1. Neutrophils' dual role in lymphoma: A
paradigm shift
Contemporary research is redefining the traditional
view of neutrophils only as tumor supporters. Current studies
underscore their nuanced participation in the immune response to
lymphoma, going beyond conventional associations with inflammation
and infection defense (1).
Recognizing this complexity is pivotal for pioneering novel
treatments to enhance patient outcomes (2).
Defining neutrophils: Guardians of the
immune frontline
Neutrophils, a subtype of white blood cells, are
vital contributors to the immune system, acting as frontline
defenders against infections and injuries (3). Their fundamental role centers around
engulfing and destroying harmful bacteria and other pathogens,
making them indispensable components of the body's initial defense
mechanism against foreign invaders (4). As key participants in the innate
immune response, these cells are generated in the bone marrow and
exist in abundant numbers in the bloodstream and various tissues
throughout the body (5).
Neutrophils also play a pivotal role in the inflammatory process,
which is the body's orchestrated response to tissue damage or
infection (6,7).
While the intricate functions of neutrophils in
immune surveillance and response have been extensively studied,
their precise involvement in lymphoma, a cancer that affects the
immune system, remains enigmatic. However, research studies are
shedding light on the potential roles of neutrophils in lymphoma
progression (8-10).
While traditionally recognized for their role in combating
infections, neutrophils are now being examined to reveal their
possible contributions or influences in the intricate landscape of
lymphoma development (11,12).
Understanding the nuanced interactions between
neutrophils and lymphoma cells may be key to unraveling the
mysteries surrounding lymphoma progression (13). Further insights may emerge as
ongoing research endeavors delve into the complex interplay between
these immune cells and lymphoma (9), opening new therapeutic avenues and
advancing our comprehension of the intricate dynamics within the
immune system.
Lymphoma unveiled: Categories,
symptoms and diagnosis
Lymphoma, a formidable type of cancer, takes its
toll on the lymphatic system, a crucial component responsible for
supporting the body's immune response against infections (14). This malignancy develops when white
blood cells, specifically lymphocytes and plasma cells, undergo a
chaotic process of uncontrolled growth, culminating in the
formation of tumors (15).
Lymphoma presents in two primary forms: Hodgkin lymphoma,
distinguished by the presence of Reed-Sternberg cells, and
non-Hodgkin lymphoma, a diverse array of subtypes, each requiring
tailored treatment (16).
Recognizing the onset of lymphoma involves an
awareness of its symptoms, which may manifest as swollen lymph
nodes, fatigue, persistent fever, night sweats and unexplained
weight loss (17). The diagnostic
process, integral to effective intervention, typically entails a
comprehensive combination of blood tests, imaging scans and biopsy
procedures targeting affected tissue. The treatment approach is
multifaceted, comprising chemotherapy, radiation therapy, targeted
therapies and stem cell transplantation (18).
This complex nature of lymphoma and its diverse
manifestations and treatment modalities underscores the complexity
of tackling this challenging disease. As research continues to
delve into the intricate details of lymphoma subtypes and their
unique characteristics, innovative treatments and therapeutic
strategies are poised to emerge, promising improved outcomes and
enhanced quality of life for those affected by this challenging
disease (19,20).
Navigating neutrophils in lymphoma:
Current understanding and ongoing research
As the most abundant white blood cells in the human
body, neutrophils traditionally stand at the forefront of the
immune system, undertaking a critical role in the defense against
infections (21). However, the
narrative surrounding neutrophils is evolving, with recent studies
illuminating an unexpected dimension to their function-one that
extends beyond infection control to encompass a substantial role in
lymphoma development and progression (22).
Of note, neutrophils are not mere bystanders in
lymphoma; they actively engage with lymphoma cells, influencing the
complex milieu known as the tumor microenvironment (TME) (21,22).
This influence has multiple facets, with neutrophils acting as
architects of inflammation and wielders of immune response
suppression within the TME (23,24).
The consequences of these interactions extend beyond the immediate
cellular level, potentially contributing to the overall progression
of lymphoma (25).
Despite the pivotal insights from recent studies,
the precise mechanisms orchestrating these interactions between
neutrophils and lymphoma cells remain enigmatic. The research
community is presently engaged in a dynamic pursuit to unravel the
intricate relationship between neutrophils and lymphoma (26). This ongoing exploration is poised
to uncover the molecular intricacies and signaling pathways that
govern this interplay, potentially unlocking novel avenues for
therapeutic intervention and refining our understanding of the
complex dynamics involved.
As the scientific community delves deeper into the
multifaceted roles of neutrophils in lymphoma, the evolving
research landscape holds promise for a paradigm shift in our
comprehension of lymphoma (27,28).
This review serves as a snapshot of the current understanding,
recognizing that the journey of discovery is far from completed,
with ongoing research poised to shape the future landscape of
lymphoma diagnostics and therapeutics.
The neutrophil-to-lymphocyte ratio
(NLR) in lymphoma prognosis
In recent studies, the NLR has garnered attention as
a critical prognostic indicator (29). The NLR is calculated by dividing
the number of neutrophils by the number of lymphocytes in the
blood, and it has been shown to hold significant prognostic value
across various cancer types, including lymphoma.
Clinical significance of the NLR. An elevated
NLR is generally associated with a poor prognosis. In patients with
lymphoma, a higher NLR may reflect a heightened inflammatory
response, which is often associated with tumor progression and
their overall immune status (30).
High NLRs may indicate a more substantial tumor burden and a worse
therapeutic response (31).
Specifically, a high NLR may indicate several key factors. Firstly,
the inflammatory state of the TME, often indicated by an increase
in neutrophils, can promote tumor cell growth and metastasis.
Secondly, a suppressed immune state, often reflected by a high NLR
indicating a relative decrease in lymphocytes, can weaken the
immune response against the tumor since lymphocytes, particularly T
cells, are crucial for anti-tumor immunity. Thirdly, the
therapeutic response, often associated with a high NLR, which some
studies suggest is linked to a worse response to treatments such as
chemotherapy or radiotherapy in patients with lymphoma, affects
their overall survival (30,31).
Application of the NLR as a prognostic
indicator. Given these reasons, the NLR can serve as a simple
and effective prognostic indicator to assist clinicians in risk
assessment during treatment planning. For instance, at the initial
diagnosis stage, measuring the NLR can provide a preliminary
prognosis and help tailor treatment strategies. During treatment,
monitoring changes in the NLR can provide insights into treatment
efficacy, allowing timely adjustments to therapeutic approaches. In
addition, the simplicity of measuring the NLR, which requires no
complex equipment or technical support, adds to its clinical
utility (32,33).
In conclusion, as an important prognostic indicator,
the NLR holds significant potential in lymphoma. Future research
should further explore the specific mechanisms of the NLR in
different types and stages of lymphoma to validate its reliability
and effectiveness as a prognostic tool.
By redefining the role of neutrophils in lymphoma, a
comprehensive understanding of their multifaceted functions within
the TME may be gained. Neutrophils are not only simple immune cells
but are actively involved in tumor initiation and progression.
Understanding these complex functions is crucial for developing
novel therapeutic strategies.
Investigation of the relationship
between neutrophils and lymphoma
Research on the relationship between neutrophils and
lymphoma has a long history, evolving from early fundamental
discoveries to current, in-depth explorations of their mechanisms,
gradually revealing their complex roles in tumorigenesis and
progression.
Early discoveries. In the mid-20th century,
researchers first observed the abnormal accumulation of neutrophils
in tumor tissues, sparking interest in the role of neutrophils in
tumor biology. Early studies focused on neutrophils' inflammatory
response and their role within the TME. Histological analyses
showed high densities of neutrophils surrounding and infiltrating
tumors, suggesting their involvement in the immune response to
tumors (34).
Progress in the late-20th century. During the
1980s and 1990s, significant advancements were made in
understanding the relationship between neutrophils and tumors.
Researchers found that neutrophils were not only inflammatory cells
but could directly influence tumor cell growth and metastasis by
secreting various cytokines and growth factors. For instance,
studies showed that neutrophils could promote tumor angiogenesis by
secreting vascular endothelial growth factors, providing nutrients
and oxygen to tumor cells. Neutrophils were also found to
facilitate tumor cell invasion and metastasis by releasing matrix
metalloproteinases (35,36).
Modern research focus. At the start of the
21st century, advances in molecular biology techniques have
deepened our understanding of neutrophils' roles within the TME.
Modern research has revealed that neutrophils not only support
tumor growth but also participate in immune suppression and tumor
immune evasion. Specific mechanisms include neutrophils suppressing
T cell anti-tumor activity by secreting inhibitory cytokines such
as interleukin (IL)-10 and chemokines such as C-X-C motif chemokine
ligand 8, aiding tumor cells in evading immune surveillance
(37-40).
The recent discovery of neutrophil extracellular
traps (NETs) added another layer of complexity to neutrophils'
roles in cancer. Initially identified as structures for trapping
and killing pathogens, NETs were found to promote tumor cell
migration and metastasis, providing new insights into neutrophils'
complex functions in cancer (41).
Clinical research and applications. As basic
research progressed, the potential of neutrophils in cancer therapy
began to emerge. Clinical studies indicated that modulating
neutrophil functions could significantly impact tumor progression
and treatment outcomes. For instance, certain drugs targeting
neutrophils are being evaluated in clinical trials to inhibit their
tumor-promoting activities or enhance their anti-tumor functions to
improve patient prognosis (42,43).
In summary, research on the relationship between
neutrophils and lymphoma reflects continuous efforts to explore
this complex field. From early discoveries to modern mechanistic
studies, the evolving understanding of neutrophils in the TME
underscores their multifaceted roles. These historical data not
only provide essential background information for current research
but also guide future studies. By comprehensively understanding
neutrophils' roles in lymphoma, it may be possible to develop more
effective therapeutic strategies and improve patient survival and
quality of life.
Reviewing the historical progress in the research on
neutrophils and lymphoma may improve the understanding of the
significance and complexity of this field. Future studies should
continue to explore neutrophils' specific roles within the TME to
provide a solid foundation for developing new therapeutic
approaches.
2. Neutrophils and lymphoma pathogenesis: A
complex interplay
The intricate interplay between neutrophils and
lymphoma pathogenesis presents as a complex relationship with both
promotional and inhibitory influences on the disease trajectory
(27,44). Neutrophils, traditionally regarded
as immune warriors, can induce DNA damage in lymphoma cells,
potentially triggering apoptosis and providing a therapeutic avenue
for impeding uncontrolled growth (26,45).
Conversely, their secretion of pro-inflammatory cytokines
paradoxically enhances lymphoma growth and contributes to tumor
angiogenesis (27,46). The real-world manifestation of
neutrophil influence through tissue infiltration is a potential
biomarker and target for therapeutic intervention, indicating
active involvement in tumorigenesis (47). Despite significant findings, the
role of neutrophils in lymphoma pathogenesis is still incompletely
understood and further investigations into the underlying
mechanisms are required. The ongoing journey of exploration holds
the promise of revealing novel therapeutic strategies and refining
approaches to lymphoma treatment, advancing our comprehension of
the complexities of neutrophil involvement and lymphoma
pathogenesis.
Inflammation: Balancing act in the
immune response
As a fundamental biological response to injury or
infection, inflammation involves intricate molecular reactions
orchestrated by neutrophils, key contributors to the immune
system's response (48,49). While acute inflammation is a
transient and protective defense, chronic inflammation,
characterized by prolonged neutrophil engagement, may lead to
sustained tissue damage and contribute to diseases such as cancer,
including lymphoma (50). Research
has revealed that neutrophils actively promote tumor growth and
ensure cancer cell survival in lymphoma by releasing cytokines and
chemokines (34,51). The complex interplay among
neutrophils, inflammation and lymphoma goes beyond conventional
immune responses, providing crucial insights into lymphoma's
pathophysiology and potential therapeutic targets (52). Delving deeper into the molecular
intricacies of neutrophil-mediated inflammation offers a tangible
prospect of unveiling novel therapeutic avenues, signifying a
crucial step in translating scientific understanding into practical
applications that could revolutionize the treatment landscape for
inflammatory diseases, including lymphoma.
Types of inflammation. Inflammation, a
fundamental and natural process, is the body's orchestrated
response to tissue injury or infection. This dynamic phenomenon is
categorized into two primary types, acute and chronic, each
characterized by distinct temporal and molecular features (53,54).
Acute inflammation is characterized by its rapid
onset and short-lived nature, unfolding over seconds to minutes.
This immediate response involves the release of chemical mediators,
including histamine and cytokines, triggering a cascade of events
aimed at restoring tissue homeostasis (55,56).
A hallmark of acute inflammation is the swift migration of
neutrophils to the site of injury or infection (52,57).
These white blood cells play a crucial role in the immune response,
phagocytizing and destroying invading microorganisms, thereby
preventing the spread of infection (58).
In contrast, chronic inflammation represents a
protracted and enduring response, persisting over weeks, months or
even years (59). This sustained
reaction involves the infiltration of immune cells, such as
macrophages and lymphocytes, into the affected tissue (49). While these immune cells are
essential components of the body's defense mechanism, their
prolonged presence in chronic inflammation can lead to tissue
damage. Notably, chronic inflammation is implicated in the
development of various diseases, including cancer (46,50).
Within the cancer context, chronic inflammation
creates an environment conducive to the initiation and progression
of malignant processes (46,50).
The infiltrating immune cells release pro-inflammatory signals,
contributing to the formation of a TME that supports the survival
and growth of cancer cells (60).
Understanding the intricate dynamics of acute and chronic
inflammation is crucial not only for deciphering the body's
response to injury and infection but also for unraveling the
complexities underlying the development of diseases, particularly
those with an inflammatory component, such as cancer.
Cytokine signaling during inflammation. In
the intricate landscape of inflammation, cytokines emerge as
pivotal orchestrators, playing a crucial role in the complex
signaling processes that facilitate communication between cells
(61,62). Cytokines are small proteins
secreted by various cell types, particularly immune cells, in
response to infection or injury. Their release, a dynamic and
tightly regulated process, serves as a molecular communication
network that coordinates the immune response and contributes
significantly to the inflammatory milieu (62,63).
During inflammation, neutrophils and other immune
cells release cytokines as part of their concerted effort to
activate and recruit additional immune cells to the site of injury
or infection (64,65). This orchestrated recruitment is
fundamental to the inflammatory response, where immune cells
collaborate to neutralize threats and restore tissue homeostasis
(65). The dysregulation of
cytokine signaling has been implicated in lymphoma development,
with their uncontrolled release fostering a TME conducive to tumor
growth and metastasis (50,66).
The significance of understanding the intricate role
of cytokine signaling during inflammation extends beyond lymphoma
and encompasses a broader spectrum of cancers (67). Unraveling the molecular intricacies
of cytokine-mediated signaling pathways not only sheds light on the
pathophysiology of cancer but also holds the promise of identifying
novel therapeutic targets (68,69).
Targeting cytokine signaling pathways offers a potential avenue for
intervention, aiming to mitigate the pro-tumorigenic effects
associated with uncontrolled cytokine release (70).
In summary, exploring cytokine signaling during
inflammation is at the frontier of cancer research, providing a
foundation for developing innovative and targeted treatments. As
our comprehension of these molecular pathways advances, so too does
the potential for transformative therapeutic strategies in the
fight against lymphoma and other malignancies.
TME: Neutrophils' symphony in lymphoma
progression
The TME is a complex ecosystem surrounding tumors,
comprising various cell types such as immune cells, stromal cells
and blood vessels (71).
Traditionally known for combatting infections, neutrophils have
gained attention for their active role in the TME, influencing
molecular and cellular interactions that shape cancer progression,
particularly in lymphoma development (51). Understanding neutrophils'
contribution to the intricate interplay within the TME is crucial
for unraveling the intricacies of lymphoma. Beyond comprehension,
this knowledge paves the way for innovative therapeutic strategies.
By deciphering neutrophil interactions and their impact on the
broader TME, researchers may identify novel targets for
intervention, offering potential avenues to disrupt pro-tumorigenic
influences and enhance treatment outcomes. Exploring neutrophils in
the TME represents a frontier in cancer research, providing a
deeper understanding of the molecular intricacies driving lymphoma
development, with the promise of transformative therapeutic
strategies to disrupt the TME's role in cancer progression.
Composition of the TME. The TME is a dynamic
and heterogeneous milieu comprising diverse cell types, including
cancer, immune and stromal cells, as well as extracellular matrix
(ECM) components (71). The
intricate interactions between these components are pivotal in
shaping tumor progression, influencing metastatic potential and
determining the response to therapy. Within this complex landscape,
neutrophils, a vital type of immune cell, have recently emerged as
key players in modulating the TME, particularly in lymphoma
(72,73).
Traditionally recognized for their role in the
innate immune response against infections, neutrophils are now
gaining recognition for their critical involvement in the TME of
lymphoma (72). The TME is a
dynamic environment in which various cell populations interact and
communicate, influencing the behavior of cancer cells (73). Understanding the nuanced
interactions between neutrophils and other cell types within the
TME is pivotal for unraveling the complexities of lymphoma
progression.
Research has shown neutrophils can significantly
impact the TME by releasing signaling molecules and enzymes
(74,75). These mediators help create an
inflammatory TME, either supporting or inhibiting tumor growth. The
specific mechanisms by which neutrophils modulate the TME in
lymphoma are still being actively investigated, highlighting their
complexity.
Appreciating the dynamics of the TME and the role of
neutrophils within it may provide valuable insights into potential
therapeutic strategies for lymphoma and other cancers (76). Targeting the interplay between
neutrophils and the broader TME could offer new therapeutic avenues
to disrupt the supportive environment that drives cancer
progression (10).
In conclusion, the TME represents a complex
ecosystem in which the orchestration of various cell types
influences tumor behavior. Neutrophils, once considered primarily
first responders to infections, are now recognized as influential
contributors to the TME in lymphoma. Deciphering the intricacies of
these interactions opens up new possibilities for therapeutic
interventions, offering hope for more effective strategies to
combat lymphoma and improve patient outcomes.
Role of neutrophils in the TME. Classically
acknowledged for their crucial role in combating infections,
neutrophils are now recognized as key contributors to the TME in
lymphoma. Recent studies have revealed their substantial impact on
TME dynamics, shedding light on their involvement in tumor growth
and metastasis (77).
One notable aspect of neutrophil-mediated influence
on the TME is their ability to release specific signaling molecules
and enzymes (9). These mediators
contribute to establishing an inflammatory milieu within the tumor,
fostering a TME conducive to tumor progression. The intricate
molecular interactions orchestrated by neutrophils are pivotal in
shaping the landscape of the TME, influencing the behavior of
cancer cells.
Furthermore, the interactions between neutrophils
and other cell types within the TME further accentuate their
significance (78,79). Neutrophils engage in crosstalk with
tumor and immune cells, creating a communication network that can
amplify the support for tumor growth and progression. Understanding
the complexities of these interactions is essential for deciphering
the mechanisms underlying lymphoma development.
Collectively, the findings from these studies
suggest that targeting neutrophils in the TME is a promising
potential therapeutic avenue for improving the outcomes of patients
with lymphoma. Novel therapeutic strategies may emerge that disrupt
the pro-tumorigenic effects orchestrated by neutrophils. However,
it is imperative to delve deeper into the molecular mechanisms and
signaling pathways involved in the interplay between neutrophils
and the TME to develop targeted interventions that effectively
impede lymphoma progression.
In conclusion, the role of neutrophils in the
lymphoma TME extends beyond their classical function in infection
defense. Their influence on signaling pathways and interactions
within the TME underscores their significance as potential
therapeutic targets. Unraveling the intricacies of
neutrophil-mediated effects on the TME holds promise for devising
innovative strategies to improve the outcomes for patients with
lymphoma.
Molecular mechanisms regulating
neutrophil behavior
Understanding the complex roles of neutrophils in
lymphoma requires thoroughly exploring the molecular mechanisms
that govern their behavior. These mechanisms include signaling
pathways, transcriptional regulation and epigenetic modifications,
all of which collectively determine neutrophil function within the
TME (Fig. 1).
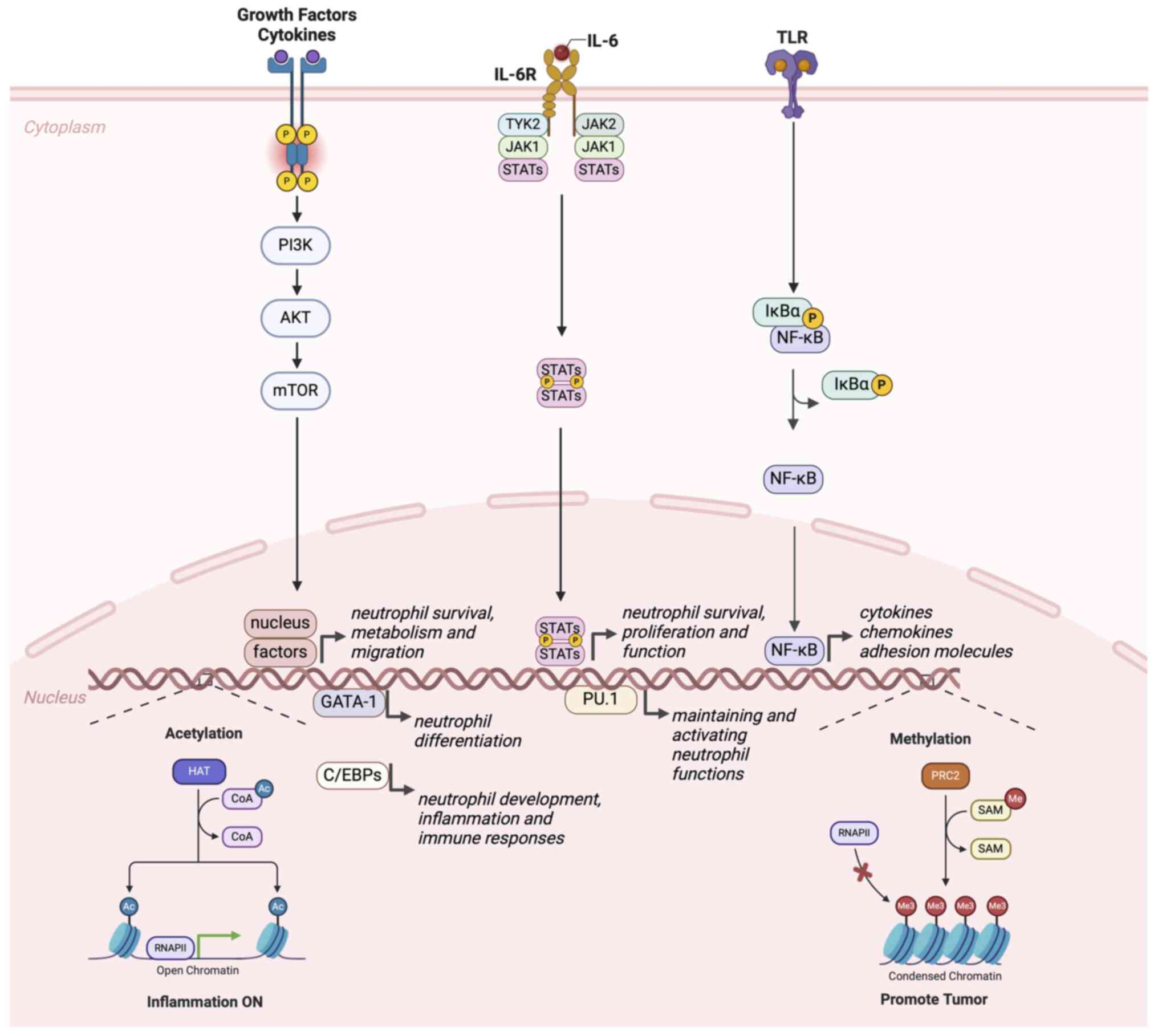 | Figure 1Molecular mechanisms regulating
neutrophil behavior in lymphoma. Schematic diagram illustrating the
NF-κB, JAK/STAT and PI3K/AKT signaling pathways, key transcription
factors and epigenetic modification processes involved in
regulating neutrophil behavior within the lymphoma
microenvironment. NF-κB, nuclear factor Κ-light-chain-enhancer of
activated B cells; IκBα, Inhibitor of κBα; PRC2, polycomb
repressive complex 2; SAM, S-adenosylmethionine; Me, methyl group;
RNAPII, RNA polymerase II; HAT, histone acetyltransferase; CoA,
coenzyme A; PI3K, phosphoinositide 3-kinase; AKT, protein kinase B;
mTOR, mechanistic target of rapamycin; TLR, Toll-like receptor;
STATs, signal transducers and activators of transcription; IL-6R,
interleukin-6 receptor; JAK1, Janus kinase 1; TYK2, tyrosine kinase
2; GATA-1, GATA binding protein 1; PU.1, Spi-1 proto-oncogene. |
Signaling pathways. Several critical
signaling pathways regulate neutrophil activation and function in
lymphoma. The nuclear factor kappa B (NF-κB) pathway is one of the
most crucial in mediating inflammatory and immune responses in
lymphogenesis with neutrophil participation. NF-κB is a
transcription factor activated in response to infection or
inflammation, translocating to the nucleus to initiate the
expression of multiple inflammatory genes, including cytokines,
chemokines and adhesion molecules. These genes are essential for
the inflammatory response and neutrophil recruitment (80-82).
This pathway is pivotal in maintaining the pro-tumorigenic
environment often seen in lymphoma.
Another vital pathway is the Janus kinase
(JAK)/signal transducer and activator of transcription (STAT)
pathway. This pathway is activated through cytokine receptors, such
as the IL-6 receptor, promoting the phosphorylation and activation
of STAT proteins, which then enter the nucleus to regulate gene
expression. The JAK/STAT pathway is critical for neutrophil
survival, proliferation and function within the lymphoma TME
(82-84).
This pathway is integral to the role of neutrophils in supporting
lymphoma progression.
The phosphoinositide 3-kinase (PI3K)/protein kinase
B (AKT) pathway also plays a significant role in regulating
neutrophil behavior in lymphogenesis, influencing cell survival,
metabolism and migration. For instance, activating the PI3K/AKT
pathway can enhance neutrophil migration and their release of
inflammatory mediators, contributing to the maintenance of a TME
conducive to lymphogenesis (85-87).
This signaling cascade underscores the dual role of neutrophils in
promoting inflammation and facilitating tumor growth.
Transcriptional regulation. In lymphoma,
transcription factors are essential for regulating neutrophil gene
expression. Beyond NF-κB and STAT transcription factor families,
the CCAAT/enhancer-binding protein (C/EBP) family plays a crucial
role in neutrophil differentiation and function. C/EBP alpha
(CEBPA) and beta (CEBPB) are key regulators of neutrophil
development and control the expression of numerous genes involved
in inflammation and immune responses within the lymphoma TME
(88-90).
GATA binding protein 1 (GATA1) and Spi-1
proto-oncogene (SPI1/PU.1) are two other significant transcription
factors with complementary roles in neutrophil development and
function. GATA1 primarily promotes neutrophil differentiation,
while PU.1 is crucial for maintaining and activating neutrophil
functions within the lymphoma TME. The interactions and regulatory
networks of these transcription factors constitute a complex
regulatory mechanism for neutrophil gene expression that drives
neutrophil behavior in lymphoma, highlighting their involvement in
both tumor-promoting and anti-tumor activities (91-93).
Epigenetic modifications. Epigenetic
modifications, including DNA methylation and histone modifications,
play vital roles in regulating neutrophil function in lymphoma. DNA
methylation is generally associated with gene silencing, while
histone acetylation and methylation can activate or repress gene
expression related to lymphogenesis.
In lymphoma, the epigenetic state of neutrophils can
significantly impact their inflammatory responses and interactions
within the TME. For instance, histone deacetylase inhibitors can
enhance neutrophils' inflammatory response, potentially
exacerbating lymphoma-promoting activities, by increasing histone
acetylation levels. Conversely, DNA methyltransferase inhibitors
can modulate neutrophil gene expression, influencing their role in
either supporting or suppressing lymphoma progression (94-96).
The multifaceted roles of neutrophils in lymphoma
pathogenesis are complex and critical. Investigating their
molecular mechanisms can enhance the understanding of their
functions in the TME, offering valuable insights for developing
targeted therapeutic approaches.
3. Neutrophils and lymphoma progression:
Unveiling the culprits
Traditionally seen as immune defenders against
infections, neutrophils have been revealed to have a dual role,
implicated in the progression of lymphoma, a cancer affecting the
immune system (9). Recent research
has revealed their active contribution to tumor progression,
promoting tumor growth through intricate molecular interactions in
the TME. Neutrophils promote angiogenesis, sustaining lymphoma
cells by creating a vascular network (97). Their immunosuppressive role aids
cancer cell evasion from surveillance mechanisms, contributing to
unchecked lymphoma growth. Collaborative interactions with other
immune cells within the TME further enhance cancer progression
(98). Recognizing these diverse
contributions opens avenues for novel therapeutic strategies, with
targeting neutrophils as a promising approach to impede lymphoma
progression. However, a comprehensive understanding of the specific
molecular mechanisms is crucial for developing effective and
targeted therapies. The emerging understanding of neutrophils in
lymphoma progression necessitates further research to unravel the
specific mechanisms and holds promise for reshaping the landscape
of lymphoma treatment.
Neutrophils and tumor growth: A
complex interplay
Traditionally merely considered defenders against
infections, neutrophils are now known to play a key role in tumor
biology, having a dual impact on tumor growth (13). Recent studies suggest their
potential to impede tumor growth, adding a counterintuitive aspect
to their conventional function (99). However, their pro-metastatic and
tumor-promoting capabilities create a dualistic nature in the TME.
Neutrophils contribute to angiogenesis, forming new blood vessels
crucial for tumor sustenance (10). They also orchestrate a
pro-inflammatory milieu by releasing cytokines, fostering
conditions for tumor progression and metastasis, which is crucial
in aggressive cancers such as lymphoma. Furthermore, neutrophils
suppress the immune system, facilitating immune evasion by tumor
cells. The intricate interplay between neutrophils and various
immune cells within the TME adds complexity, emphasizing the need
for a nuanced understanding. Unraveling this interplay is
imperative for advancing cancer therapies, particularly for
lymphoma. As research progresses, targeting neutrophil-tumor
interactions may offer a promising avenue for innovative and
effective cancer therapies.
Neutrophils and tumor initiation. Neutrophils
have recently come under greater scrutiny in tumor biology.
Contrary to their conventional role, emerging research suggests
that neutrophils may have a dual role, potentially contributing to
tumor initiation and growth (78),
thus opening new avenues for exploring the challenging landscape of
cancers such as lymphoma.
One aspect of neutrophil involvement in tumor
biology centers around their ability to produce reactive oxygen
species and other inflammatory factors (24). When released by neutrophils, these
molecular entities can damage cellular DNA. In the tumor context,
this DNA damage can stimulate aberrant cellular responses,
promoting the survival and uncontrolled proliferation of tumor
cells (24). This revelation
underscores the complex interplay between neutrophils and the
genomic integrity of cells within the TME, providing a compelling
rationale for investigating their role in initiating malignancies
such as lymphoma.
Furthermore, neutrophils contribute to the formation
of blood vessels through a process known as angiogenesis. By
promoting the growth of new blood vessels, neutrophils facilitate
the supply of oxygen and nutrients to the growing tumor, creating a
TME supportive of sustained tumor growth (97). In lymphoma research, understanding
how neutrophils influence angiogenesis within lymphomatous tissues
remains an active area of investigation. Insights into this process
may be vital to deciphering the mechanisms underlying lymphoma
progression and could potentially inform the development of
targeted therapeutic interventions.
While the precise nature of neutrophil involvement
in lymphoma is still under investigation, ongoing research is
unraveling the intricacies of their interactions with lymphoma
cells (79). Determining the role
of neutrophils in tumor initiation and progression, particularly in
the context of lymphoma, is a dynamic field that presents promising
opportunities for developing innovative treatments.
Understanding these complex interactions provides a
foundation for devising novel therapeutic strategies tailored to
the unique challenges posed by lymphoma. By deciphering the
intricate language of neutrophil-tumor crosstalk, researchers aim
to unveil potential vulnerabilities that can be exploited to
develop targeted therapies (99).
As research progresses, the evolving narrative of neutrophils in
tumor biology holds the promise of not only enhancing our
understanding of cancer initiation and progression but also paving
the way for more effective and tailored treatments for
lymphoma.
Neutrophils and tumor promotion. Long
heralded as guardians of the immune system, neutrophils are central
players in the intricate process of cancer progression (13). While their conventional role
involves combating cancer cells, recent research has revealed a
paradoxical dimension wherein neutrophils can, under certain
conditions, promote tumor growth and metastasis. This revelation
underscores the complexity of the immune response within the TME
and necessitates a nuanced understanding to develop effective
strategies for countering their pro-tumor effects and ultimately
improving cancer outcomes.
In the context of tumor promotion, inflammation
emerges as a pivotal player in orchestrating the behavior of
neutrophils within the TME (10).
The chronic inflammatory milieu in cancer tissues stimulates
neutrophils to release diverse pro-tumor factors that exert
multifaceted effects, enhancing angiogenesis, suppressing immune
cell activity, and fostering the survival and migration of cancer
cells. The net result is the creation of a TME that drives tumor
growth and facilitates metastasis.
Angiogenesis, the formation of new blood vessels, is
a critical process for sustaining tumor growth and metastasis
(97). In response to signals from
the inflamed TME, neutrophils release factors that promote
angiogenesis. These newly formed blood vessels provide the growing
tumor with a dedicated supply of oxygen and nutrients, supporting
its relentless growth. Understanding the intricate interplay
between neutrophils and angiogenesis is essential for developing
interventions that disrupt this supportive network and slow tumor
expansion.
Designed to recognize and eliminate aberrant cells,
the immune system paradoxically experiences suppression when
confronted with neutrophil-driven tumor promotion (98). Neutrophils release factors that
attenuate the activity of immune cells, creating an
immunosuppressive TME conducive to tumor immune evasion. Unraveling
the mechanisms underlying neutrophil-mediated immune suppression
holds promise for devising strategies that enhance the immune
response against cancer cells.
The survival and migration of cancer cells, crucial
determinants of metastasis, are also influenced by
neutrophil-derived factors (9,10,97).
By fostering a TME that shields cancer cells from immune
surveillance and promotes their mobility, neutrophils contribute
significantly to the metastatic process. Targeting these specific
aspects of neutrophil involvement in tumor promotion may offer
novel therapeutic avenues for impeding metastasis and improving
overall cancer outcomes.
In conclusion, while neutrophils are stalwart
defenders in the immune system's arsenal, their dynamic role in the
TME introduces complexities that demand careful consideration.
Understanding the dual nature of neutrophil behavior-as guardians
and inadvertent promoters of tumor growth-is pivotal for advancing
cancer research and therapeutic development. Harnessing this
understanding to modulate neutrophil behavior within the TME holds
promise for refining treatment strategies, ultimately improving
outcomes for patients with cancer.
Neutrophils and tumor metastasis. The
intricate interplay between neutrophils and tumor metastasis
involves molecular interactions that significantly shape cancer
progression (10,13,78).
Once considered foot soldiers in the body's immune defense,
neutrophils have emerged as pivotal orchestrators of the metastatic
process, controlling events that pave the way for cancer cells to
colonize distant organs.
Central to this process is the activation of
neutrophils by tumor cells, which sets off a cascade of events,
with neutrophils releasing various factors crucial in metastasis
(10,97,98).
One such factor is the promotion of blood vessel formation, a
process known as angiogenesis. Driven by signals from tumor cells,
neutrophils release pro-angiogenic factors that induce the
formation of new blood vessels. This neo-vascular network provides
an essential conduit for cancer cell migration, facilitating their
journey to distant organs.
Moreover, the interaction between neutrophils and
cancer cells goes beyond merely creating a structural support
system. Neutrophils actively help to suppress the immune response,
tipping the balance in favor of cancer cells (98). By releasing factors that inhibit
the immune system's vigilance, neutrophils mask cancer cells,
allowing them to evade immune surveillance and establish footholds
in new tissues.
The establishment of a favorable TME for cancer cell
growth and survival is another aspect of neutrophil involvement in
metastasis (99). By secreting
factors that create a nurturing milieu, neutrophils contribute to
the proliferation and survival of cancer cells in distant organs.
This supportive TME enhances the adaptability and resilience of
cancer cells, further driving metastatic progression.
Understanding the intricate mechanisms that govern
the interplay between neutrophils and tumor metastasis is paramount
in developing effective anti-cancer strategies (10). Targeting specific steps in this
process, such as neutrophil activation or their pro-angiogenic
activities, may provide novel therapeutic opportunities (98). Furthermore, unraveling the complex
network of molecular signals exchanged between neutrophils and
cancer cells may offer insight into disrupting the immune evasion
strategies used by metastatic cancer cells.
In conclusion, the role of neutrophils in tumor
metastasis extends beyond their conventional function in immune
defense. Instead, they emerge as active participants in the complex
process of cancer progression, influencing key events that underpin
metastasis. As research delves deeper into this intricate
relationship, new avenues for therapeutic interventions may emerge,
holding the promise of more effective strategies to prevent the
metastatic spread of cancer.
NETs: Unraveling a paradox
Initially celebrated for their role in immune
defense, the intricate web of NETs is now being scrutinized for its
potential involvement in cancer development, notably in lymphoma
(100,101). NETs comprise DNA, histones and
antimicrobial proteins. Initially recognized for their role in
combating infections, NETs are now known to be implicated in the
complex landscape of cancer pathogenesis (102,103). Their ability to promote
inflammation, particularly in lymphoma, suggests a role in creating
a TME conducive to tumor progression (104,105). NETs also contribute to forming
supportive niches for tumor growth and metastasis, acting as
scaffolding for cell migration (106,107). Understanding this interplay
offers avenues for therapeutic interventions, targeting NETs to
disrupt pro-inflammatory and pro-metastatic effects (108). Deciphering molecular signals
driving NET deployment may reveal potential biomarkers for disease
prognosis or therapeutic responsiveness. Exploring the dual nature
of NETs provides a fresh perspective on cancer dynamics,
particularly in lymphoma, with the potential to improve treatment
strategies and patient outcomes through targeted interventions.
Function of NETs. NETs are a dynamic and
crucial component of the body's immune response, having
contradictory roles that safeguard against pathogens and,
paradoxically, contribute to tissue damage and inflammation
(109). These intricate
structures, composed of chromatin and antimicrobial peptides, are
deployed in response to infection, inflammation or various stimuli
(110).
NETs are designed as a defense mechanism, acting as
a formidable barrier against invading pathogens (111). When faced with microbial threats,
neutrophils undergo a specialized form of cell death called
NETosis. During NETosis, the neutrophil releases its chromatin,
decorated with antimicrobial peptides, into the extracellular space
(109). The resulting web-like
structures effectively trap and immobilize pathogens, preventing
their further spread and facilitating their destruction. This
process is a crucial first line of defense against infections,
exemplifying the proactive nature of the immune system.
Beyond their role in pathogen containment, NETs are
also pivotal in modulating the immune response (112). The release of NETs activates
nearby immune cells, orchestrating a coordinated effort to
eliminate the threat. This immune activation is a finely tuned
response that involves the recruitment and activation of various
immune components to the site of infection or inflammation. In this
context, NETs act as signals that engage the immune system in a
targeted and localized manner.
However, the multifaceted nature of NETs becomes
apparent when considering their potential to contribute to tissue
damage and inflammation (113).
Under certain conditions, the release of NETs can exacerbate
inflammation, leading to damage to healthy tissues. This dual
role-protective against pathogens, yet potentially harmful to host
tissues-underscores the delicate balance within the immune
system.
In diseases such as cancer, including lymphoma, the
role of NETs takes on additional complexity (114). While NETs are traditionally
associated with microbial defense, emerging research suggests their
involvement in shaping the TME and influencing cancer progression.
The pro-inflammatory nature of NETs may contribute to chronic
inflammation, a known driver of cancer development (115). Furthermore, the ability of NETs
to create a TME conducive to tumor growth and metastasis adds
another layer of complexity to their role in cancer biology.
In conclusion, NETs represent a sophisticated arm of
the immune system, exhibiting a nuanced interplay between
protective and potentially detrimental effects. The intricate
interplay of NETs in the immune response reflects the adaptive and
responsive nature of the body's defense mechanisms. As our
understanding of NETs deepens, so too does the potential for
harnessing their power for therapeutic interventions, not only in
the context of infections but also in the intricate landscape of
cancer, including lymphoma.
Role of NETs in lymphoma progression. NETs
have emerged as intricate players in the complex landscape of
inflammation, with recent studies revealing their involvement in
the progression of lymphoma, a cancer originating in lymphocytes
(116). These web-like
structures, composed of chromatin and antimicrobial peptides,
contribute to various aspects of lymphoma development, including
cancer cell migration, invasion and metastasis, while fostering an
immunosuppressive TME (104)
(Fig. 2).
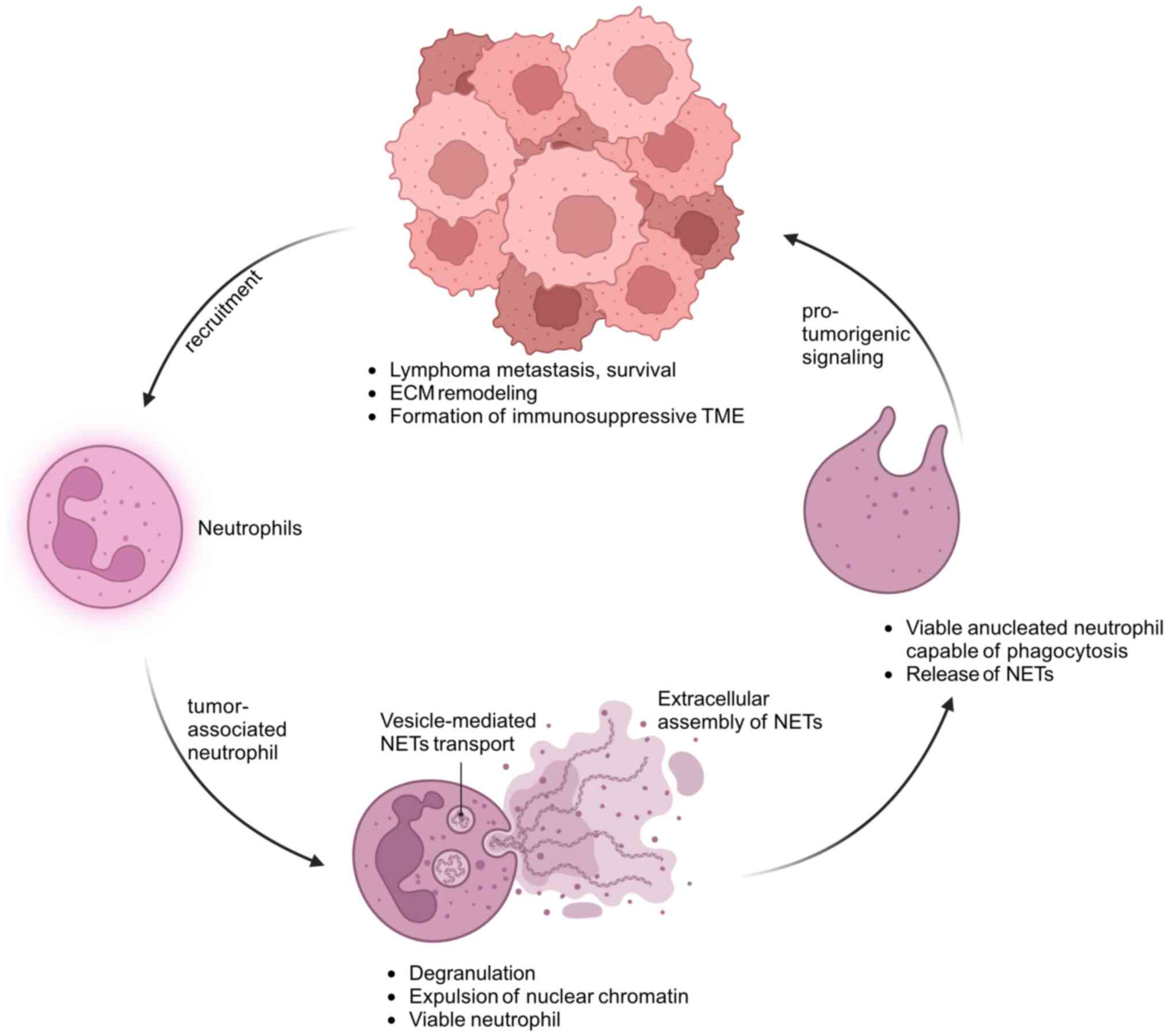 | Figure 2Role of tumor-associated neutrophils
and neutrophil extracellular traps in lymphoma progression.
Schematic diagram illustrating the interplay between neutrophils,
tumor-associated neutrophils and lymphoma cells, highlighting the
role of NETs in lymphoma progression. Lymphoma cells recruit
neutrophils from the bloodstream into the TME, where they
differentiate into tumor-associated neutrophils. Tumor-associated
neutrophils undergo vesicle-mediated NETs transport, releasing
chromatin into the extracellular space while remaining viable. NETs
are then extracellularly assembled, composed of DNA and
antimicrobial peptides. The NETs interact with lymphoma cells,
promoting tumorigenic signaling. These interactions contribute to
the survival and metastasis of lymphoma cells, ECM remodeling and
the formation of an immunosuppressive TME. The pro-tumorigenic
signaling from the NETs reinforces further recruitment of
neutrophils to the tumor site, perpetuating the cycle of tumor
progression. NTEs, neutrophil extracellular traps; TME, tumor
microenvironment; ECM, extracellular matrix. |
One key aspect of NET-mediated lymphoma progression
is their ability to influence cancer cell behavior (117). NETs have been implicated in
facilitating the migration and invasion of cancer cells, thereby
promoting the metastatic spread of lymphoma (104). This phenomenon is intricately
connected to the immunosuppressive characteristics of the TME
orchestrated by NETs.
The mechanism through which NETs drive lymphoma
progression involves the activation of inflammatory signaling
pathways and the remodeling of ECM proteins (114). Inflammatory signaling pathways
are crucial orchestrators of cancer development and NETs appear to
drive this process by activating pathways that sustain a
pro-tumorigenic TME (107).
Simultaneously, the remodeling of ECM proteins by NETs may create a
favorable niche for cancer cell survival, proliferation and
invasion, a critical determinant in the metastatic process.
Remarkably, the relationship between cancer cells
and neutrophils appears to be reciprocal, as cancer cells can
induce the production of NETs by neutrophils (101), creating a feedback loop that
amplifies the pro-tumorigenic effects of NETs, contributing to a
TME conducive to tumor growth (105). Understanding the intricate
interplay between cancer cells and neutrophils and the role of NETs
in this communication opens new avenues for therapeutic
exploration.
Modulating NET formation and function emerges as a
promising strategy to intervene in lymphoma progression and
potentially improve patient outcomes (116). Strategies aimed at targeting NETs
could involve developing drugs that inhibit NET formation or
neutralize the pro-tumorigenic factors released by these structures
(118). Gaining insights into the
specific signaling pathways activated by NETs in the context of
lymphoma could also reveal potential targets for precision
therapies.
In conclusion, the discovery of NETs as active
participants in lymphoma progression adds complexity to our
understanding of cancer biology. Unraveling the intricacies of the
interplay between NETs, cancer cells and the TME provides a
foundation for developing innovative therapeutic approaches.
Targeting NETs may not only offer a means to slow cancer
progression but also represent a novel avenue for immunomodulation
in the fight against lymphoma. As research progresses, the
potential for translating these findings into tangible clinical
benefits for patients with lymphoma becomes an exciting prospect in
cancer therapeutics.
4. Neutrophil-mediated therapeutic
approaches in lymphoma: A promising frontier
Neutrophil-mediated therapeutic strategies have
garnered significant attention as innovative approaches in treating
lymphoma (119). These strategies
aim to harness the natural immune response and enhance neutrophil
activity to increase the destruction of tumor cells. Several
promising avenues have emerged, showcasing the potential for
improved treatment outcomes and potentially reducing the need for
more aggressive interventions (78).
One notable approach involves leveraging antibodies
to mediate tumor cell destruction, effectively recruiting
neutrophils to engage in targeted and specific anti-tumor
activities. Designed to recognize and bind to specific markers on
lymphoma cells, antibodies serve as guiding signals for
neutrophils. Neutrophils become activated once bound to the target
tumor cells, leading to the latter's destruction. This targeted
antibody-mediated approach holds promise in minimizing collateral
damage to healthy tissues and enhancing the precision of
therapeutic interventions (120).
Combination therapies represent another frontier in
neutrophil-mediated strategies for lymphoma treatment. Integrating
neutrophil-targeted approaches with conventional chemotherapy aims
to capitalize on the synergistic effects of both modalities. By
enhancing the recruitment and activation of neutrophils in the TME,
these combination therapies seek to enhance the overall treatment
efficacy (121). This approach
not only maximizes the impact on cancer cells but may also
contribute to minimizing drug resistance and expanding the
therapeutic window.
NETs have emerged as a particularly intriguing
target for therapeutic intervention in lymphoma (114). As web-like structures composed of
chromatin and antimicrobial peptides released by neutrophils, NETs
have been implicated in lymphoma progression. Targeting NETs as a
therapeutic strategy involves modulating their formation or
function to disrupt their pro-tumorigenic effects (101). Preventing the ability of NETs to
facilitate cancer cell migration, invasion and immune evasion aims
to impede lymphoma progression.
In the context of NETs, another potential avenue
involves understanding and manipulating the interaction between
cancer cells and neutrophils (117). Disrupting the feedback loop where
cancer cells induce NET formation by neutrophils may represent a
novel therapeutic approach (105), which could involve developing
drugs that specifically inhibit this induction, potentially
attenuating the pro-tumorigenic effects associated with NETs.
Using neutrophil-mediated therapeutic approaches to
treat lymphoma reflects a paradigm shift towards exploiting the
body's defenses to fight cancer (122). By enhancing the role of
neutrophils in recognizing and destroying cancer cells, these
strategies offer a more nuanced and targeted approach to treatment
(123). As research in this field
progresses, there is optimism that these innovative approaches may
contribute to improved treatment outcomes for patients with
lymphoma, potentially reducing the reliance on more aggressive and
potentially debilitating interventions (124). Continued exploration of the
complex interplay between neutrophils and lymphoma cells is vital
to unlocking the full therapeutic potential of these
immune-mediated strategies.
5. Conclusions: Neutrophils in lymphoma - a
continuing journey of discovery
In summary, neutrophils have complex and
multifaceted roles in lymphoma. The existing evidence indicates
their dual potential in promoting and inhibiting lymphoma growth,
emphasizing the need for ongoing research to unravel the
intricacies of their role. The recognition of neutrophils as
potential prognostic markers and therapeutic targets highlights the
importance of sustained research in this area. As our comprehension
of the interactions between neutrophils and lymphoma improves,
these cells are poised to maintain a central role in shaping the
trajectory of lymphoma research and refining treatment
strategies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
KW wrote most of the manuscript. XW helped with the
topic selection and wrote a draft of the manuscript. KW and XW
edited the manuscript. LS edited the manuscript. All authors have
read and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rapoport BL, Steel HC, Theron AJ, Smit T
and Anderson R: Role of the neutrophil in the pathogenesis of
advanced cancer and impaired responsiveness to therapy. Molecules.
25(1618)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sounbuli K, Mironova N and Alekseeva L:
diverse neutrophil functions in cancer and promising
neutrophil-based cancer therapies. Int J Mol Sci.
23(15827)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Borregaard N: Neutrophils, from marrow to
microbes. Immunity. 33:657–670. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nauseef WM and Borregaard N: Neutrophils
at work. Nat Immunol. 15:602–611. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hidalgo A, Chilvers ER, Summers C and
Koenderman L: The neutrophil life cycle. Trends Immunol.
40:584–597. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kolaczkowska E and Kubes P: Neutrophil
recruitment and function in health and inflammation. Nat Rev
Immunol. 13:159–175. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ley K, Hoffman HM, Kubes P, Cassatella MA,
Zychlinsky A, Hedrick CC and Catz SD: Neutrophils: New insights and
open questions. Sci Immunol. 3(eaat4579)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sionov RV, Fridlender ZG and Granot Z: The
multifaceted roles neutrophils play in the tumor microenvironment.
Cancer Microenviron. 8:125–158. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Coffelt SB, Wellenstein MD and de Visser
KE: Neutrophils in cancer: Neutral no more. Nat Rev Cancer.
16:431–446. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Powell DR and Huttenlocher A: Neutrophils
in the tumor microenvironment. Trends Immunol. 37:41–52.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mantovani A, Cassatella MA, Costantini C
and Jaillon S: Neutrophils in the activation and regulation of
innate and adaptive immunity. Nat Rev Immunol. 11:519–531.
2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Galdiero MR, Bonavita E, Barajon I,
Garlanda C, Mantovani A and Jaillon S: Tumor associated macrophages
and neutrophils in cancer. Immunobiology. 218:1402–1410.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fridlender ZG, Sun J, Kim S, Kapoor V,
Cheng G, Ling L, Worthen GS and Albelda SM: Polarization of
tumor-associated neutrophil phenotype by TGF-beta: ‘N1’ versus ‘N2’
TAN. Cancer Cell. 16:183–194. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Swerdlow SH, Campo E, Pileri SA, Harris
NL, Stein H, Siebert R, Advani R, Ghielmini M, Salles GA, Zelenetz
AD and Jaffe ES: The 2016 revision of the World Health Organization
classification of lymphoid neoplasms. Blood. 127:2375–2390.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dhodapkar MV, Borrello I, Cohen AD and
Stadtmauer EA: Hematologic malignancies: Plasma cell disorders. Am
Soc Clin Oncol Educ Book. 37:561–568. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Parente P, Zanelli M, Sanguedolce F,
Mastracci L and Graziano P: Hodgkin Reed-Sternberg-like cells in
non-hodgkin lymphoma. Diagnostics (Basel). 10(1019)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Armitage JO, Gascoyne RD, Lunning MA and
Cavalli F: Non-Hodgkin lymphoma. Lancet. 390:298–310.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Matasar MJ and Zelenetz AD: Overview of
lymphoma diagnosis and management. Radiol Clin North Am.
46:175–198, vii. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Xing AY, Dong XZ, Zhu LQ, Liu L, Sun D and
Guo S: Clinicopathological characteristics and molecular phenotypes
of primary hepatic lymphoma. Front Oncol. 12(906245)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wang HW, Balakrishna JP, Pittaluga S and
Jaffe ES: Diagnosis of Hodgkin lymphoma in the modern era. Br J
Haematol. 184:45–59. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liew PX and Kubes P: The Neutrophil's role
during health and disease. Physiol Rev. 99:1223–1248.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sureda A and Martinez C: Classical
Hodgkin's lymphoma. In: The EBMT Handbook: Hematopoietic Stem Cell
Transplantation and Cellular Therapies. Carreras E, Dufour C, Mohty
M and Kroger N (eds): 7th edition. Springer, Cham, CH, pp653-662,
2019.
|
|
23
|
Euler M and Hoffmann MH: The double-edged
role of neutrophil extracellular traps in inflammation. Biochem Soc
Trans. 47:1921–1930. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pillay J, Kamp VM, van Hoffen E, Visser T,
Tak T, Lammers JW, Ulfman LH, Leenen LP, Pickkers P and Koenderman
L: A subset of neutrophils in human systemic inflammation inhibits
T cell responses through Mac-1. J Clin Invest. 122:327–336.
2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Upadhyay R, Hammerich L, Peng P, Brown B,
Merad M and Brody JD: Lymphoma: Immune evasion strategies. Cancers
(Basel). 7:736–762. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hirz T, Matera EL, Chettab K, Jordheim LP,
Mathé D, Evesque A, Esmenjaud J, Salles G and Dumontet C:
Neutrophils protect lymphoma cells against cytotoxic and targeted
therapies through CD11b/ICAM-1 binding. Oncotarget. 8:72818–72834.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu S, Wu W, Du Y, Yin H, Chen Q, Yu W,
Wang W, Yu J, Liu L, Lou W and Pu N: The evolution and
heterogeneity of neutrophils in cancers: Origins, subsets,
functions, orchestrations and clinical applications. Mol Cancer.
22(148)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang X, Qiu L, Li Z, Wang XY and Yi H:
Understanding the multifaceted role of neutrophils in cancer and
autoimmune diseases. Front Immunol. 9(2456)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Heshmat-Ghahdarijani K, Sarmadi V, Heidari
A, Falahati Marvasti A, Neshat S and Raeisi S: The
neutrophil-to-lymphocyte ratio as a new prognostic factor in
cancers: A narrative review. Front Oncol.
13(1228076)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ohashi K, Nishito Y, Fukuda H, Sadahiro R,
Yoshida Y, Watanabe SI, Motoi N, Sonobe Y, Mizuno H, Tsunoda H, et
al: Neutrophil-to-lymphocyte ratio is a prognostic factor
reflecting immune condition of tumor microenvironment in squamous
cell lung cancer. Sci Rep. 14(429)2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kim SI, Cassella CR and Byrne KT: Tumor
burden and immunotherapy: Impact on immune infiltration and
therapeutic outcomes. Front Immunol. 11(629722)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Pradeep U, Chiwhane A, Acharya S, Kumar S,
Daiya V, Kasat PR, Gupta A and Bedi GN: The role of
neutrophil-to-lymphocyte ratio in predicting outcomes of acute
organophosphorus poisoning: A comprehensive review. Cureus.
16(e60854)2024.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zhang G, Yang C, Zhao C, Xian F, Qing D,
Guo Q, Song J, Liu X and Bie J: Prognostic value of the
neutrophil-to-lymphocyte ratio in patients treated with definitive
chemoradiotherapy for locally advanced oesophageal squamous cell
carcinoma. Cancer Manag Res. 15:101–112. 2023.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Masucci MT, Minopoli M and Carriero MV:
Tumor associated neutrophils. Their role in tumorigenesis,
metastasis, prognosis and therapy. Front Oncol.
9(1146)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Quintero-Fabian S, Arreola R,
Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V,
Lara-Riegos J, Ramírez-Camacho MA and Alvarez-Sánchez ME: Role of
matrix metalloproteinases in angiogenesis and cancer. Front Oncol.
9(1370)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Christoffersson G, Vagesjo E, Vandooren J,
Lidén M, Massena S, Reinert RB, Brissova M, Powers AC, Opdenakker G
and Phillipson M: VEGF-A recruits a proangiogenic MMP-9-delivering
neutrophil subset that induces angiogenesis in transplanted hypoxic
tissue. Blood. 120:4653–4662. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yu X, Li C, Wang Z, Xu Y, Shao S, Shao F,
Wang H and Liu J: Neutrophils in cancer: Dual roles through
intercellular interactions. Oncogene. 43:1163–1177. 2024.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kwantwi LB: Interplay between
tumor-derived factors and tumor-associated neutrophils:
Opportunities for therapeutic interventions in cancer. Clin Transl
Oncol. 25:1963–1976. 2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Xiong X, Liao X, Qiu S, Xu H, Zhang S,
Wang S, Ai J and Yang L: CXCL8 in tumor biology and its
implications for clinical translation. Front Mol Biosci.
9(723846)2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Teijeira A, Garasa S, Ochoa MC, Villalba
M, Olivera I, Cirella A, Eguren-Santamaria I, Berraondo P, Schalper
KA, de Andrea CE, et al: IL8, Neutrophils, and NETs in a collusion
against cancer immunity and immunotherapy. Clin Cancer Res.
27:2383–2393. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
De Meo ML and Spicer JD: The role of
neutrophil extracellular traps in cancer progression and
metastasis. Semin Immunol. 57(101595)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Huang X, Nepovimova E, Adam V, Sivak L,
Heger Z, Valko M, Wu Q and Kuca K: Neutrophils in cancer
immunotherapy: Friends or foes? Mol Cancer. 23(107)2024.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zhang Y, Guoqiang L, Sun M and Lu X:
Targeting and exploitation of tumor-associated neutrophils to
enhance immunotherapy and drug delivery for cancer treatment.
Cancer Biol Med. 17:32–43. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Armstrong H, Bording-Jorgensen M, Dijk S
and Wine E: The Complex Interplay between chronic inflammation, the
microbiome, and cancer: Understanding disease progression and what
we can do to prevent it. Cancers (Basel). 10(83)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wu TH, Hsieh SC, Li TH, Lu CH, Liao HT,
Shen CY, Li KJ, Wu CH, Kuo YM, Tsai CY and Yu CL: Molecular basis
for paradoxical activities of polymorphonuclear neutrophils in
inflammation/anti-inflammation, bactericide/autoimmunity,
pro-cancer/anticancer, and antiviral infection/SARS-CoV-II-induced
immunothrombotic dysregulation. Biomedicines.
10(773)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899.
2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Coletto LA, Rizzo C, Guggino G, Caporali
R, Alivernini S and D'Agostino MA: The role of neutrophils in
spondyloarthritis: A journey across the spectrum of disease
manifestations. Int J Mol Sci. 24(4108)2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Herrero-Cervera A, Soehnlein O and Kenne
E: Neutrophils in chronic inflammatory diseases. Cell Mol Immunol.
19:177–191. 2022.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng
J, Li Y, Wang X and Zhao L: Inflammatory responses and
inflammation-associated diseases in organs. Oncotarget.
9:7204–7218. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhao H, Wu L, Yan G, Chen Y, Zhou M, Wu Y
and Li Y: Inflammation and tumor progression: Signaling pathways
and targeted intervention. Signal Transduct Target Ther.
6(263)2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Xiong S, Dong L and Cheng L: Neutrophils
in cancer carcinogenesis and metastasis. J Hematol Oncol.
14(173)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Rosales C: Neutrophil: A cell with many
roles in inflammation or several cell types? Front Physiol.
9(113)2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Tu Z, Zhong Y, Hu H, Shao D, Haag R,
Schirner M, Lee J, Sullenger B and Leong KW: Design of therapeutic
biomaterials to control inflammation. Nat Rev Mater. 7:557–574.
2022.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Mata R, Yao Y, Cao W, Ding J, Zhou T, Zhai
Z and Gao C: The dynamic inflammatory tissue microenvironment:
Signality and disease therapy by biomaterials. Research (Wash D C).
2021(4189516)2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Hannoodee S and Nasuruddin DN: Acute
Inflammatory Response. StatPearls, Treasure Island, FL, 2023.
|
|
56
|
Ward PA and Lentsch AB: The acute
inflammatory response and its regulation. Arch Surg. 134:666–669.
1999.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Filep JG and Ariel A: Neutrophil
heterogeneity and fate in inflamed tissues: Implications for the
resolution of inflammation. Am J Physiol Cell Physiol.
319:C510–C532. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hirayama D, Iida T and Nakase H: The
phagocytic function of macrophage-enforcing innate immunity and
tissue homeostasis. Int J Mol Sci. 19(92)2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Lawrence T and Gilroy DW: Chronic
inflammation: A failure of resolution? Int J Exp Pathol. 88:85–94.
2007.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Whiteside TL: The tumor microenvironment
and its role in promoting tumor growth. Oncogene. 27:5904–5912.
2008.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Megha KB, Joseph X, Akhil V and Mohanan
PV: Cascade of immune mechanism and consequences of inflammatory
disorders. Phytomedicine. 91(153712)2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Zhang JM and An J: Cytokines,
inflammation, and pain. Int Anesthesiol Clin. 45:27–37.
2007.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Altan-Bonnet G and Mukherjee R:
Cytokine-mediated communication: A quantitative appraisal of immune
complexity. Nat Rev Immunol. 19:205–217. 2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Fajgenbaum DC and June CH: Cytokine Storm.
N Engl J Med. 383:2255–2273. 2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Prame Kumar K, Nicholls AJ and Wong CHY:
Partners in crime: Neutrophils and monocytes/macrophages in
inflammation and disease. Cell Tissue Res. 371:551–565.
2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Yang L, Shi P, Zhao G, Xu J, Peng W, Zhang
J, Zhang G, Wang X, Dong Z, Chen F and Cui H: Targeting cancer stem
cell pathways for cancer therapy. Signal Transduct Target Ther.
5(8)2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Shao S, Miao H and Ma W: Unraveling the
enigma of tumor-associated macrophages: Challenges, innovations,
and the path to therapeutic breakthroughs. Front Immunol.
14(1295684)2023.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Khilwani R and Singh S: Systems biology
and cytokines potential role in lung cancer immunotherapy targeting
autophagic axis. Biomedicines. 11(2706)2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Yang L, Xie X, Tu Z, Fu J, Xu D and Zhou
Y: The signal pathways and treatment of cytokine storm in COVID-19.
Signal Transduct Target Ther. 6(255)2021.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Anderson NM and Simon MC: The tumor
microenvironment. Curr Biol. 30:R921–R925. 2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Yan M, Zheng M, Niu R, Yang X, Tian S, Fan
L, Li Y and Zhang S: Roles of tumor-associated neutrophils in tumor
metastasis and its clinical applications. Front Cell Dev Biol.
10(938289)2022.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Xiao Y and Yu D: Tumor microenvironment as
a therapeutic target in cancer. Pharmacol Ther.
221(107753)2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Xiong T, He P, Zhou M, Zhong D, Yang T, He
W, Xu Z, Chen Z, Liu YW and Dai SS: Glutamate blunts cell-killing
effects of neutrophils in tumor microenvironment. Cancer Sci.
113:1955–1967. 2022.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Giese MA, Hind LE and Huttenlocher A:
Neutrophil plasticity in the tumor microenvironment. Blood.
133:2159–2167. 2019.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Mantovani A and Allavena P: The
interaction of anticancer therapies with tumor-associated
macrophages. J Exp Med. 212:435–445. 2015.PubMed/NCBI View Article : Google Scholar
|
|
77
|
McFarlane AJ, Fercoq F, Coffelt SB and
Carlin LM: Neutrophil dynamics in the tumor microenvironment. J
Clin Invest. 131(e143759)2021.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Galdiero MR, Garlanda C, Jaillon S, Marone
G and Mantovani A: Tumor associated macrophages and neutrophils in
tumor progression. J Cell Physiol. 228:1404–1412. 2013.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Di Carlo E, Forni G, Lollini P, Colombo
MP, Modesti A and Musiani P: The intriguing role of
polymorphonuclear neutrophils in antitumor reactions. Blood.
97:339–345. 2001.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Li MY, Chong LC, Duns G, Lytle A, Woolcock
B, Jiang A, Telenius A, Ben-Neriah S, Nawaz W, Slack GW, et al:
TRAF3 loss-of-function reveals the noncanonical NF-κB pathway as a
therapeutic target in diffuse large B cell lymphoma. Proc Natl Acad
Sci USA. 121(e2320421121)2024.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Mondragon L, Mhaidly R, De Donatis GM,
Tosolini M, Dao P, Martin AR, Pons C, Chiche J, Jacquin M, Imbert
V, et al: GAPDH Overexpression in the T cell lineage promotes
angioimmunoblastic T cell lymphoma through an NF-κB-Dependent
Mechanism. Cancer Cell. 36:268–287 e10. 2019.PubMed/NCBI View Article : Google Scholar
|
|
82
|
von Hoff L, Kargel E, Franke V, McShane E,
Schulz-Beiss KW, Patone G, Schleussner N, Kolesnichenko M, Hübner
N, Daumke O, et al: Autocrine LTA signaling drives NF-kappaB and
JAK-STAT activity and myeloid gene expression in Hodgkin lymphoma.
Blood. 133:1489–1494. 2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Gluud M, Pallesen EMH, Buus TB, Gjerdrum
LMR, Lindahl LM, Kamstrup MR, Bzorek M, Danielsen M, Bech R,
Monteiro MN, et al: Malignant T cells induce skin barrier defects
through cytokine-mediated JAK/STAT signaling in cutaneous T-cell
lymphoma. Blood. 141:180–193. 2023.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Ramis-Zaldivar JE, Gonzalez-Farre B,
Nicolae A, Pack S, Clot G, Nadeu F, Mottok A, Horn H, Song JY, Fu
K, et al: MAPK and JAK-STAT pathways dysregulation in plasmablastic
lymphoma. Haematologica. 106:2682–2693. 2021.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Gehringer F, Weissinger SE, Moller P,
Wirth T and Ushmorov A: Physiological levels of the PTEN-PI3K-AKT
axis activity are required for maintenance of Burkitt lymphoma.
Leukemia. 34:857–871. 2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Takashima Y, Hayano A and Yamanaka R:
Metabolome analysis reveals excessive glycolysis via PI3K/AKT/mTOR
and RAS/MAPK signaling in methotrexate-resistant primary CNS
Lymphoma-Derived Cells. Clin Cancer Res. 26:2754–2766.
2020.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Wang G, Liu H, An L, Hou S and Zhang Q:
CAPG facilitates diffuse large B-cell lymphoma cell progression
through PI3K/AKT signaling pathway. Hum Immunol. 83:832–842.
2022.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Sato A, Kamio N, Yokota A, Hayashi Y,
Tamura A, Miura Y, Maekawa T and Hirai H: C/EBPβ isoforms
sequentially regulate regenerating mouse hematopoietic
stem/progenitor cells. Blood Adv. 4:3343–3356. 2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Wang W, Xia X, Mao L and Wang S: The
CCAAT/Enhancer-Binding protein family: Its Roles in MDSC expansion
and function. Front Immunol. 10(1804)2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Avellino R and Delwel R: Expression and
regulation of C/EBPα in normal myelopoiesis and in malignant
transformation. Blood. 129:2083–2091. 2017.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Hosokawa H, Koizumi M, Masuhara K,
Romero-Wolf M, Tanaka T, Nakayama T and Rothenberg EV:
Stage-specific action of Runx1 and GATA3 controls silencing of PU.1
expression in mouse pro-T cells. J Exp Med.
218(e20202648)2021.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Inage E, Kasakura K, Yashiro T, Suzuki R,
Baba Y, Nakano N, Hara M, Tanabe A, Oboki K, Matsumoto K, et al:
Critical Roles for PU.1, GATA1, and GATA2 in the expression of
human FcƐRI on mast cells: PU.1 and GATA1 transactivate FCER1A, and
GATA2 transactivates FCER1A and MS4A2. J Immunol. 192:3936–3946.
2014.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Zakrzewska A, Cui C, Stockhammer OW,
Benard EL, Spaink HP and Meijer AH: Macrophage-specific gene
functions in Spi1-directed innate immunity. Blood. 116:e1–e11.
2010.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Wu S, Wang H, Yang Q, Liu Z, Du J, Wang L,
Chen S, Lu Q and Yang DH: METTL3 regulates M6A methylation-modified
EBV-pri-miR-BART3-3p to promote NK/T cell lymphoma growth. Cancer
Lett. 597(217058)2024.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Zhao A, Zhou H, Yang J, Li M and Niu T:
Epigenetic regulation in hematopoiesis and its implications in the
targeted therapy of hematologic malignancies. Signal Transduct
Target Ther. 8(71)2023.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Zhuang S, Yang Z, Cui Z, Zhang Y and Che
F: Epigenetic alterations and advancement of lymphoma treatment.
Ann Hematol. 103:1435–1454. 2024.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Tecchio C and Cassatella MA:
Neutrophil-derived cytokines involved in physiological and
pathological angiogenesis. Chem Immunol Allergy. 99:123–137.
2014.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Shaul ME and Fridlender ZG: Neutrophils as
active regulators of the immune system in the tumor
microenvironment. J Leukoc Biol. 102:343–349. 2017.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Jablonska J, Leschner S, Westphal K,
Lienenklaus S and Weiss S: Neutrophils responsive to endogenous
IFN-beta regulate tumor angiogenesis and growth in a mouse tumor
model. J Clin Invest. 120:1151–1164. 2010.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Matta B, Battaglia J and Barnes BJ:
Detection of neutrophil extracellular traps in patient plasma:
Method development and validation in systemic lupus erythematosus
and healthy donors that carry IRF5 genetic risk. Front Immunol.
13(951254)2022.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Demers M, Krause DS, Schatzberg D,
Martinod K, Voorhees JR, Fuchs TA, Scadden DT and Wagner DD:
Cancers predispose neutrophils to release extracellular DNA traps
that contribute to cancer-associated thrombosis. Proc Natl Acad Sci
USA. 109:13076–13081. 2012.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Saffarzadeh M, Juenemann C, Queisser MA,
Lochnit G, Barreto G, Galuska SP, Lohmeyer J and Preissner KT:
Neutrophil extracellular traps directly induce epithelial and
endothelial cell death: A predominant role of histones. PLoS One.
7(e32366)2012.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Fuchs TA, Abed U, Goosmann C, Hurwitz R,
Schulze I, Wahn V, Weinrauch Y, Brinkmann V and Zychlinsky A: Novel
cell death program leads to neutrophil extracellular traps. J Cell
Biol. 176:231–241. 2007.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Cools-Lartigue J, Spicer J, McDonald B,
Gowing S, Chow S, Giannias B, Bourdeau F, Kubes P and Ferri L:
Neutrophil extracellular traps sequester circulating tumor cells
and promote metastasis. J Clin Invest. 123:3446–3458.
2013.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Berger-Achituv S, Brinkmann V, Abed UA,
Kühn LI, Ben-Ezra J, Elhasid R and Zychlinsky A: A proposed role
for neutrophil extracellular traps in cancer immunoediting. Front
Immunol. 4(48)2013.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Jehannin-Ligier K, Belot A, Guizard AV,
Bossard N, Launoy G and Uhry Z: FRANCIM network. Incidence trends
for potentially human papillomavirus-related and -unrelated head
and neck cancers in France using population-based cancer registries
data: 1980-2012. Int J Cancer. 140:2032–2039. 2017.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Tohme S, Yazdani HO, Al-Khafaji AB, Chidi
AP, Loughran P, Mowen K, Wang Y, Simmons RL, Huang H and Tsung A:
Neutrophil extracellular traps promote the development and
progression of liver metastases after surgical stress. Cancer Res.
76:1367–1380. 2016.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Shimada K, Crother TR, Karlin J, Dagvadorj
J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, et
al: Oxidized mitochondrial DNA activates the NLRP3 inflammasome
during apoptosis. Immunity. 36:401–414. 2012.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Brinkmann V and Zychlinsky A: Neutrophil
extracellular traps: Is immunity the second function of chromatin?
J Cell Biol. 198:773–783. 2012.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Papayannopoulos V, Metzler KD, Hakkim A
and Zychlinsky A: Neutrophil elastase and myeloperoxidase regulate
the formation of neutrophil extracellular traps. J Cell Biol.
191:677–691. 2010.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Kaplan MJ and Radic M: Neutrophil
extracellular traps: Double-edged swords of innate immunity. J
Immunol. 189:2689–2695. 2012.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Porto BN and Stein RT: Neutrophil
extracellular traps in pulmonary diseases: Too much of a good
thing? Front Immunol. 7(311)2016.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Schonrich G, Raftery MJ and Samstag Y:
Devilishly radical NETwork in COVID-19: Oxidative stress,
neutrophil extracellular traps (NETs), and T cell suppression. Adv
Biol Regul. 77(100741)2020.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Cedervall J, Zhang Y, Huang H, Zhang L,
Femel J, Dimberg A and Olsson AK: Neutrophil extracellular traps
accumulate in peripheral blood vessels and compromise organ
function in tumor-bearing animals. Cancer Res. 75:2653–2662.
2015.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Albrengues J, Shields MA, Ng D, Park CG,
Ambrico A, Poindexter ME, Upadhyay P, Uyeminami DL, Pommier A,
Küttner V, et al: Neutrophil extracellular traps produced during
inflammation awaken dormant cancer cells in mice. Science.
361(eaao4227)2018.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Demers M, Wong SL, Martinod K, Gallant M,
Cabral JE, Wang Y and Wagner DD: Priming of neutrophils toward
NETosis promotes tumor growth. Oncoimmunology.
5(e1134073)2016.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Najmeh S, Cools-Lartigue J, Rayes RF,
Gowing S, Vourtzoumis P, Bourdeau F, Giannias B, Berube J, Rousseau
S, Ferri LE and Spicer JD: Neutrophil extracellular traps sequester
circulating tumor cells via β1-integrin mediated interactions. Int
J Cancer. 140:2321–2330. 2017.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Park J, Wysocki RW, Amoozgar Z, Maiorino
L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH,
et al: Cancer cells induce metastasis-supporting neutrophil
extracellular DNA traps. Sci Transl Med. 8(361ra138)2016.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Belaaouaj A, McCarthy R, Baumann M, Gao Z,
Ley TJ, Abraham SN and Shapiro SD: Mice lacking neutrophil elastase
reveal impaired host defense against gram negative bacterial
sepsis. Nat Med. 4:615–618. 1998.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Geyer CE, Forster J, Lindquist D, Chan S,
Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A,
Kaufman B, et al: Lapatinib plus capecitabine for HER2-positive
advanced breast cancer. N Engl J Med. 355:2733–2743.
2006.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Allen TM: Ligand-targeted therapeutics in
anticancer therapy. Nat Rev Cancer. 2:750–763. 2002.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Mayadas TN, Cullere X and Lowell CA: The
multifaceted functions of neutrophils. Annu Rev Pathol. 9:181–218.
2014.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Nemeth T, Mocsai A and Lowell CA:
Neutrophils in animal models of autoimmune disease. Semin Immunol.
28:174–186. 2016.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Tecchio C, Micheletti A and Cassatella MA:
Neutrophil-derived cytokines: Facts beyond expression. Front
Immunol. 5(508)2014.PubMed/NCBI View Article : Google Scholar
|
















