoided in all three trimesters of pregnancy (13,14).
Management of APL in the second and
third trimesters of pregnancy
During the second and third trimesters, ATRA and
chemotherapy can exclusively be administered after childbirth.
Another choice is the coadministration of ATRA with chemotherapy
during pregnancy. This coadministration offers increased cure rates
but it is associated with increased risk of abortion, prematurity,
neonatal neutropenia and sepsis. Monotherapy with ATRA has the same
percentages of disease recession but increases the risk of relapse
and causes leukocytosis. For this reason, in cases of monotherapy
with ATRA, regular check-ups with PCR and check-ups for disease
relapse are necessary. The preferred method of delivery is vaginal,
due to the lower risk of hemorrhage (15).
5. Acute lymphoblastic leukemia
ALL is an aggressive hematological tumor of lymphoid
origin that causes immature lymphoblasts to proliferate
uncontrollably in the bone marrow, blood and tissues. B-cell ALL is
more common and is characterized by the presence of the
Philadelphia chromosome and the B-cell receptor (BCR)/ABL (p190)
oncoprotein, which can be targeted with tyrosine kinase inhibitors
(TKIs). If it remains untreated, it can lead to mortality rapidly
(16).
Pathophysiology of ALL
ALL is considered to occur after DNA damage. As a
result, abnormal lymphocytes and their progenitors undergo
uncontrolled proliferation and spread throughout the body,
resulting in the replacement of the bone marrow and other lymphoid
organs (17). Immunophenotyping
using multi-channel flow cytometry (MFC) has become the standard
procedure for diagnosing and subclassifying ALL. Additionally, it
serves as a valuable tool for detecting and monitoring MRD.
According to the European Group for the Immunological
Characterization of Leukemias consensus, blast cells are considered
positive for a given monoclonal antibody if they exhibit a
threshold of 20% expression, except for markers such as MPO, CD3,
CD79a and TdT, which are deemed positive at 10% expression. The
EuroFlow consortium has developed novel MFC strategies to ensure
the accuracy and reproducibility of diagnostic tests. In total,
75-80% of adult ALL cases are of B-cell lineage, while 20-25%
belong to the T-cell lineage (18).
Risk factors of ALL
Genetic syndromes, Down syndrome, severe combined
immunodeficiency, genetic mutations in the GATA3 gene, prior
chemotherapy and radiotherapy are related to ALL (19).
General therapeutic approach for
ALL
Intensive chemotherapy regimens [hyperfractionated
cyclophosphamide, vincristine, doxorubicin (adriamycin) and
dexamethasone or gemtuzumab ozogamicin, mitoxantrone, cytarabine
(cytosine arabinoside), liposomal daunorubicin (GMAL)] and
intrathecal central nervous system (CNS) prophylaxis is the
mainstay of treatment. Prednisone, vincristine, anthracyclines,
methotrexate and aracytin are commonly used. Other drugs such as
L-asparaginase, cyclophosphamide and rituximab in CD20+
ALL can be used as well (19).
Relapse of the disease is usual, but bone marrow transplantation
after bridging therapy with bispecific antibodies can be employed
as salvage treatment (20). The
technology of chimeric antigen receptor (CAR) T cells with chimeric
receptors against CD19 provides another opportunity for remission
in patients with B-ALL.
ALL in pregnancy
Studies on the coexistence of ALL in pregnancy are
limited because it affects 1.3 in 100,000 gestations (20,21).
Clinical presentation of ALL in
pregnancy
Clinical presentation includes fatigue, lethargy,
headache, vomiting, hemorrhage, bone pain, joint pain, weight and
appetite loss, as well as liver, spleen and lymph node enlargement,
in addition to edema of the lower limbs and abdomen, metabolic
disorders such as hyperuricemia, CNS paralysis due to malfunction,
confusion, fever and susceptibility to infections (21).
Diagnosis of ALL in pregnancy
The presence of lymphoblasts in the complete blood
count and peripheral blood smear are indicators of disease.
Diagnosis is confirmed with a bone marrow biopsy. Cytogenetics of
bone marrow samples direct the prognosis and treatment, especially
in patients with the Philadelphia chromosome (22).
Management of ALL until the 20th
gestational week
Pregnancy does not change the course of the disease,
but a delay in treatment can adversely affect the outcome. Thus,
when ALL is diagnosed in pregnancy, immediate treatment is
recommended. The majority of protocols advocate the use of
methotrexate, even though methotrexate has been reported to cause
fetal aminopterin syndrome (cranial dysostosis and micrognathia).
If ALL appears before the 20th gestational week, then pregnancy
termination is suggested (23).
Management of ALL after the 20th
gestational week
Prednisone is recommended for 1-2 weeks before the
initiation of chemotherapy. Preferred chemotherapeutic agents
include cytarabine, cyclophosphamide, anthracyclines, vincristine
and steroids. Methotrexate is recommended only in the third
trimester. Administration of chemotherapy in the last weeks of
pregnancy is not recommended because it increases the risk of
infections and hematological malignancies in newborn. After the
32nd gestational week, induction of labor is suggested to avoid
fetal pancytopenia (23).
6. Chronic myeloid leukemia
CML
CML comprises 8% of all leukemia cases in the UK,
and it appears most frequently during the sixth decade of life. CML
is characterized by the presence of the Philadelphia chromosome t
(9:22), leading to the excessive production of white blood cells in
the bone marrow (24).
Pathophysiology of CML
The pathophysiology of CML involves a genetic
exchange between chromosomes 9 and 22, resulting in the fusion of
the BCR gene from chromosome 22 with the ABL gene from chromosome
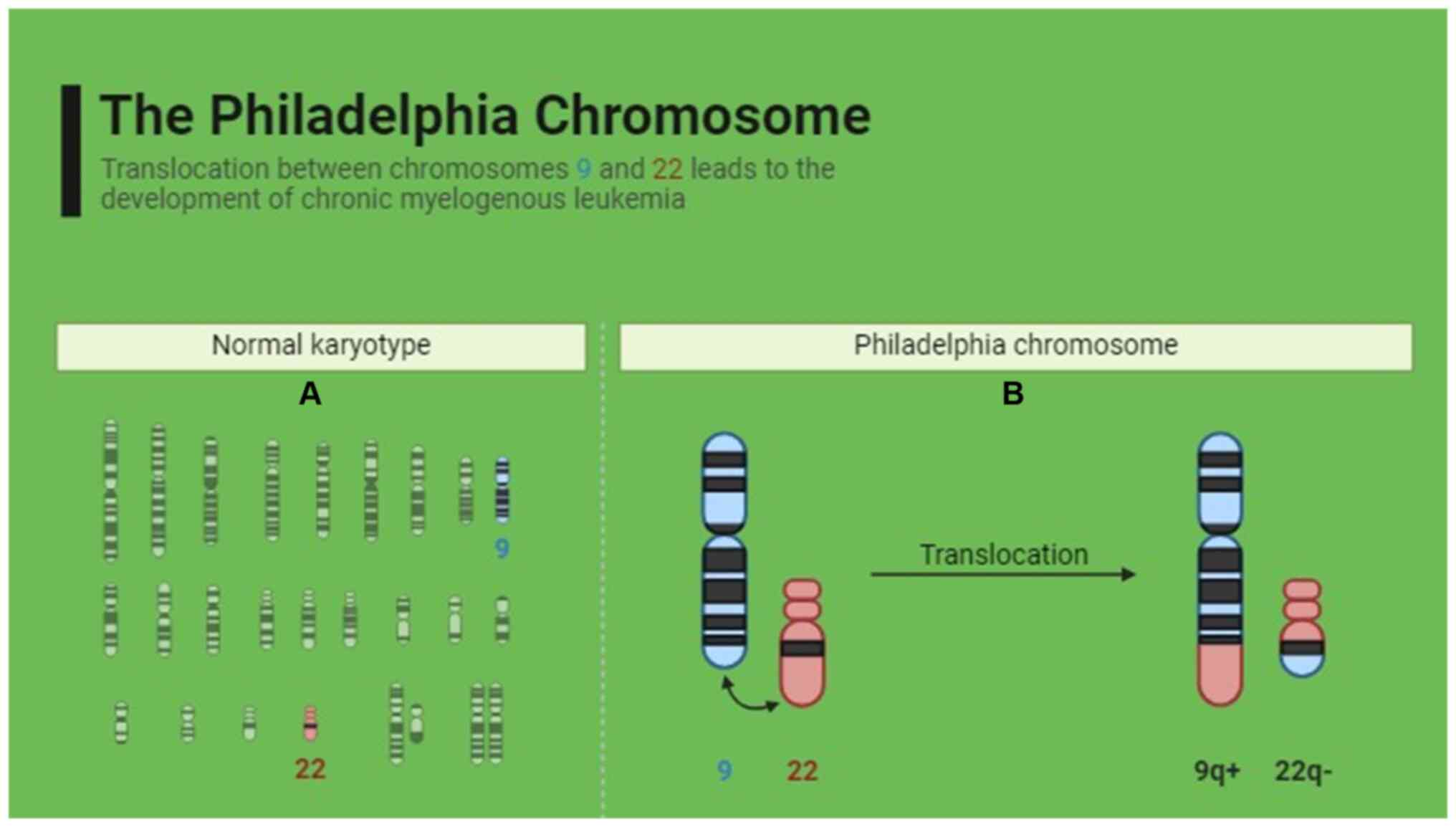
9. This translocation forms the Philadelphia chromosome, which
generates a novel hybrid chimeric oncoprotein (24), as shown in Fig. 1. Reverse transcription-quantitative
PCR (RT-qPCR) analysis of BCR-ABL1 is the most sensitive method for
detecting and monitoring abnormal fusion transcripts associated
with certain leukemias. This analysis can be performed on
peripheral blood or bone marrow samples. Unless there are specific
clinical indications, it is generally not necessary to obtain bone
marrow specifically for molecular testing (24).
Risk factors of CML
Exposure to ionizing radiation, advanced age and
male sex may be associated with the development of CML (25).
General therapeutic approach for
CML
TKIs, leukapheresis and interferon-α (INF-α) are
used for the treatment of CML, while marrow transplantation is
rarely used (26).
CML in pregnancy
CML constitutes ≤10% of all leukemias during
pregnancy, with an annual incidence of 1 in 100,000 pregnancies.
Its diagnosis during pregnancy is complicated. The disease is
usually diagnosed in the second and third trimesters, while its
progression is not affected by pregnancy (26).
Clinical presentation of CML in
pregnancy
There are three phases of CML: Blast crisis,
accelerated and chronic. A total of 90% of patients with CML are
diagnosed during the chronic phase. If left untreated, CML
progresses to the accelerated phase or the blast crisis phase.
Blast crisis represents the ultimate phase of CML and is
characterized by blast cells constituting >20% of total white
blood cells (27). Prompt
treatment is crucial for achieving remission and preventing
progression to advanced stages. The clinical presentation of CML
depends on the stage of the disease. Most patients diagnosed in the
chronic phase are usually asymptomatic. If symptoms appear, these
include right and left hypochondriac pain (due to
hepatosplenomegaly), low-grade fever and night sweats. Patients in
the accelerated stage have an increased risk of hemorrhage, while
patients in the blast crisis phase have joint pain, fever and bone
marrow fibrosis (28).
Diagnosis of CML in pregnancy
Suspicion of the disease is based on increased
numbers of neutrophils, basophils and eosinophils in complete blood
count. Bone marrow biopsy confirms the diagnosis. The common
translocation between chromosomes 9 and 22 is confirmed by
cytogenetic analysis, while the detection of BCR-ABL genes is
performed with hybridization or PCR (29).
Therapeutic approach for CML in
pregnancy
The therapeutic approach for CML includes
leukapheresis, chemotherapy, INF-α and TKIs. However, the use of
TKIs in the first trimester of pregnancy appears to lead to
abortions and fetal anomalies such as craniosynostosis, hypoplastic
lungs, double kidneys, cerebellar hypoplasia, scoliosis,
polydactyly and premature closure of skull bones (30). There are not sufficient data on the
safety of TKIs in the second and third trimesters. In the first
trimester, for the safety of the fetus, leukapheresis is only
suggested. Leukapheresis and INF-α are the safest therapeutic
options in the second and third trimesters of pregnancy, while TKIs
are a possible therapeutic option during the third trimester
(31). Professional advice and
follow-up from specialized oncologists-hematologists and
gynecologists are recommended for the potential maternal effects
from the termination of therapy and the potential dangers for the
fetus due to therapy. Full administration of treatment should be
stopped 10 months before conception and continued after delivery.
Follow-up of the BCR-ABL gene is monthly recommended, and treatment
must be administered if BCR-ABL increases >1% (32).
7. Chronic lymphocytic leukemia
Chronic lymphocytic leukemia
(CLL)
CLL is the most common type of leukemia in Western
countries, with a positive association with age (usually diagnosed
at ≥50 years of age) and male sex. In CLL, the bone marrow produces
a large number of B lymphocytes. CLL is related to genetic
mutations and epigenetic changes (33).
Pathophysiology of CLL
CLL is characterized by the clonal expansion of
CD5+CD23+ B cells in blood, marrow and
lymphoid tissues. Additionally, recurrent genetic lesions are
implicated in CLL pathogenesis. Furthermore, attention has been
focused on BCR and chemokine receptors that help CLL cells to home
to lymphoid tissues, resulting in establishing a microenvironment
for the development of leukemia (33). Flow-cytometric demonstration of the
typical immunophenotype is essential for diagnosing CLL. CLL is
characterized by a specific immunophenotype, which includes the
expression of CD5, CD19, dim CD20, dim CD22, CD23, bright CD43, dim
CD45, dim-to-negative CD79b, dim CD81, CD200 and dim monoclonal
surface immunoglobulin (Ig). This distinct immunophenotype enables
a definitive diagnosis of CLL and helps exclude other types of
leukemia or lymphoma (33).
Risk factors of CLL
Known risk factors of CLL are family history,
autoimmune diseases, exposure to pesticides and insecticides, and
hepatitis C infection (34).
General therapeutic approach for
CLL
If symptoms and treatment indications are absent,
follow-up is only recommended. Depending on the stage, genetic
features and clinical characteristics of the disease,
pharmacological options include chemoimmunotherapy with an
anti-CD20 monoclonal antibody or targeted therapies with BTK or
BCL2 inhibitors. which can achieve disease control (35).
CLL in pregnancy
CLL in pregnancy is extremely rare and can present
with cytopenia, which leads to an increased risk of infections and
bleeding. For this reason, erythrocyte and platelet transfusions
are necessary, with desirable hemoglobin values >10 g/dl and
platelet values >50,000/µl. CLL in pregnancy is related to
diabetes mellitus, especially in women who receive corticosteroids
and in intrauterine growth restriction (36).
Clinical presentation of CLL in
pregnancy
CLL is mainly asymptomatic, but with disease
progression, painless lymphadenopathy, fever, fatigue, weight loss,
anemia and splenomegaly appear. Patients are vulnerable to
infections due to hypogammaglobulinemia and can develop autoimmune
hemolytic anemia (36).
Diagnosis of CLL in pregnancy
Patients with swollen lymph nodes may be suspected
of having the illness. In a peripheral blood smear, ‘smudge cells’
or ‘basket cells’ are apparent, and lymphocytosis manifests in a
complete blood count. The primary basis for diagnosis is the
presence of pathogenic B lymphocytes, which are clonal and
genetically identical cells with surface expression of CD5 and CD23
molecules, in blood, bone marrow and tissues. Therefore, to
diagnose CLL, peripheral smear microscopical inspection in
conjunction with flow cytometric analysis of B lymphocytes (which
verifies the clonality of these cells and highlights the expression
of molecules on their cellular surface) is required. Imaging
analysis, including computed tomography, completes the diagnosis of
the disease. Confirmation of the diagnosis is established with
immunophenotype of peripheral blood, while bone marrow biopsy is
rarely required (37).
Therapeutic approach for CLL in
pregnancy
Few cases of pregnant women with CLL (34) have been reported in the literature,
so there are no leading directions. This results in the
personalization of management of patients. Follow-up is suggested
in most cases, without treatment until labor if the disease is not
progressive. The therapeutic approaches in pregnancy are mainly
related to complications of the disease. The patient should be
examined systematically with physical examination, blood tests,
follow-up of the fetus and ultrasound of lymph nodes. If therapy is
needed, chlorambucil is preferred after the 15th gestational week
because it is related to fetal abnormalities if received in the
first trimester (35). Rituximab
belongs to Pregnancy Category C according to the Food and Drug
Administration (FDA) of the USA and is contraindicated in
pregnancy. Pregnancy should be avoided if the patient is treated
with rituximab until 12 months after the last dose. Complications
of the treatment in the second and third trimesters are neonatal
lymphopenia and reduction of B-lymphocytes. In addition, cladribine
belongs to Pregnancy Category D according to the FDA, and is not
recommended in pregnancy (36).
CLL does not affect fertility and is not an inhibiting factor for
pregnancy progression. Family planning and consultation are
suggested if a patient receives or will receive pharmacological
treatment, since numerous therapeutic agents used for the treatment
of CLL are contraindicated during pregnancy. Cooperation of a
hematologist, gynecologist and the patient is useful during
pregnancy (36,37). Ibrutinib is considered teratogenic
because it causes heart malformation in pregnant rats and its use
is not recommended during pregnancy as it belongs to Pregnancy
Category D. Furthermore, Bruton's tyrosine kinase inhibitors can
cause severe hypoglobulinemia in the fetus and severe
immunodeficiency (38).
8. Hairy cell leukemia
Hairy cell leukemia (HCL) in
pregnancy
HCL exhibits a unique immunophenotype characterized
by staining with antibodies. In HCL, the cells typically show
negativity for CD5, CD10 and CD23. Conversely, they display
abnormally high expression of CD20, CD22, CD11c and CD25.
Furthermore, HCL cells are positive for CD103 and CD123. This
distinctive immunophenotypic profile aids in the diagnosis and
differentiation of HCL from other leukemias and lymphomas (39).
Therapeutic approach for HCL in
pregnancy
HCL is not treated unless excessive cytopenia
symptomatic splenomegaly, recurrent infections, extra lymphatic
involvement, autoimmune complications and/or progressive
deterioration of the disease appear. Purine analogues, mainly
cladribine, are widely used as first-line treatment, with
percentages of disease eradication of 76-80% (39). Purine analogues belong to Pregnancy
Category D due to their teratogenic effects and fetal mortality,
but there are not sufficient data on the use of cladribine in
pregnancy. INF-α is used for the treatment of HCL. Pregnant women
in which INF-α was administered, had non-complicated pregnancy and
physiological development of the child after labor. Splenectomy is
a treatment of choice during the second trimester of pregnancy. In
the medical literature, 4 cases of pregnant women were reported in
which splenectomy was performed, and 2 of them needed cladribine
after labor (39). Monoclonal
antibodies, mainly rituximab, have been used in pregnancy for the
treatment of acute HCL. As aforementioned, rituximab belongs to
Pregnancy Category C, and its use in pregnancy slightly increases
the risk of premature labor (40).
Types, pathophysiology, risk factors and treatment of leukemias in
pregnancy are summarized in Table
II.
 | Table IITypes, pathophysiology, risk factors
and treatment of leukemias in pregnancy. |
Table II
Types, pathophysiology, risk factors
and treatment of leukemias in pregnancy.
| Type of
leukemia |
Pathophysiology | Risk factors | Treatment |
|---|
| Acute myeloid
leukemia | Accumulation of
blasts in the bone marrow | Inherited
conditions such as Fanconi and Diamond-Blackfan anemias; genetic
mutations in the germline, namely RUNX1, CEBPA and GATA2 genes;
epidemiological factors such as smoking and chemical exposure;
prior chemotherapy, radiotherapy; and myelodysplastic
syndromes | Termination of
pregnancy is mainly recommended in the first trimester. Risk of
teratogenicity is negligible in the second and third trimesters.
The preferred chemotherapeutic schemes in the second and third
trimesters are anthracyclines, especially doxorubicin. |
| Acute promyelocytic
leukemia | Accumulation of
immature granulocytes-chromosomal translocation involving the
α-receptor of retinoic acid on chromosome 17 and the PML gene on
chromosome 15; and dysfunctional hybrid protein, which interferes
with the normal differentiation of promyelocytes into mature
granulocytes | Advanced age,
chemical solvents, prior myelodysplastic syndromes and germline
mutations | Daunorubicin is the
anthracycline of choice in the first trimester, while
coadministration of all-trans retinoic acid and chemotherapy
is recommended in the second and third trimesters |
| ALL | Damage to DNA,
which causes abnormal lymphocytes and their progenitors to undergo
uncontrolled proliferation and spread throughout the body, leading
to the replacement of the bone marrow and other lymphoid organs.
B-cell ALL is more common and is manifested by the presence of the
Philadelphia chromosome and the BCR/ABL (p190) oncoprotein | Genetic syndromes,
Down syndrome, severe combined immunodeficiency, genetic mutations
in the GATA3 gene, and prior chemotherapy and radiotherapy | If ALL appears
before the 20th gestational week, pregnancy termination is
suggested. After the 20th gestational week, prednisone is
recommended for 1-2 weeks before the initiation of chemotherapy.
The preferred chemotherapeutic agents includecytarabine,
cyclophosphamide, anthracyclines, vincristine and steroids
Methotrexate is recommended only in the third trimester. |
| Chronic myeloid
leukemia | Philadelphia
chromosome t (9:22), leading to the excessive production of white
blood cells in the bone marrow | Exposure to
ionizing radiation, advanced age and male sex | Leukapheresis,
chemotherapy, INF-α and tyrosine kinase inhibitors |
| Chronic lymphocytic
leukemia | Clonal expansion of
CD5+ CD23+ B cells in blood, marrow and
lymphoid tissues | Family history,
autoimmune diseases, exposure to pesticides, and insecticides, and
hepatitis C infection | There are no
leading directions due to limited data |
| Hairy cell
leukemia | Expression of a
range of B-cell antigens (surface Ig, CD19, CD20 and CD22);
expression ofa single light-chain type and clonal rearrangement of
Ig | Age, sex (more
common in men than in women) and ethnicity (more often found in the
family history of white patients) | INF-α, cladribine
and rituximab |
9. Lymphomas
Lymphomas in pregnancy diagnosis
therapeutic approach
In the present review epidemiological, diagnostic
and therapeutic data on lymphomas and multiple myeloma (MM) in
pregnancy are presented. The current study mainly focuses on their
management during pregnancy per trimester, the possibility of
future pregnancy in women affected by lymphoma or MM, and the
association between the therapy for these diseases and the danger
of future infertility. It must be emphasized that the data and
conclusions are based on the results of small-group cases of
pregnant women that have been reported in the literature. Thus,
further research in the field of hematological malignancies and
pregnancy is necessary to validate the results. The term ‘lymphoma’
refers to a tumor that develops from immune system cells called
lymphocytes (T or B cells), with B lymphocyte-derived lymphomas
being more prevalent. Hodgkin and non-Hodgkin lymphomas are the two
primary forms of lymphomas (41).
Hodgkin lymphoma (HL)
HL is a tumor that arises from B-cell lymphocytes.
The disease is characterized by the appearance of symptoms in
advanced stages. HL appears more often in two age groups: 15-35 and
>55 years of age (41).
Pathophysiology of HL
One type of cancer that affects the lymphatic system
and is typically detected in the lymph nodes is HL. Large cells
known as Reed-Sternberg (RS) cells are a hallmark of HL, which
often begins in the lymph nodes and occasionally invades other
organs (42).
Risk factors of HL
The risk factors of HL include male sex, age (15-40
and >55 years of age), positive family history, history of
infectious mononucleosis, immunosuppression, extended use of growth
hormone and exposure to exotoxins (43).
General therapeutic approach for
HL
Patients at the early stage of the disease are
treated with radiotherapy or chemotherapy, while patients at the
advanced stage are treated with chemotherapy. The most known
chemotherapy treatment is the doxorubicin, bleomycin, vinblastine
and dacarbazine (ABVD) scheme. In addition, there are other
chemotherapeutic schemes such as the bleomycin, etoposide,
doxorubicin, cyclophosphamide, vincristine, procarbazine and
prednisolone (BEACOPP) regimen. In advanced disease stages, the
addition to the doxorubicin, vinblastine and dacarbazine (AVD)
regimen of brentuximab vendotin, an anti-CD30 monoclonal antibody
conjugated with a toxin that inhibits cell mitosis, has recently
been approved as first-line therapy of HL (38).
HL in pregnancy
In pregnancy, the incidence of HL ranges between
1:1,000 and 1:3,000(44).
Clinical presentation of HL in
pregnancy
One of the most common features of HL is the
painless enlargement of ≥1 lymph nodes. The lymph nodes appear
edematous during clinical examination, while Β symptoms (fever
>38˚C, weight loss >10% in 6 months and night sweats)
characterize this lymphoma (45).
Diagnosis of HL in pregnancy
Pregnancy-related HL is the sixth most common
malignancy. RS cells, which are large cells expressing CD30 and
CD15 antigens on their surface, are what define HL, and their
presence is required for the diagnosis of HL. Diagnosis of the
disease is accomplished by lymph node biopsy. Concerning the
imaging methods used for staging, computed tomography (CT) and
positron emission tomography (PET) scans are not used, while
magnetic resonance imaging (MRI) and abdominal ultrasound are the
most common methods (46).
Therapeutic approach for HL in the
first trimester
If HL is diagnosed in the first trimester, delay of
the treatment is preferred, since the fetus can be affected by
chemotherapy during the stage of organogenesis. In case of
aggressive lymphomas, termination of pregnancy is suggested, but if
a woman does not wish to terminate the pregnancy, immediate
chemotherapy is proposed, mainly vinblastine, which is less harmful
than other chemotherapeutic agents. Adriamycin, bleomycin,
vinblastine and dacarbazine are also suggested in markedly
aggressive lymphomas, while radiotherapy is postponed until after
birth due to the risk of teratogenesis and cancer development in
childhood (47).
Therapeutic approach for HL in the
second and third trimesters of pregnancy
Management of HL in the second and third trimesters
is easier due to a wide range of therapeutic options. If
asymptomatic HL is diagnosed during the second half of the
pregnancy, then therapy is not provided, but treatment is suggested
after the completion of childbirth. If immediate treatment is
crucial, then there are chemotherapeutic schemes such as ABVD. Some
possible toxic effects of this chemotherapeutic scheme are low
bodyweight embryos, limited endometrial development, birth of a
dead embryo, mental retardation and learning difficulties during
childhood. In addition to these possible effects, previous research
has shown that embryo exposure to these agents is limited.
Radiation is not suggested during pregnancy due to its long-term
impact on embryos. If radiation is necessary, it is used after the
first trimester and the abdomen is covered to prevent exposure to
radiation (48). The timing of
childbirth is a shared decision of gynecologists, hematologists,
oncologists and patients. Whenever it is possible and permitted by
the health of the mother and the fetus, the pregnancy is allowed to
be completed. If this is impossible, the fetus should be born after
the 34th week of gestation. Childbirth by cesarean section is not
necessary, except if there are other obstetrical reasons. A
previous study has shown that fertility is possibly affected after
chemotherapy or radiotherapy. There are chemotherapeutic schemes
that appear to be less harmful for infertility such as ABVD, and
others that are more harmful such as BEACOPP. Additionally, the
larger the dose of medications is, the greater the possibility of
infertility. In addition, it is not possible to estimate in detail
the effect of these medications or radiotherapy on the fertility of
women. Chemotherapy can lead to premature ovarian insufficiency and
premature menopause. Specific medications such as procarbazine and
cyclophosphamide result in 5-25% premature ovarian insufficiency
mainly in women aged <30 years. Doxorubicin, bleomycin,
vincristine and dacarbazine pose a smaller risk of premature
ovarian insufficiency (49). For
this reason, cryopreservation of ovaries is essential before the
beginning of therapy. Another promising method is the
cryopreservation of ovarian tissue, which has been used in a few
cases of women with HL who desire future pregnancy. Planning for a
possible pregnancy is recommended 2 years after the completion of
therapy, since patients with HL can relapse in the first decade
(49).
Non-HL (NHL)
NHL is a hematological malignancy of either B- or
T-cell origin. The incidence of this disease increases with age and
is usually diagnosed in patients >60 years of age. NHL is
classified into two groups, aggressive and indolent, which have
different prognoses. The most aggressive type of NHL is Burkitt
lymphoma, which is also one of the most rapidly developing types of
tumor (50). Aggressive lymphoma
cells rapidly divide in the body, and, if left untreated, they can
result in mortality within 6 months. Patients that are diagnosed in
the early stages of aggressive disease, have more possibilities to
be cured and less possibilities of relapse.
Pathophysiology of NHL
Chromosome translocation causes NHL to develop in B,
T or natural killer cells. The most prevalent chromosomal
aberration in NHL is the t (14;18) translocation. Oncogenes are
activated and tumor suppressor genes are inactivated due to
translocations (51). Biological
markers play a crucial role in the clinical management of NHL by
supporting morphologic diagnosis, staging, prognostic assessment
and monitoring of MRD. Serological markers such as β2-microglobulin
(β2-M), lactate dehydrogenase (LDH) and cancer antigen 125 reflect
tumor load, proliferative activity and invasive potential,
respectively. LDH and β2-M are important prognostic parameters
included in widely used staging systems. Immunophenotypic analysis
helps to identify lineage (B or T cells), maturation level, cell
proliferation and clonality, thus providing valuable insights into
the disease biology and guiding treatment decisions (51,52).
Risk factors of NHL
Risk factors involved in the disease are genetic
predisposition, Epstein-Barr infection, immunosuppression, human
immunodeficiency virus, Helicobacter pylori or hepatitis C
infections, obesity, exposure to chemical substances (benzene,
ethylene oxide and formaldehyde) and autoimmune diseases, in
addition to silicone implants in the case of T-cell anaplastic
lymphoma (52).
General therapeutic approach for
NHL
The therapeutic approach for NHL includes the use of
immunochemotherapy, radiotherapy, targeted therapies and
transplantation of hemopoietic progenitor cells, as well as the
recent use of CAR T-cell technology. The most known widely used
chemotherapeutic regimen is rituximab, cyclophosphamide,
doxorubicin, vincristine and prednisone (R-CHOP) (53).
NHL in pregnancy
NHL is rare in pregnancy, and it affects
extranodular locations such as the breasts, ovaries and vagina. The
most frequent subtype of NHL in pregnancy is diffuse B-cell
lymphoma (54).
Clinical presentation of NHL in
pregnancy
The symptoms of NHL are not different from those
that appear in non-pregnant women. Regarding these, painless
lymphadenopathy is often manifested, while the presence of systemic
symptoms (known as B symptoms), such as fever, abundant night
sweats and loss of >10% body weight over 6 months is frequent
(54).
Diagnosis of NHL in pregnancy
The diagnosis of NHL in pregnancy should always
include history, physical examination and blood tests. Bone marrow
biopsy and/or cerebrospinal fluid evaluations establish the final
diagnosis of the disease (55).
Therapeutic approach for NHL in
pregnancy
The decision regarding therapy in pregnancy is
dependent on several factors, including the type of lymphoma, week
of the pregnancy and patient's preference. Asymptomatic and
non-aggressive lymphomas can be managed with supervision, while
aggressive lymphomas require systematic treatment (55). As for the treatment, combined
chemotherapy can be used with certain safety in the second and
third trimesters, but is contraindicated during the first
trimester. Regarding non-aggressive NHLs (nodular and lymphocytic),
treatment can be delayed until the appearance of symptoms. If the
patient remains asymptomatic, it is suggested to start the
treatment after childbirth. If it is symptomatic and the treatment
in the first trimester is necessary, rituximab is exclusively
recommended. The use of rituximab in pregnancy is associated with
abortions in the first trimester in a percentage of 21%, with
neonatal prematurity in a percentage of 24%, and with neutropenia,
thrombocytopenia and a reduced number of B cells in the neonate
after birth. The additional therapeutic choice in the second and
third trimesters for non-aggressive lymphomas is cyclophosphamide,
vincristine and prednisolone (CVP) (56). The treatment of non-aggressive
lymphomas can be initiated after the first trimester. Aggressive
lymphomas are diagnosed often during pregnancy, and must be treated
immediately and in combination with other agents. When the
diagnosis is made early in the first trimester, termination of
pregnancy is suggested. If the diagnosis is made at the end of the
first trimester, CHOP is suggested in the second trimester, while
the combination of rituximab with CHOP is considered the most
effective one in pregnancy. Concerning the most aggressive NHLs,
termination of pregnancy in the first trimester is advised
(56,57). According to limited bibliographic
data, methotrexate has been used in the second and third
trimesters, and it is not considered to have teratogenic effects
but can cause myelosuppression of the embryo (56,57).
Treatment for NHL can have adverse effects on fertility, mainly the
combination of chemotherapeutic agents and radiotherapy (58). A temporary period of infertility
may occur subsequent to treatment of the disease, while
irreversible infertility rarely occurs.
10. Multiple myeloma
MM
MM is defined as a multifocal neoplasm characterized
by the expansion of clonal plasma cells in the bone marrow, soft
tissues and bones. MM is characterized by the presence of
monoclonal proteins in serum and urine (59). In the majority of cases,
asymptomatic myeloma exists prior to the development of MM
(60). The production of
monoclonal paraproteins results in their accumulation in several
organs (kidney, heart, liver and peripheral neurons) and in the
manifestation of symptoms such as amyloidosis or hyperviscosity
syndrome.
Pathophysiology of MM
Monoclonal plasma cells in myeloma proliferate and
produce excessive M protein levels, abnormal light chain proteins
and cytokines that stimulate osteoclasts and suppress osteoblasts,
and angiogenesis factors that lead to new blood vessel formation.
Therefore, the overproduction of M protein level can cause
hyperviscosity and light chain protein-associated end organ damage,
especially in the kidneys, as well as bone lesions, osteoporosis
and hypercalcemia (Fig. 2). Bone
marrow invasion can result in anemia and recurrent infections
(61).
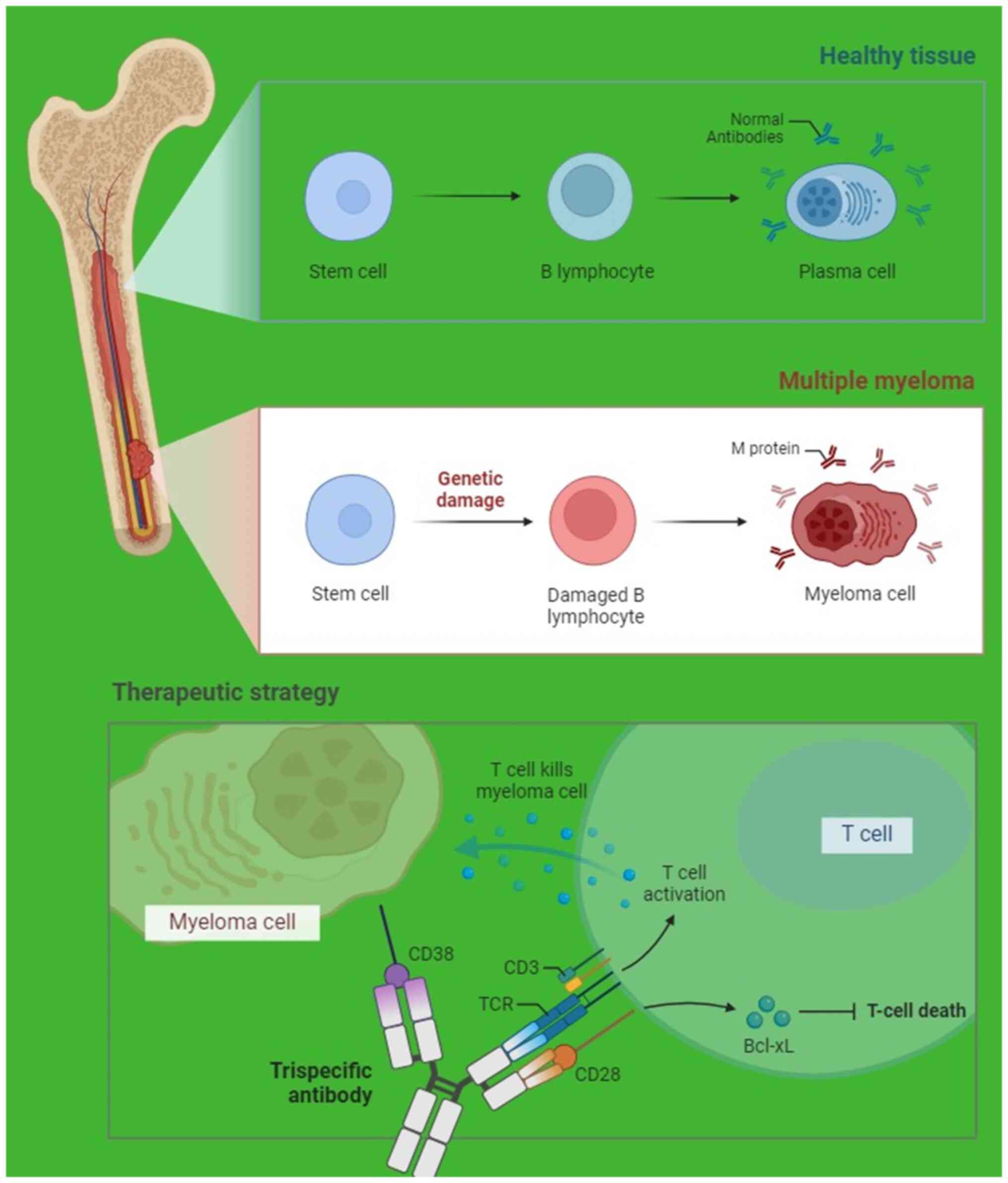 | Figure 2Representation of healthy plasma
cells, damaged plasma cells with M protein and therapeutic
approaches in MM. The healthy plasma cell image is a depiction of a
normal, functioning plasma cell, responsible for producing
antibodies to fight infections. The cell exhibits regular
morphology and produces a diverse range of antibodies to maintain
the immune system function. For representing a damaged plasma cell
with M protein, the illustration of a plasma cell afflicted by a
cancer of plasma cells is shown. The cell displays aberrant
morphology and produces excessive levels of monoclonal antibodies
known as M proteins. These abnormal proteins can interfere with
normal immune functions, and cause complications such as kidney
damage and bone lesions. Regarding therapeutic approaches for MM,
various treatment modalities for MM are proposed, including
chemotherapy, immunomodulatory drugs, proteasome inhibitors, stem
cell transplantation and targeted therapies. These interventions
aim to eliminate malignant plasma cells, reduce M protein levels
and alleviate symptoms associated with the disease, ultimately
improving the outcome and quality of life of patients. MM, multiple
myeloma; BcL-xL, B-cell lymphoma-extra large. |
Risk factors of MM
Age >55 years old, male sex, black ethnicity and
history of monoclonal gammopathy of undetermined significance are
associated with the development of MM (62).
General therapeutic approach for
MM
The treatment of MM depends on the age of the
patient. Induction chemotherapy with bortezomib and
lenalidomide-dexamethasone is used in patients <65 years old and
is followed by stem cell transplantation (63). In patients >65 years old that
cannot tolerate stem cell transplantation, chemotherapy is
recommended. Combination of daratumumab (an anti-CD38 monoclonal
antibody) with either bortezomib or lenalidomide can possibly offer
an overall improved life expectancy. On average, the 5-year
survival rate is 35-50% (64).
MM in pregnancy
The coexistence of MM and pregnancy is rare, since
the disease affects mainly older patients, and is usually found in
the third trimester (65).
Clinical presentation of MM in
pregnancy
Hypercalcemia, renal impairment, anemia and bone
osteolysis require immediate treatment (66), as well as patients with ≥1 focus
lesion on MRI imaging (67) or
infiltration by plasma cells in the bone marrow. The symptoms that
are more often reported during diagnosis are lower back pain, pale
skin, anemia, weight loss and fatigue. MM usually leads to frequent
infections and osteolytic lesions resulting in bone fractures, and
can cause severe disability and permanent neurological impairment
(65).
Diagnosis of MM in pregnancy
Suspicion of the disease arises from laboratory
pathological tests, such as complete blood count, elevated
erythrocyte sedimentation rate, and measurement of calcium,
albumin, lactic dehydrogenase, urea and creatinine in blood. The
results reflect bone marrow and renal insufficiency, and bone
lesions attributed to MM, while measurement of β2-M and serum
albumin are recommended to determine the stage of the disease.
Increased β2-M levels determine the prognosis, while the IgG or IgA
levels reflect the tumor burden (68). Serum and urine protein
electrophoresis should not be omitted. Urine tests measure red
blood cells, glucose and proteins; however, the existence of MM is
indicated by a 24-h urine collection for the detection of light
chains of proteins (Bence-Jones proteins). Additional diagnostic
techniques include CT, MRI, PET scans and X-rays. A bone marrow
biopsy is used to establish the final diagnosis (69).
Therapeutic approach for MM in
pregnancy
Regarding the treatment for MM, glucocorticoids
until childbirth are suggested. Chemotherapy in the first trimester
is considered dangerous for the fetus, while its administration
during the second and third trimesters is not considered to be
harmful. In the advanced cases of disease, in which chemotherapy is
necessary in the first trimester, termination of pregnancy is
suggested. Thalidomide, lenalidomide and pomalidomide are
medications that are used in the treatment of MM, but their
administration during pregnancy and during puerperium is strictly
contraindicated. The consequences of bortezomib in embryo
development are not clear because there are no clinical studies on
it. Cyclophosphamide is used because it inhibits the development
and multiplication of malignant cells (70). Dexamethasone and prednisone are
considered safe in pregnancy. Bisphosphonates prevent bone
fractures and are considered safe in pregnancy. The most frequently
used bisphosphonates are pamidronate and zoledronic acid. It is
still unknown if pregnancy deteriorates or not as the disease
progresses (71). Fertility is
reduced in women affected by MM who are treated with chemotherapy.
One approach that is used for the maintenance of fertility in women
affected by MM is the cryopreservation of embryos before
chemotherapy, which can be implanted in the uterus after recovery
from the disease (72).
Pathophysiology, risk factors and treatment of HL, non-HL and MM
are summarized in Table III.
 | Table IIIPathophysiology, risk factors and
treatment of HL, non-HL and MM. |
Table III
Pathophysiology, risk factors and
treatment of HL, non-HL and MM.
| Types |
Pathophysiology | Risk factors | Treatment |
|---|
| HL | Large cells known
as Reed-Sternberg cells are a hallmark of HL, while other aberrant
cell types may also be present | Male sex, age
(15-40 and >55 years), positive family history of HL, history of
infectious mononucleosis, immunosuppression (including HIV), use of
growth hormone and exposure to exotoxins | In case of
aggressive lymphomas, termination of pregnancy is suggested, while
in the second and third trimesters of pregnancy, adriamycin,
bleomycin, vinblastine and dacarbazine are recommended |
| Non-HL | Chromosome
translocation causes non-HL to develop in B, T or natural killer
cells. The most prevalent chromosomal aberration innon-HL is the t
(14;18) translocation | Genetic
predisposing, Epstein-Barr infection, immunosuppression
(iatrogenic, hereditary or HIV-related), HIV infection,
Helicobacter pylori or hepatitis C infections, obesity,
exposure to chemical substances, autoimmune diseases and silicone
implants (for T-cell anaplastic lymphoma) | Asymptomatic and
non-aggressive lymphomas, supervision; aggressive lymphomas,
systematic treatment. Combined chemotherapy can be used with
relative safety in the second and third trimesters, but is
contraindicated during the first trimester |
| MM | Monoclonal plasma
cells proliferate and produce excessive M protein levels, abnormal
light chain proteins and cytokines that stimulate osteoclasts and
suppress osteoblasts, and angiogenesis factors that lead to new
blood vessel formation | Advanced age
(>55 years), sex (more common in males), ethnicity (double risk
in black vs. Caucasian men) and history of monoclonal gammopathy of
undetermined significance | Glucocorticoids
until childbirth are suggested; chemotherapy in the first trimester
is dangerous for the fetus, while chemotherapy during the second
and third trimesters is not considered to be harmful |
11. AML cases vs. bone marrow metastatic
cancer
Exploring clinical treatment
strategies and differential diagnosis in typical AML cases vs. bone
marrow metastatic cancer
There is limited literature addressing the
differences in treatment approaches between cases of leukemia and
bone marrow metastases during pregnancy. However, data regarding
gastric cancer metastasizing to the bone marrow are available.
Performing a bone marrow biopsy for histopathological diagnosis is
the most reliable method for distinguishing between these two
clinical entities if the primary cancer has not been identified.
Although it is beyond the scope of the present review, it remains
important to differentiate between general bone marrow metastasis
and disseminated carcinomatosis of the bone marrow (DCBM). In
typical bone metastasis, the metastatic pattern manifests as a
nodular pattern, whereas in DCBM, it presents as diffuse
infiltration, leading to hematological disorders such as
disseminated intravascular coagulation, leukoerythroblastosis and
microangiopathic hemolytic anemia. DCBM arises from the metastasis
of solid tumors, with ~90% of cases originating from gastric cancer
(73-75).
The management of such patients poses significant
challenges. It is advised to consider termination of pregnancy
before 22 weeks of gestation and early delivery after 28 weeks of
gestation when initiating treatment. However, the recommended
treatment approach between 22 and 28 weeks of gestation is subject
to debate. Immediate interventions such as systemic chemotherapy
are beneficial for patients, although there is a risk of adverse
effects on the fetus (74,75).
Administration of chemotherapy during the first
trimester carries an elevated risk of congenital malformations.
Unfavorable experiences of chemotherapy in the first trimester
resulted in the recommendation for therapeutic abortion; in
particular, the association of cytarabine and 6-thioguanine with
congenital abnormalities led to the recommendation that both of
these drugs should be avoided during this time period.
Regimen-induced toxicity during the first trimester is well
accepted, but as not all fetuses are adversely affected, there may
be a genetic predisposition to teratogenesis. Thus, when acute
leukemia is diagnosed in the first trimester, termination of
pregnancy is strongly recommended, followed by the initiation of
induction therapy. Conversely, if leukemia is diagnosed later in
pregnancy, conventional management strategies akin to those in
non-pregnant individuals can be employed, typically obviating the
need for pregnancy termination. The risk of fetal malformation is
generally acknowledged to decrease as the pregnancy progresses.
Exposure to chemotherapy after the first trimester is associated
with a higher incidence of intrauterine growth retardation, which
is also influenced by maternal nutritional status throughout
pregnancy, as well as preterm delivery and fetal mortality.
However, there is no observed increase in the occurrence of
congenital abnormalities, and specifically, no documented increase
in childhood malignancy or adverse neurological development,
despite ongoing neurological development throughout gestation.
Treatment during the third trimester typically yields the fewest
complications; however, the timing of chemotherapy must be
meticulously planned to avoid inducing pancytopenia immediately
prior to delivery. Patients diagnosed with CML during pregnancy can
often be managed with leukapheresis, chemotherapy
(hydroxycarbamide), IFN-α and imatinib. Those with established CML
and a sustained complete molecular response prior to conception may
be monitored without treatment throughout the pregnancy. In the
rare occurrence of CLL during pregnancy, treatment can typically be
postponed until after delivery (76,77).
12. Future perspectives and conclusions
Future perspectives
Van Calsteren et al (78) explored the transplacental
transmission of various chemotherapeutic agents from mother to
fetus using both mouse and baboon models. The baboon model is
considered a pertinent animal model due to its close phylogenetic
resemblance to humans in terms of embryological development,
reproductive physiology, placental structure and function, as well
as drug metabolism. In the baboon model, notable discrepancies were
observed in fetal plasma concentrations for each chemotherapeutic
drug. Anthracyclines (doxorubicin and epirubicin) and taxanes
(paclitaxel) exhibited limited transplacental transfer rates
(<10 and <2%, respectively). Conversely, carboplatin showed
the highest fetal compartment concentration (>55%), indicative
of its considerable transplacental passage (78).
Building upon the aforementioned findings, future
perspectives could include: i) Further investigation on
transplacental transfer by conducting studies to explore the
transplacental transfer of chemotherapeutic drugs in different
animal models, including non-human primates, to validate and expand
upon the observed patterns; ii) mechanistic understanding by
delving deeper into the mechanisms underlying the varying
transplacental transfer rates of different chemotherapeutic agents,
including factors such as drug properties, placental transport
mechanisms and fetal metabolism; iii) clinical implications by
translating these findings into clinical practice by informing
treatment strategies for pregnant patients with cancer, which may
involve refining dosing regimens, selecting drugs with lower fetal
exposure or developing novel approaches to minimize fetal harm
while ensuring maternal therapeutic efficacy; iv) pharmacokinetic
modeling by developing pharmacokinetic models to predict the
transplacental transfer of chemotherapeutic agents in pregnant
individuals, thus aiding clinicians in optimizing treatment
regimens and monitoring fetal exposure; and v) long-term follow-up
by conducting long-term follow-up studies to assess the impact of
prenatal exposure to chemotherapeutic agents on fetal development,
birth outcomes and childhood health, thus providing valuable
insights into the safety profile of these treatments during
pregnancy.
Overall, future research should aim to enhance the
current understanding of the complex interplay between maternal
cancer treatment, placental physiology and fetal development,
ultimately improving the care and outcomes of pregnant individuals
with cancer.
Conclusions
The incidence of leukemias in pregnancy is low,
with acute leukemia and mainly AML predominance. For acute
leukemias, immediate treatment after diagnosis is necessary.
Therapeutic approach and management should be personalized
according to the trimester of pregnancy. More specifically,
termination of pregnancy is recommended in the first trimester,
while chemotherapeutic schemes and labor induction are preferred
after the 32nd gestational week.
Management of pregnant women with chronic leukemias
differs from management of those with acute ones. Asymptomatic
pregnant women are not treated unless symptoms appear. Various
therapeutic schemes have been used as monotherapy or combined
therapy. Leukemias do not inhibit a future pregnancy. Regarding the
estimated time of childbearing, women receiving or about to receive
medication for leukemias, family planning and counseling from a
specialized medical team is recommended.
The clinical picture and diagnosis of lymphomas are
not different in pregnant women compared to the rest of the
population. Most lymphomas are treatable during pregnancy. One
pregnancy can end up full-term when the mother's and embryo's
health allows it. In asymptomatic lymphomas, follow-up of the
disease is recommended, while in symptomatic and/or aggressive
lymphomas, immediate therapy is suggested during the second and
third trimesters of pregnancy. In symptomatic lymphomas, during the
first trimester, administration of chemotherapeutic agents or
monoclonal antibodies is necessary. For women who manifest the
disease at the beginning of the first trimester, termination of
pregnancy is recommended. Temporary infertility of patients is
affected by using chemotherapeutics because they lead to premature
ovarian insufficiency. If the disease is manifested mainly during
the third trimester of pregnancy with atypical symptoms, therapy is
based on glucocorticoids. In women affected by MM, cryopreservation
of egg cells or embryos is suggested before the beginning of
chemotherapy.
The present review emphasizes the importance of
prompt diagnosis and tailored management of leukemias and lymphomas
during pregnancy. Immediate treatment is crucial for acute
leukemias, with consideration given to trimester-specific
approaches and potential termination in the first trimester.
Chronic leukemias may not require immediate intervention unless
symptoms arise. Family planning and counseling are essential for
women receiving or planning to receive leukemia treatment.
Lymphomas may require immediate therapy depending on their symptoms
and aggressiveness, while fertility preservation measures are
recommended before chemotherapy for MM. Overall, the current review
underscores the need for personalized care and timely intervention
to optimize outcomes for both the mother and fetus.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AGG participated in the review process and prepared
the manuscript. MNG collected data and relevant literature. EO
contributed to manuscript corrections, and collected data and
relevant literature. KN, AB, SK, NK, SA and TN collected data and
literature. ES, GI, CD, NG and NN corrected and modified the
manuscript. PT was responsible for the study protocol and
contributed to the study design, participated in the review
process, prepared the manuscript and made substantive intellectual
contributions to the published study. All authors read and approved
the final version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Estey E, Hasserjian RP and Döhner H:
Distinguishing AML from MDS: A fixed blast percentage may no longer
be optimal. Blood. 139:323–332. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bispo JAB, Pinheiro PS and Kobetz EK:
Epidemiology and etiology of leukemia and lymphoma. Cold Spring
Harb Perspect Med. 10(a034819)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rausch C, Rothenberg-Thurley M, Dufour A,
Schneider S, Gittinger H, Sauerland C, Görlich D, Krug U, Berdel
WE, Woermann BJ, et al: Validation and refinement of the 2022
European LeukemiaNet genetic risk stratification of acute myeloid
leukemia. Leukemia. 37:1234–1244. 2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li Y, Yang W, Devidas M, Winter SS,
Kesserwan C, Yang W, Dunsmore KP, Smith C, Qian M, Zhao X, et al:
Germline RUNX1 variation and predisposition to childhood acute
lymphoblastic leukemia. J Clin Invest. 131(e147898)2021.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
5
|
Voulgaris E, Pentheroudakis G and Pavlidis
N: Cancer and pregnancy: A comprehensive review. Surg Oncol.
20:e175–e185. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Thomas X: Acute myeloid leukemia in the
pregnant patient. Eur J Haematol. 95:124–136. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhu D, Tang D, Chai X, Zhang G and Wang Y:
Acute leukemia in pregnancy: A single institutional experience with
21 cases at 10 years and a review of the literature. Ann Med.
53:567–575. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ali S, Jones GL, Culligan DJ, Marsden PJ,
Russell N, Embleton ND and Craddock C: British Committee for
Standards in Haematology. Guidelines for the diagnosis and
management of acute myeloid leukaemia in pregnancy. Br J Haematol.
170:487–495. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Grove CS and Vassiliou GS: Acute myeloid
leukaemia: A paradigm for the clonal evolution of cancer? Dis Model
Mech. 7:941–951. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lavudi K, Nuguri SM, Olverson Z,
Dhanabalan AK, Patnaik S and Kokkanti RR: Targeting the retinoic
acid signaling pathway as a modern precision therapy against
cancers. Front Cell Dev Biol. 11(1254612)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Park JH, Qiao B, Panageas KS, Schymura MJ,
Jurcic JG, Rosenblat TL, Altman JK, Douer D, Rowe JM and Tallman
MS: Early death rate in acute promyelocytic leukemia remains high
despite all-trans retinoic acid. Blood. 118:1248–1254.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tsuda H, Doi H, Inada T and Shirono K:
Successful treatment of acute promyelocytic leukemia in a pregnant
woman by using all-trans retinoic acid. Rinsho Ketsueki.
35:717–719. 1994.PubMed/NCBI(In Japanese).
|
|
13
|
Sanz MA, Fenaux P, Tallman MS, Estey EH,
Löwenberg B, Naoe T, Lengfelder E, Döhner H, Burnett AK, Chen SJ,
et al: Management of acute promyelocytic leukemia: Updated
recommendations from an expert panel of the European LeukemiaNet.
Blood. 133:1630–1643. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Santolaria A, Perales A, Montesinos P and
Sanz MA: Acute promyelocytic leukemia during pregnancy: A
systematic review of the literature. Cancers (Basel).
12(968)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Sanz MA, Grimwade D, Tallman MS, Lowenberg
B, Fenaux P, Estey EH, Naoe T, Lengfelder E, Büchner T, Döhner H,
et al: Management of acute promyelocytic leukemia: Recommendations
from an expert panel on behalf of the European LeukemiaNet. Blood.
113:1875–1891. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ssenyonga N, Stiller C, Nakata K, Shalkow
J, Redmond S, Bulliard JL, Girardi F, Fowler C, Marcos-Gragera R,
Bonaventure A, et al: Worldwide trends in population-based survival
for children, adolescents, and young adults diagnosed with
leukaemia, by subtype, during 2000-14 (CONCORD-3): Analysis of
individual data from 258 cancer registries in 61 countries. Lancet
Child Adolesc Health. 6:409–431. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
DeRenzo C, Krenciute G and Gottschalk S:
The landscape of CAR T cells beyond acute lymphoblastic leukemia
for pediatric solid tumors. Am Soc Clin Oncol Educ Book.
38:830–837. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chiaretti S, Zini G and Bassan R:
Diagnosis and subclassification of acute lymphoblastic leukemia.
Mediterr J Hematol Infect Dis. 6(e2014073)2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ticku J, Oberoi S, Friend S, Busowski J,
Langenstroer M and Baidas S: Acute lymphoblastic leukemia in
pregnancy: A case report with literature review. Ther Adv Hematol.
4:313–319. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Farhadfar N, Cerquozzi S, Hessenauer MR,
Litzow MR, Hogan WJ, Letendre L, Patnaik MM, Tefferi A and Gangat
N: Acute leukemia in pregnancy: A single institution experience
with 23 patients. Leuk Lymphoma. 58:1052–1060. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nadeem S, Elahi E, Iftikhar I, Umar S,
Ahsan B, Ahmad U and Bokhari SW: Management of acute lymphoblastic
leukemia during pregnancy: A case report and review of the
literature. Cureus. 16(e52489)2024.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Theodora M, Theofanakis C, Fasoulakis ZN,
Barbarousi D, Daskalakis G and Rodolakis A: EP685 Management of
acute lymphoblastic leukemia in a 26-year-old primigravida. Int J
Gynecol Cancer. 29 (Suppl 4):A392.3–A393. 2019.
|
|
23
|
Vlijm-Kievit A, Jorna NGE, Moll E, Pajkrt
E, Pals ST, Middeldorp S, Biemond BJ, Zeerleder SS, Tio MA, Kemper
EM and Hazenberg MD: Acute lymphoblastic leukemia during the third
trimester of pregnancy. Leuk Lymphoma. 59:1274–1276.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Romero-Morelos P, González-Yebra AL,
Muñoz-López D, Lara-Lona E and González-Yebra B: Frequencies of
BCR::ABL1 transcripts in patients with chronic myeloid leukemia: A
meta-analysis. Genes (Basel). 15(232)2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
American Cancer Society: Risk Factors for
Chronic Myeloid Leukemia. https://www.cancer.org/cancer/types/chronic-myeloid-leukemia/causes-risks-prevention/risk-factors.html.
|
|
26
|
Zhao D, Long X and Wang J:
Pharmacovigilance study of BCR-ABL1 tyrosine kinase inhibitors: A
safety analysis of the FDA adverse event reporting system. BMC
Pharmacol Toxicol. 25(20)2024.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Brehme M, Koschmieder S, Montazeri M,
Copland M, Oehler VG, Radich JP, Brümmendorf TH and Schuppert A:
Combined population dynamics and entropy modelling supports patient
stratification in chronic myeloid leukemia. Sci Rep.
6(24057)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Xue M, Wang Q, Huo L, Wen L, Yang X, Wu Q,
Pan J, Cen J, Ruan C, Wu D and Chen S: Clinical characteristics and
prognostic significance of chronic myeloid leukemia with rare
BCR-ABL1 transcripts. Leuk Lymphoma. 60:3051–3057. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yadav U, Solanki SL and Yadav R: Chronic
myeloid leukemia with pregnancy: Successful management of pregnancy
and delivery with hydroxyurea and imatinib continued till delivery.
J Cancer Res Ther. 9:484–486. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pye SM, Cortes J, Ault P, Hatfield A,
Kantarjian H, Pilot R, Rosti G and Apperley JF: The effects of
imatinib on pregnancy outcome. Blood. 111:5505–5508.
2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Abruzzese E, Mauro M, Apperley J and
Chelysheva E: Tyrosine kinase inhibitors and pregnancy in chronic
myeloid leukemia: Opinion, evidence, and recommendations. Ther Adv
Hematol. 11(2040620720966120)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Soverini S, De Benedittis C, Mancini M and
Martinelli G: Best practices in chronic myeloid leukemia monitoring
and management. Oncologist. 21:626–633. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang S and Kipps TJ: The pathogenesis of
chronic lymphocytic leukemia. Annu Rev Pathol. 9:103–118.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kipps TJ, Stevenson FK, Wu CJ, Croce CM,
Packham G, Wierda WG, O'Brien S, Gribben J and Rai K: Chronic
lymphocytic leukaemia. Nat Rev Dis Primers. 3(16096)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rogers A and Woyach JA: BTK inhibitors and
anti-CD20 monoclonal antibodies for treatment-naive elderly
patients with CLL. Ther Adv Hematol.
11(2040620720912990)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hamad N, Kliman D, Best OG, Caramins M,
Hertzberg M, Lindeman R, Porter R and Mulligan SP: Chronic
lymphocytic leukaemia, monoclonal B-lymphocytosis and pregnancy:
Five cases, a literature review and discussion of management. Br J
Haematol. 168:350–360. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Hallek M, Cheson BD, Catovsky D,
Caligaris-Cappio F, Dighiero G, Döhner H, Hillmen P, Keating MJ,
Montserrat E, Rai KR, et al: Guidelines for the diagnosis and
treatment of chronic lymphocytic leukemia: a report from the
International workshop on chronic lymphocytic leukemia updating the
national cancer institute-working group 1996 guidelines. Blood.
111:5446–5456. 2008.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Luttwak E, Gurevich-Shapiro A, Azem F,
Lishner M, Klieger C, Herishanu Y, Perry C and Avivi I: Novel
agents for the treatment of lymphomas during pregnancy: A
comprehensive literature review. Blood Rev.
49(100831)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Daver N, Nazha A, Kantarjian HM, Haltom R
and Ravandi F: Treatment of hairy cell leukemia during pregnancy:
Are purine analogues and rituximab viable therapeutic options. Clin
Lymphoma Myeloma Leuk. 13:86–89. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cannon T, Mobarek D, Wegge J and Tabbara
IA: Hairy cell leukemia: Current concepts. Cancer Invest.
26:860–865. 2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Munir F, Hardit V, Sheikh IN, AlQahtani S,
He J, Cuglievan B, Hosing C, Tewari P and Khazal S: Classical
Hodgkin lymphoma: from past to future-A comprehensive review of
pathophysiology and therapeutic advances. Int J Mol Sci.
24(10095)2023.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mazharul M: Complications and
Pathophysiology of Hodgkin Lymphoma. J Hematol Thrombo Dis.
10(515)2023.
|
|
43
|
American Cancer Society: Hodgkin Lymphoma
Risk Factors, Risk Factors for Hodgkin Disease. https://www.cancer.org/cancer/types/hodgkin-lymphoma/causes-risks-prevention/risk-factors.html.
|
|
44
|
Smith LH, Danielsen B, Allen ME and Cress
R: Cancer associated with obstetric delivery: Results of linkage
with the California cancer registry. Am J Obstet Gynecol.
189:1128–1135. 2003.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Connors JM: Clinical manifestations and
natural history of Hodgkin's lymphoma. Cancer J. 15:124–128.
2009.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Bachanova V and Connors JM: Hodgkin
lymphoma in pregnancy. Curr Hematol Malig Rep. 8:211–217.
2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Gurevich-Shapiro A and Avivi I: Current
treatment of lymphoma in pregnancy. Expert Rev Hematol. 12:449–459.
2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Borchmann P, Eichenauer DA and Engert A:
State of the art in the treatment of Hodgkin lymphoma. Nat Rev Clin
Oncol. 9:450–459. 2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ciccarone M, Cavaceppi P, Tesei C,
Brunetti S, Pulsoni A, Annibali O, Gasparoli C, Battistini R,
Hohaus S, Pelliccia S, et al: Effects of ABVD chemotherapy on
ovarian function: Epidemiology, hormonal dosages and ultrasound
morphologic analyses in 270 patients with Hodgkin's disease. Front
Oncol. 13(1059393)2023.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Armitage JO, Gascoyne RD, Lunning MA and
Cavalli F: Non-Hodgkin lymphoma. Lancet. 390:298–310.
2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Jiang L and Li N: B-cell non-Hodgkin
lymphoma: Importance of angiogenesis and antiangiogenic therapy.
Angiogenesis. 23:515–529. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Gequelin LCF, Riediger IN, Nakatani SM,
Biondo AW and Bonfim CM: Epstein-Barr virus: General factors,
virus-related diseases and measurement of viral load after
transplant. Rev Bras Hematol Hemoter. 33:383–388. 2011.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Câmara AB and Brandão IA: The non-Hodgkin
lymphoma treatment and side effects: A systematic review and
meta-analysis. Recent Pat Anticancer Drug Discov: Jan 17, 2023
(Epub ahead of print).
|
|
54
|
Mohseni S, Shojaiefard A, Khorgami Z,
Alinejad S, Ghorbani A and Ghafouri A: Peripheral lymphadenopathy:
Approach and diagnostic tools. Iran J Med Sci. 39 (2
Suppl):S158–S170. 2014.PubMed/NCBI
|
|
55
|
Pirosa MC and Peccatori FA: Lymphomas in
pregnancy. Hematol Oncol. 41 (Suppl 1):S70–S74. 2023.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Dunleavy K and McLintock C: How I treat
lymphoma in pregnancy. Blood. 136:2118–2124. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Evens AM, Advani R, Press OW, Lossos IS,
Vose JM, Hernandez-Ilizaliturri FJ, Robinson BK, Otis S, Nadav
Dagan L, Abdallah R, et al: Lymphoma occurring during pregnancy:
Antenatal therapy, complications, and maternal survival in a
multicenter analysis. J Clin Oncol. 31:4132–4139. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Nooh MM, Rizk SM, Saied NM and Abdelazim
SM: Carnosine remedial effect on fertility of male rats receiving
cyclophosphamide, hydroxydaunomycin, oncovin and prednisone (CHOP).
Andrologia. 53(e14233)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Antoine-Pepeljugoski C and Braunstein MJ:
Management of newly diagnosed elderly multiple myeloma patients.
Curr Oncol Rep. 21(64)2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ribourtout B and Zandecki M: Plasma cell
morphology in multiple myeloma and related disorders. Morphologie.
99:38–62. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Michels TC and Petersen KE: Multiple
myeloma: Diagnosis and treatment. Am Fam Physician. 95:373–383.
2017.PubMed/NCBI
|
|
62
|
Landgren O, Gridley G, Turesson I,
Caporaso NE, Goldin LR, Baris D, Fears TR, Hoover RN and Linet MS:
Risk of monoclonal gammopathy of undetermined significance (MGUS)
and subsequent multiple myeloma among African American and white
veterans in the United States. Blood. 107:904–906. 2006.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kyle RA and Rajkumar SV: Multiple myeloma.
N Engl J Med. 351:1860–1873. 2004.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Kyle RA and Rajkumar SV: Multiple myeloma.
Blood. 111:2962–2972. 2008.PubMed/NCBI View Article : Google Scholar
|
|
65
|
International Myeloma Working Group.
Criteria for the classification of monoclonal gammopathies,
multiple myeloma and related disorders: A report of the
international myeloma working group. Br J Haematol. 121:749–757.
2003.PubMed/NCBI
|
|
66
|
Kristinsson SY, Minter AR, Korde N, Tan E
and Landgren O: Bone disease in multiple myeloma and precursor
disease: Novel diagnostic approaches and implications on clinical
management. Expert Rev Mol Diagn. 11:593–603. 2011.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Regelink JC, Minnema MC, Terpos E,
Kamphuis MH, Raijmakers PG, Pieters-van den Bos IC, Heggelman BGF,
Nievelstein RJ, Otten RHJ, van Lammeren-Venema D, et al: Comparison
of modern and conventional imaging techniques in establishing
multiple myeloma-related bone disease: A systematic review. Br J
Haematol. 162:50–61. 2013.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Rajkumar SV: MGUS and smoldering multiple
myeloma: Update on pathogenesis, natural history, and management.
Hematology Am Soc Hematol Educ Program. 2005:340–345.
2005.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Greipp PR, San Miguel J, Durie BG, Crowley
JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H,
Kyle RA, et al: International staging system for multiple myeloma.
J Clin Oncol. 23:3412–3420. 2005.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Elgabry G, Spencer L, Siddiqi H, Ojha S
and Wandroo F: A report of a symptomatic progressive myeloma during
pregnancy and postpartum period from asymptomatic state. Hematol
Rep. 15:305–311. 2023.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Rizack T, Mega A, Legare R and Castillo J:
Management of hematological malignancies during pregnancy. Am J
Hematol. 84:830–841. 2009.PubMed/NCBI View Article : Google Scholar
|
|
72
|
de Montalembert M, Ferster A, Colombatti
R, Rees DC and Gulbis B: European Network for Rare and Congenital
Anaemias. ENERCA clinical recommendations for disease management
and prevention of complications of sickle cell disease in children.
Am J Hematol. 86:72–75. 2011.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Kinoshita S, Yamashita K, Iwatsuki M, Sato
H, Matsumoto C, Matsumoto T, Shiraishi Y, Yoshida N and Baba H:
Disseminated carcinomatosis of the bone marrow from gastric cancer
during pregnancy. Clin J Gastroenterol. 12:447–452. 2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Sakamoto K, Kanda T, Ohashi M, Kurabayashi
T, Serikawa T, Matsunaga M and Hatakeyama K: Management of patients
with pregnancy-associated gastric cancer in Japan: A mini-review.
Int J Clin Oncol. 14:392–396. 2009.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Song MJ, Park YS, Song HJ, Park SJ, Ahn
JY, Choi KD, Lee GH, Jung HY, Yook JH and Kim BS: Prognosis of
pregnancy-associated gastric cancer: An age-, sex-, and
stage-matched case-control study. Gut Liver. 10:731–738.
2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Milojkovic D and Apperley JF: How I treat
leukemia during pregnancy. Blood. 123:974–984. 2014.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Krashin E and Lishner M: Managing leukemia
during pregnancy. In: Azim H Jr (ed). Managing Cancer during
Pregnancy. Springer, Cham, pp175-184, 2016.
|
|
78
|
Van Calsteren K, Verbesselt R, Beijnen J,
Devlieger R, De Catte L, Chai DC, Van Bree R, Heyns L, de Hoon J
and Amant F: Transplacental transfer of anthracyclines,
vinblastine, and 4-hydroxy-cyclophosphamide in a baboon model.
Gynecol Oncol. 119:594–600. 2010.PubMed/NCBI View Article : Google Scholar
|