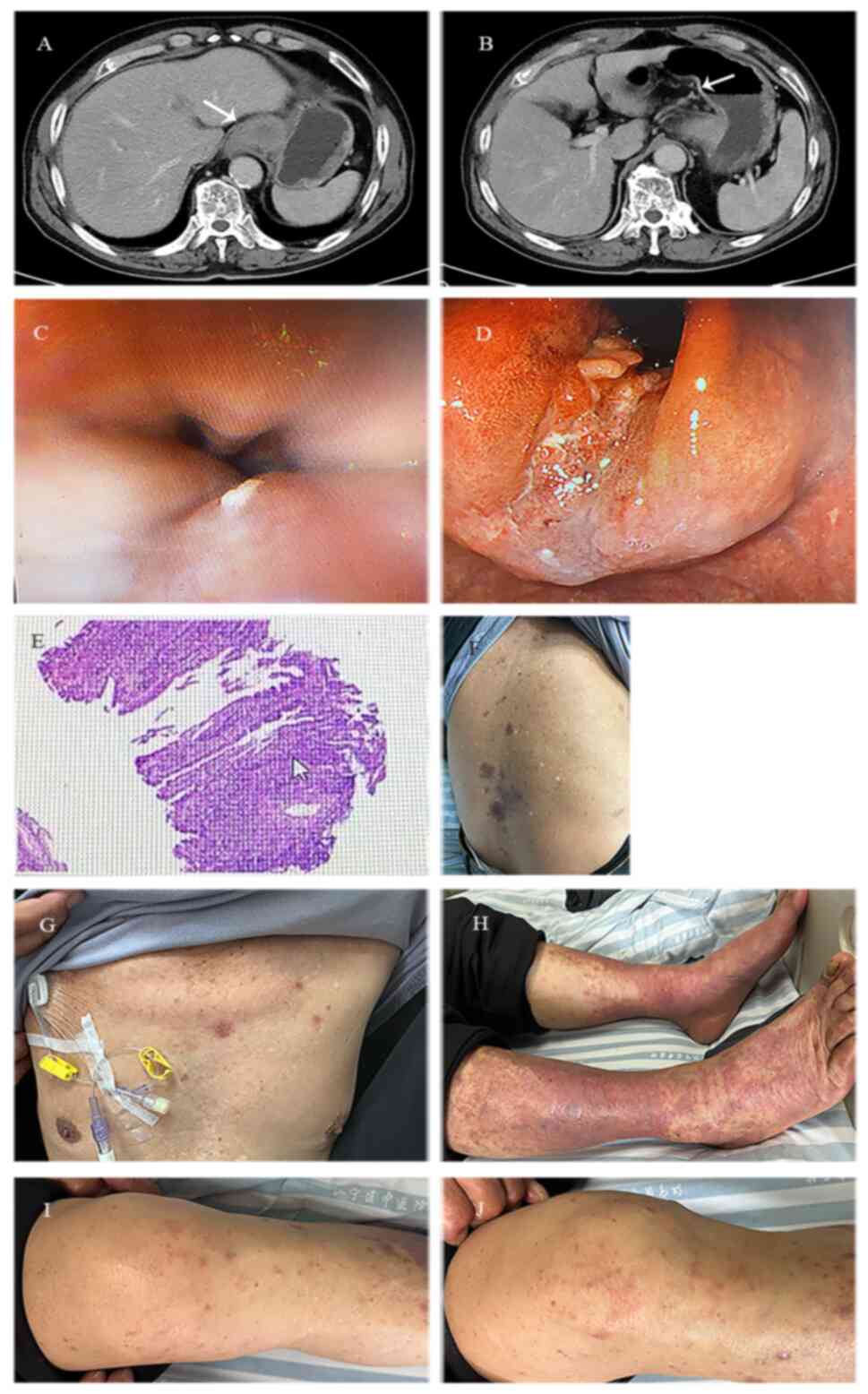
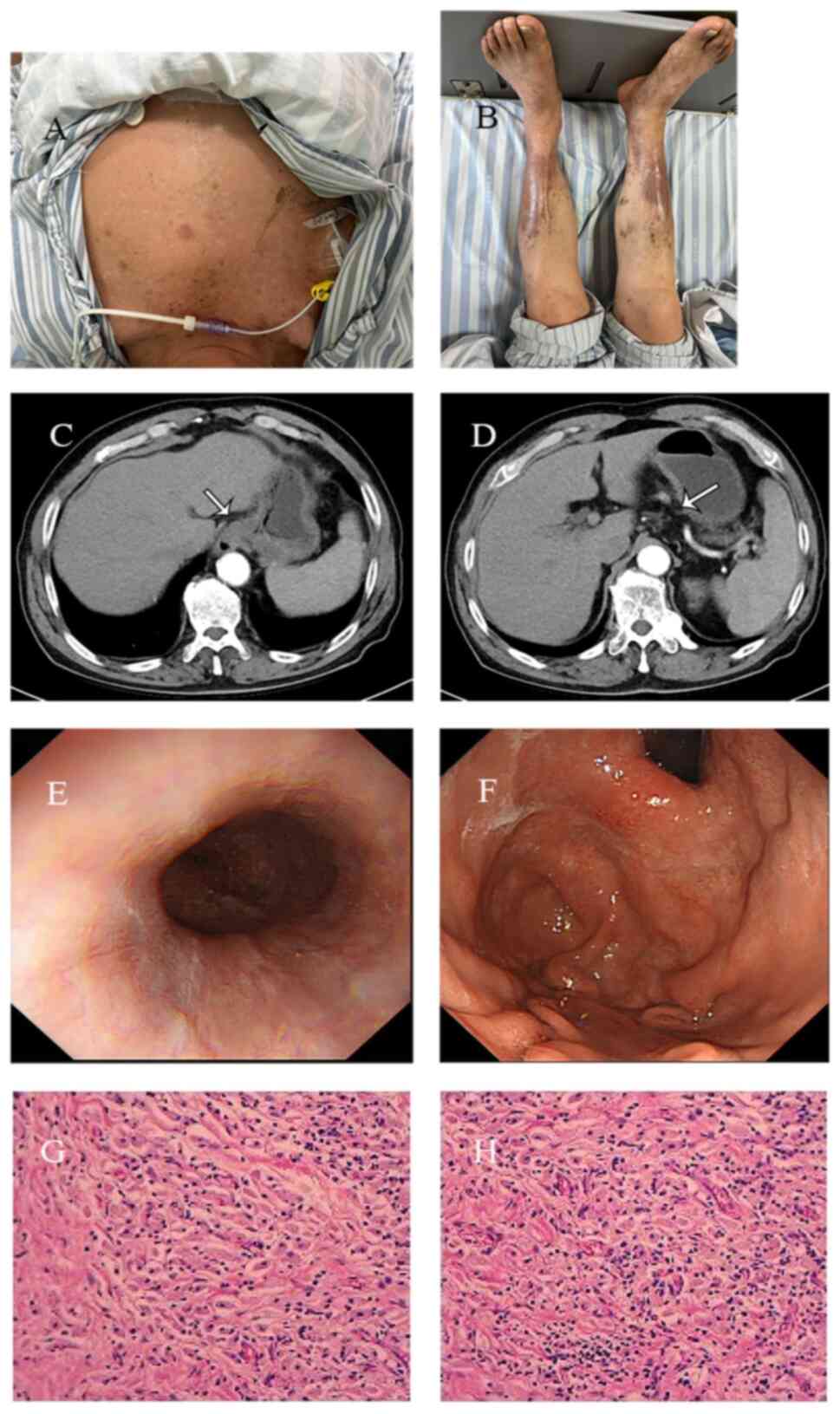
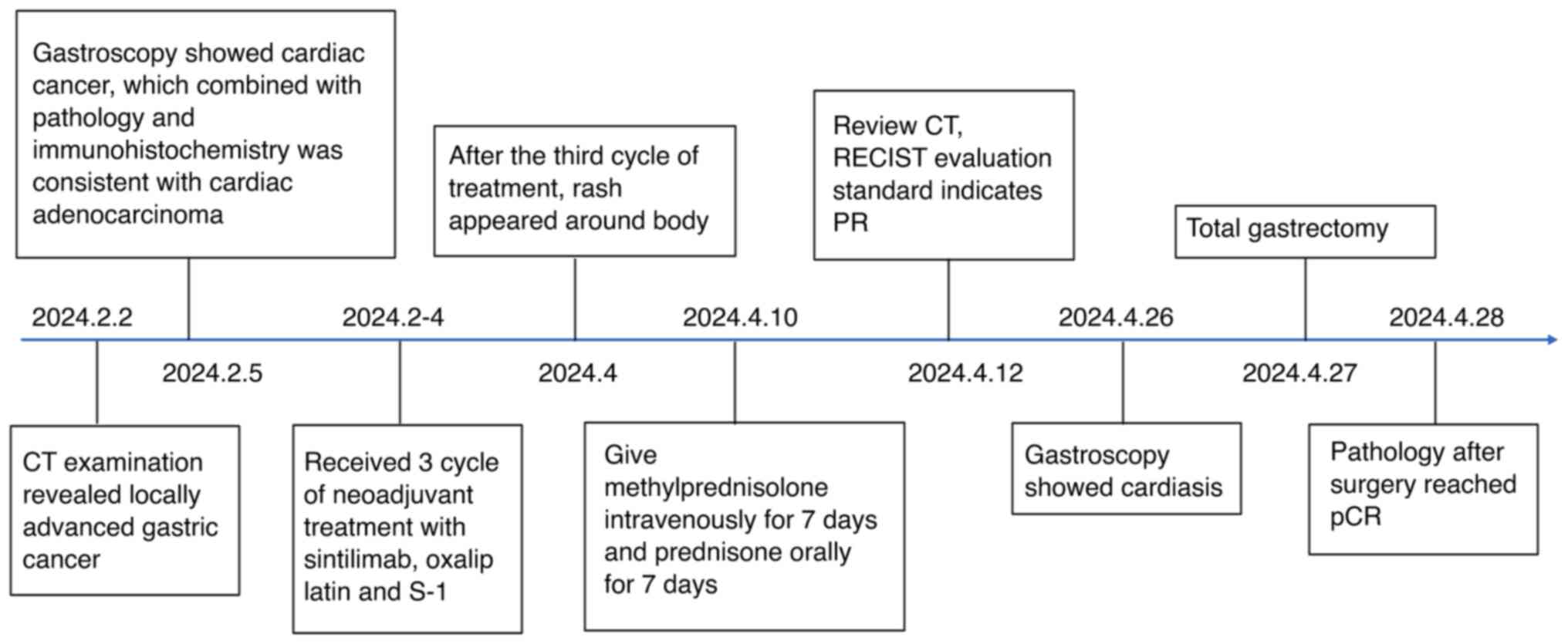
|
1
|
Banday AH and Abdalla M: Immune checkpoint
inhibitors: Recent clinical advances and future prospects. Curr Med
Chem. 30:3215–3237. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hoy SM: Sintilimab: First global approval.
Drugs. 79:341–346. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L,
Shu Y, Li J, Zhao J, Pan H, et al: Sintilimab plus chemotherapy for
unresectable gastric or gastroesophageal junction cancer: The
ORIENT-16 randomized clinical trial. JAMA. 330:2064–2074.
2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang L, Wu Q, Chi H and Yang G: Letter to
the editor for the article ‘Efficacy and safety of neoadjuvant
sintilimab in combination with FLOT chemotherapy in patients with
HER2-negative locally advanced gastric or gastroesophageal junction
adenocarcinoma: An investigator-initiated, single-arm, open-label,
phase II study’. Int J Surg. 110:6026–6027. 2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ramos-Casals M, Brahmer JR, Callahan MK,
Flores-Chavez A, Keegan N, Khamashta MA, Lambotte O, Mariette X,
Prat A and Suarez-Almazor ME: Immune-related adverse events of
checkpoint inhibitors. Nat Rev Dis Primers. 6(38)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ruggiero R, Fraenza F, Scavone C, di Mauro
G, Piscitelli R, Mascolo A, Ferrajolo C, Rafaniello C, Sportiello
L, Rossi F and Capuano A: Immune checkpoint inhibitors and
immune-related adverse drug reactions: Data from Italian
pharmacovigilance database. Front Pharmacol. 11(830)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu S, Wang Z, Ge Y and Zhao Y: Prognostic
significance of an innovative staging system based on the
logarithmic odds of positive lymph nodes for resectable
gastroesophageal cancer after neoadjuvant chemoradiation: A
population-based study with external validation of data. J Transl
Med. 22(801)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yan J, Ma N, Qiao WL, Liu KQ, Liu DW, Wang
Y, Qiao TT, Hao XQ and Zheng MD: Adverse skin reactions induced by
sintilimab in advanced lung squamous carcinoma: A case report and
review of the literature. Ann Transl Med. 10(1411)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li G, Gong S, Wang N and Yao X: Toxic
epidermal necrolysis induced by sintilimab in a patient with
advanced non-small cell lung cancer and comorbid pulmonary
tuberculosis: A case report. Front Immunol.
13(989966)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang W, Xu X, Xia D, Wang H, Jiang J and
Yang G: Toxic epidermal necrolysis associated with
chemoimmunotherapy for lymphoma: Case report and literature review.
Immunotherapy. 14:275–282. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao Y, Cao Y, Wang X and Qian T:
Treatment of PD-1 inhibitor-associated toxic epidermal necrolysis:
A case report and brief review. Onco Targets Ther. 15:345–351.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhou S, Zhang Z, Feng X, Zhao C and Jiang
L: Lichenoid mucocutaneous reactions associated with sintilimab
therapy in a non-small cell lung adenocarcinoma patient: Case
report and review. Front Pharmacol. 14(1276788)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu Y, Tang J, Yu LY and Jiang Q:
Successful treatment of immune-related lichenoid dermatitis by
Weiling decoction in a patient with non-small cell lung cancer: A
case report and review of literature. Explore (NY). 19:730–735.
2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang T, Shao Q, Xiao C and Liu L: Case
report: Bullous pemphigoid associated with sintilimab therapy for
pMMR/MSS colorectal cancer. Front Oncol. 13(1124730)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen L, Cao X, Luo X and Jiang T:
Refractory pruritus caused by sintilimab and its clinical
management: A case report. Heliyon. 10(e34107)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rawla P and Barsouk A: Epidemiology of
gastric cancer: Global trends, risk factors and prevention. Prz
Gastroenterol. 14:26–38. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zeng Y and Jin RU: Molecular pathogenesis,
targeted therapies, and future perspectives for gastric cancer.
Semin Cancer Biol. 86:566–582. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang G, Huang Y, Zhou L, Yang H, Lin H,
Zhou S, Tan Z and Qian J: Immunotherapy and targeted therapy as
first-line treatment for advanced gastric cancer. Crit Rev Oncol
Hematol. 198(104197)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang X, Fang J, Huang L, Chen H, Chen H,
Chai T, Ye Z, Chen H, Xu Q, Du Y and Yu P: SOX combined with
sintilimab versus SOX alone in the perioperative management of
locally advanced gastric cancer: A propensity score-matched
analysis. Gastric Cancer. 26:1040–1050. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jia R, Li Y, Xu N, Jiang HP, Zhao CH, Liu
RR, Shi Y, Zhang YY, Wang SY, Zhou H and Xu JM: Sintilimab in
patients with previously treated metastatic neuroendocrine
neoplasms. Oncologist. 27:e625–e632. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wongvibulsin S, Pahalyants V, Kalinich M,
Murphy W, Yu KH, Wang F, Chen ST, Reynolds K, Kwatra SG and Semenov
YR: Epidemiology and risk factors for the development of cutaneous
toxicities in patients treated with immune-checkpoint inhibitors: A
United States population-level analysis. J Am Acad Dermatol.
86:563–572. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Weber JS, Dummer R, de Pril V, Lebbé C and
Hodi FS: Patterns of onset and resolution of immune-related adverse
events of special interest with ipilimumab: Detailed safety
analysis from a phase 3 trial in patients with advanced melanoma.
Cancer. 119:1675–1682. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Teng YS and Yu S: Molecular mechanisms of
cutaneous immune-related adverse events (irAEs) induced by immune
checkpoint inhibitors. Curr Oncol. 30:6805–6819. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ye Z, Yang W, Xuan B, Li X, He J, Si H and
Ma W: Efficacy and safety evaluation of sintilimab for cancer
treatment: A systematic review and meta-analysis of randomized
controlled trials. Front Pharmacol. 13(895187)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Thompson JA, Schneider BJ, Brahmer J,
Achufusi A, Armand P, Berkenstock MK, Bhatia S, Budde LE, Chokshi
S, Davies M, et al: Management of immunotherapy-related toxicities,
version 1.2022, NCCN clinical practice guidelines in oncology. J
Natl Compr Canc Netw. 20:387–405. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Schneider BJ, Naidoo J, Santomasso BD,
Lacchetti C, Adkins S, Anadkat M, Atkins MB, Brassil KJ, Caterino
JM, Chau I, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: ASCO
guideline update. J Clin Oncol. 39:4073–4126. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhou Q, Qin Z, Wu G, Yan P, Wang Q, Qu J,
Jiang J and Ye D: Sintilimab-induced myocarditis suspected in a
patient with esophageal cancer and followed septic shock: Case
report and literature review. Front Oncol.
14(1465395)2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ni CX, Zhao Y, Qian H, Fu H, Yan YY, Qiu
YS, Zhou CC, Huang F, Shen FM, Li DJ and Xu Q: Long survival in a
pancreatic carcinoma patient with multi-organ toxicities after
sintilimab treatment: A case report. Front Pharmacol.
14(1121122)2023.PubMed/NCBI View Article : Google Scholar
|