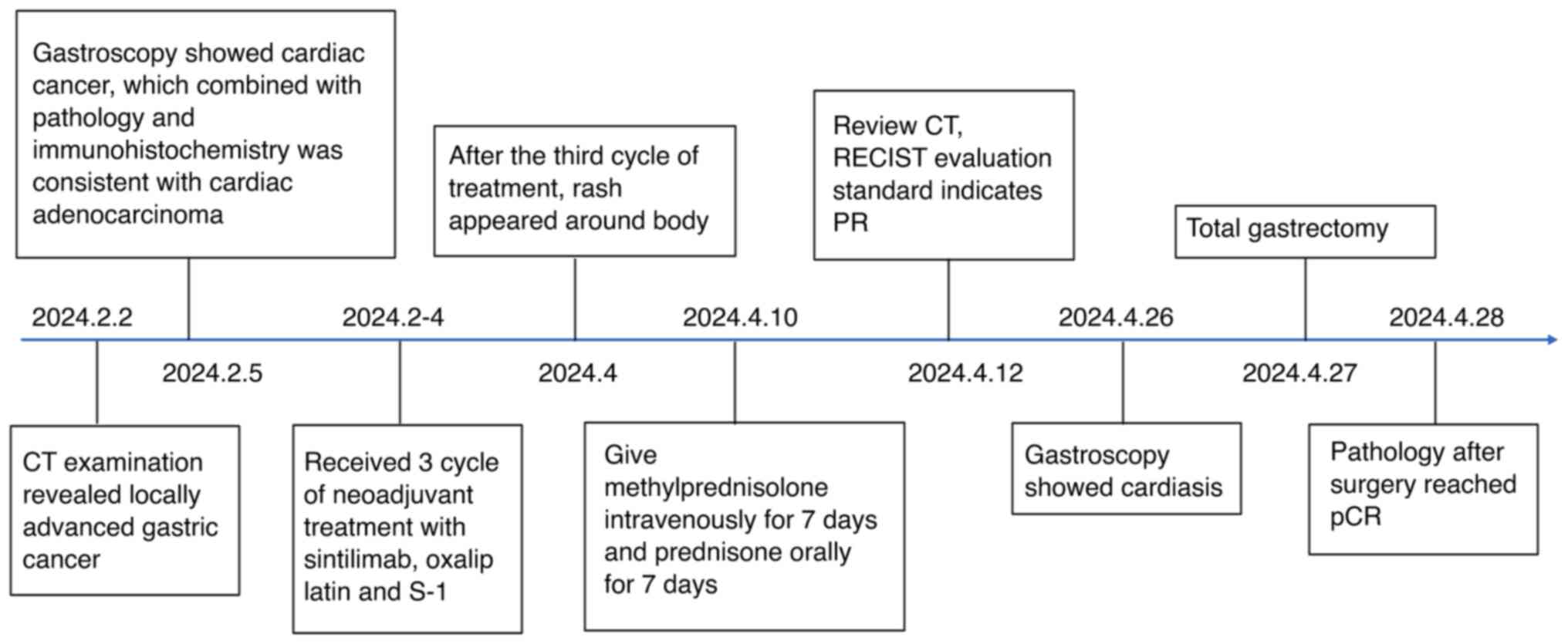
Introduction
In the past few decades, cancer treatment has
advanced into the era of immunotherapy. Immune checkpoint
inhibitors (ICIs) are increasingly used against particular types of
cancer and have achieved significant therapeutic effects (1). Sintilimab, a humanized monoclonal
immunoglobulin G4 antibody, acts as a programmed cell death
protein-1 (PD-1) antagonist. Therefore, it blocks the interaction
between PD-1 and its ligands, namely programmed cell death ligand 1
(PD-L1) and PD-L2, thus alleviating immunosuppressive effects and
activating T cell functions (2).
For advanced gastric adenocarcinoma, the combination of sintilimab
and chemotherapy as first-line treatment could notably improve
patient survival rates (3).
Currently, the combination of sintilimab and chemotherapy as
neoadjuvant therapy for gastric cancer displays significant safety
and promising efficacy (4).
However, severe skin toxicity cannot only impair the quality of
life of patients, but also limit the effectiveness of cancer
treatments (5). Although the
incidence of immune-related adverse skin reactions in patients
treated with sintilimab alone or in combination is rare, the
associated mortality rate is notably high (6). In the present study, a case of a
patient who experienced severe immune-related cutaneous adverse
reactions during neoadjuvant therapy was reported. Following
surgery, the aforementioned immune-related adverse event (irAE)
reached a pathological complete response (pCR).
Case presentation
A 78-year-old man was admitted to the Nanjing
Jiangning Hospital of Traditional Chinese Medicine (Nanjing,
China); afterwards, the patient underwent a computed tomography
(CT) examination, which revealed locally advanced gastric
adenocarcinoma. The tumor had invaded the lower end of the
esophagus and surrounding lymph nodes (Fig. 1A and B). Upon admission on February 5, 2024,
gastroscopy confirmed the diagnosis of gastric adenocarcinoma
(Fig. 1C and D). After dehydration, biopsy samples
taken via gastroscopy were embedded in paraffin, sectioned, and
subjected to pathological diagnosis and immunohistochemical
analysis. The tissue sections were 4 µm thick. The primary antibody
was a ready-to-use reagent purchased from Fuzhou Maixin Biotech
Co., Ltd., incubated at 37˚C for 32 min. The secondary antibody,
also a ready-to-use reagent from Roche Diagnostics, was incubated
at 37˚C for 32 min. Microscopic images were captured using a light
microscope with an objective lens at a x40 magnification.
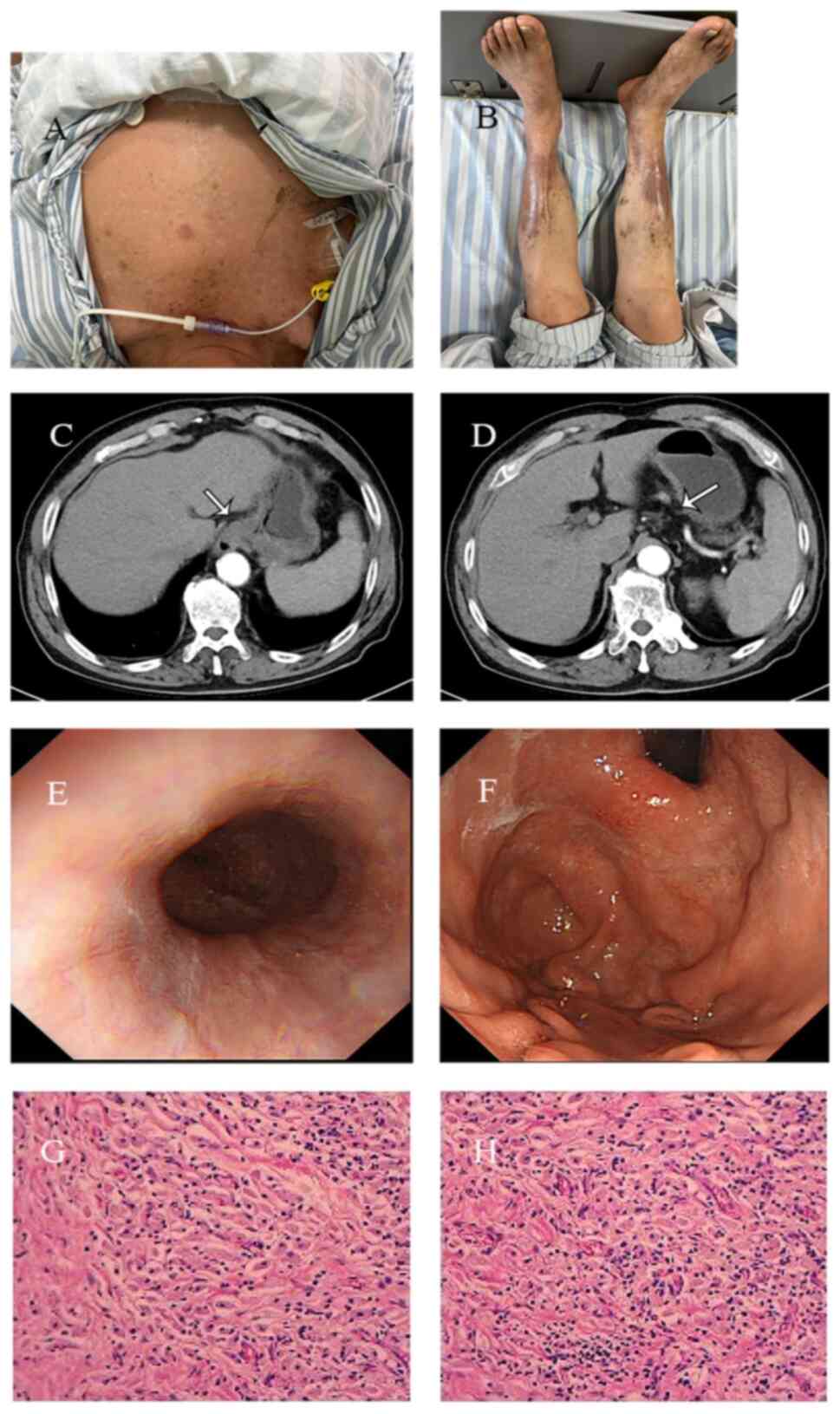
 | Figure 1Radiological, endoscopic, pathological
findings of gastric cardia adenocarcinoma and patient's skin before
and after therapy. (A) Computed tomography scan revealed a mass of
soft tissue in the fundus of the stomach, with uneven and moderate
enhancement, with more pronounced enhancement in the edges, and an
irregular serosal surface. (B) Multiple mildly enhanced and
slightly enlarged lymph nodes were detected around the fundus of
the stomach. (C) Gastroscopy revealed cardia stenosis, thus making
it difficult for the gastroscope to pass through. (D) The
gastroscopic findings indicated that the cardia extended to the
lower esophagus and the fundus of the stomach, with a large
irregular bulge, surface erosion, white coating and unclear
boundaries. The biopsy was brittle and easily bleeding. (E) The
pathological diagnosis was cardiac adenocarcinoma. (F-J) Prior
therapy, the patient developed a widespread rash all over the
body. |
Pathology results were consistent with
adenocarcinoma (Fig. 1E) and
immunohistochemistry revealed CKpan (+), CK8/18 (+), CK5/6 (-), P40
(-), Ki-67 (+; rate, 90%), Her-2 (1+), MLH (+), PMS2 (+), MSH2 (+),
MSH6 (+). The PD-L1 combined positive score was 60 % (Fig. 1E). According with the 8th edition
of the American Joint Committee on Cancer staging system for
gastric cancer and considering CT and endoscopy results, the
patient was diagnosed with TNM stage of cT3-4aN2-3M0, Stage III
(7). He was then treated with
three cycles of neoadjuvant therapy with sintilimab (200 mg on day
1), oxaliplatin (150 mg on day 1) and Tigio (S-1; 40 mg in the
morning, 60 mg in the evening on days 1-14). After the third cycle,
the patient complained of sporadic rash with pruritus on the front
chest, back and both lower limbs (Fig.
1F-H). The male patient was finally diagnosed with eczema
dermatitis at the outpatient clinic of the Institute of Dermatology
of the Chinese Academy of Medical Sciences. However, the homemade
medication containing Triamcinolone Acetonide Cream (40 g),
Allantoin Cream (40 g) and Vitamin E Cream (40 g) provided by the
hospital had no effect and therefore the eczema dermatitis became
more severe, gradually spreading from the lower limbs to the knees
(Fig. 1I and J). The skin on both lower limbs was red,
swollen and itchy (Fig. 1H).
On April 10, 2024, the patient was admitted again to
the Nanjing Jiangning Hospital of Traditional Chinese Medicine.
Upon hospital admission, the patient underwent comprehensive blood
tests. The results revealed high-sensitivity C-reactive protein
(CRP) levels of 5.52 mg/l, white blood cell (WBC) count of
3.66x109/l, elevated monocyte rate of 15.4%, eosinophil
rate of 10.2% and basophil rate of 1.3%. Additionally, hemoglobin
(HGB) levels of 121 g/l and platelet count (PLT) of
129x109/l were recorded. Coagulation tests revealed
D-dimer and fibrin degradation product (FDP) levels of 3.56 mg/l
and 7.36 µg/ml, respectively. Routine urinalysis, stool analysis,
liver and kidney function tests, blood lipid, electrolyte, troponin
I, carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP),
carbohydrate antigen (CA) 19-9, CA24-2, CA50 and CA724 levels, and
thyroid function and immune system parameters were all within
normal ranges. The patient reported unbearable itching and could
not stop scratching repeatedly. A dermatology consultation was
requested. The patient had no history of any skin diseases and he
was ultimately diagnosed with eczema dermatitis, which was likely
caused by the medication. The dermatologist recommended oral
desloratadine citrate disodium tablets and topical halometasone
ointment. The patient also received intravenous infusion of 40 mg
methylprednisolone daily for five consecutive days. After five
days, he switched to 20 mg methylprednisolone for two days,
followed by administration of 25 mg prednisone for one week. After
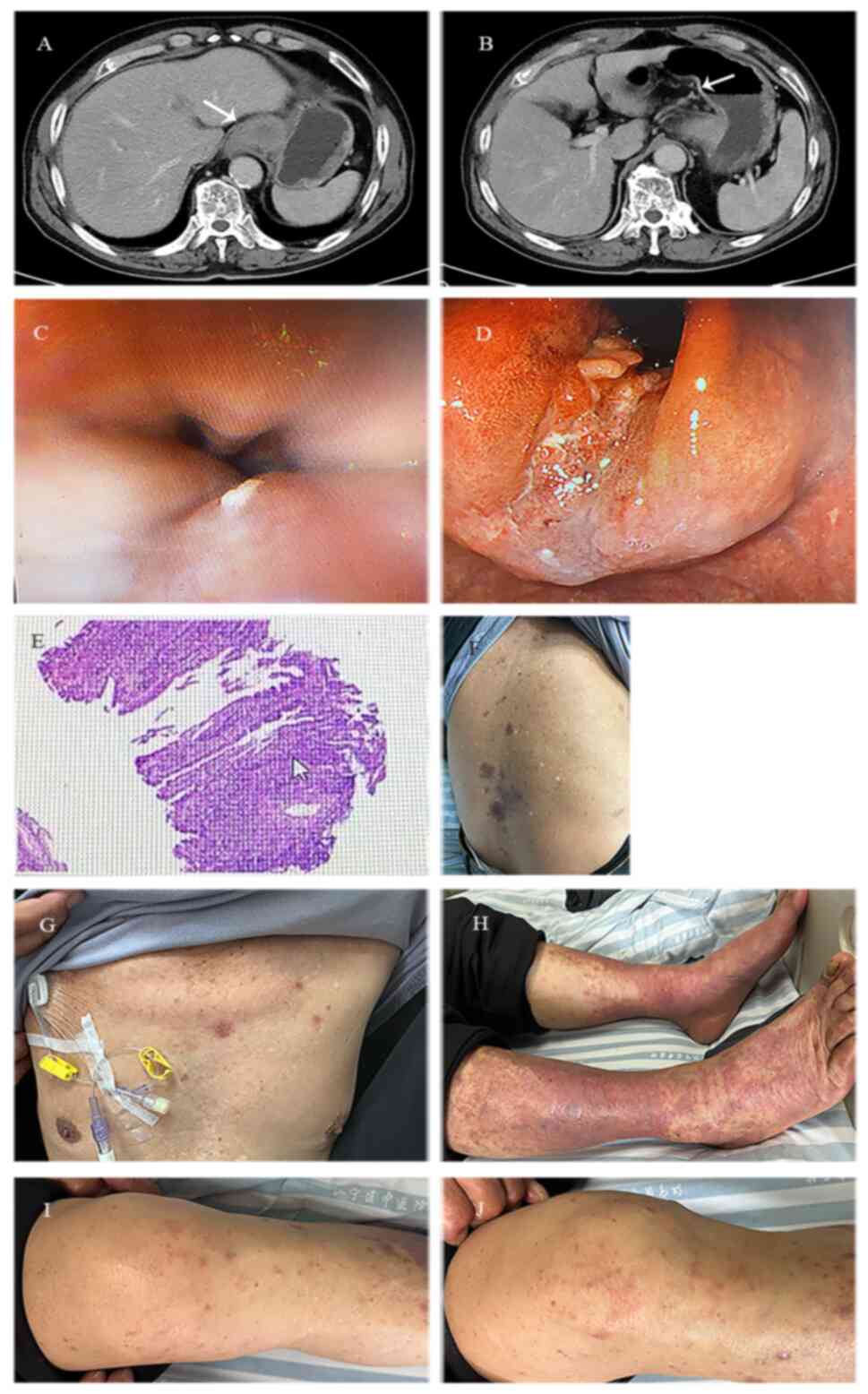
treatment, eczema dermatitis significantly improved (Fig. 2A and B), while the follow-up CT scan revealed
that the mass of the soft tissue at the fundus of the stomach was
significantly reduced, while the surrounding lymph nodes were
smaller (Fig. 2C and D).
On April 25, 2024, the patient was re-admitted to
the Nanjing Jiangning Hospital of Traditional Chinese Medicine to
evaluate the indications for surgery, since he and his family
strongly requested surgical treatment. Therefore, on April 26
gastroscopy was performed, which revealed inflammation at the
cardia and chronic gastritis with hyperplastic-like protrusions
(Fig. 2E and F). Preoperative blood tests revealed:
High-sensitivity CRP levels of 2.78 mg/l, WBC count of
8.92x109/l, lymphocyte percentage of 17.8%, and elevated
neutrophil and monocyte counts of 6.55x109/l and
0.67x109/l, respectively. HGB was 142 g/l, while PLT
count was reduced to 100x109/l. Furthermore, coagulation
tests revealed increased D-dimer (1.11 mg/l) and FDP (5.13 µg/ml)
levels. Lipid profile displayed enhanced triglyceride (2.08 mmol/l)
and total cholesterol (6.50 mmol/l). Finally, routine urinalysis,
stool analysis, liver and kidney function tests, electrolyte, CEA,
AFP, CA19-9, CA24-2, CA50 and CA724 levels and the infectious
disease panel results were all within normal limits.
On April 27, 2024, the patient underwent total
gastrectomy, esophagojejunal Roux-en-Y anastomosis and abdominal
lymph node dissection at the Gastrointestinal Surgery Department of
the Nanjing Jiangning Hospital of Traditional Chinese Medicine. The
postoperative pathology indicated chronic inflammation of the
cardiac mucosa, while no residual cancer tissue was found on the
upper and lower resection margins. No cancer metastasis was
detected in the lymph nodes around the cardia (0/13; Fig. 2G and H). Based on the pathological results, the
patient's response evaluation suggested pCR (Fig. 3).
On June 3, 2024, follow-up chest and abdominal CT
scans revealed no signs of tumor recurrence or lymph node
enlargement, indicative of malignancy. Tumor marker levels,
including those of CEA, AFP, CA199, CA24-2, CA50 and CA724, were
all within normal ranges. The patient is currently undergoing
regular follow-up examinations and has not received any further
antitumor therapy.
Discussion
A literature review identified 33 cases of adverse
reactions associated with sintilimab, including eight cases
involving skin-related complications (Table I). Among the aforementioned eight
cases, three cases of toxic epidermal necrolysis, one case of
lichenoid mucocutaneous reactions, one of lichenoid dermatitis, one
of refractory pruritus, one of bullous pemphigoid and one of eczema
dermatitis, were recorded. All eight patients demonstrated
improvement after treatment.
 | Table ICase analysis of literature on adverse
skin reaction caused by sintilimab. |
Table I
Case analysis of literature on adverse
skin reaction caused by sintilimab.
| Number | Sex | Age | Cancer | Immunotherapy
regimen | Occurrence time | Dermatological
diagnosis | Treatment | Stop/ continue
sintilimab | Outcome of the
adverse event |
|---|
| 1(8) | Male | 67 | Advanced lung
squamous carcinoma | Sintilimab combined
with paclitaxel and cisplatin | 33 days after first
cycle | Eczema
dermatitis | Hormones traditional
Chinese medicine | Stop | Skin symptoms
disappeared |
| 2(9) | Male | 59 | Centrally located
squamous cell lung carcinoma and pulmonary tuberculosis | Sintilimab combined
with paclitaxel and cisplatin | 10 days after the
post-oprerative adjuvant therapy | TEN | Intravenous
methylprednisolone and oral prednisone | Stop | Relieved |
| 3(10) | Male | 65 | Lymphoma | Sintilimab,
gemcitabine oxaliplatin | 11 days after first
cycle | TEN | Oral cetirizine
methylprednisolone immunoglobulin pipracillin sodium/tazobactam and
parenteral nutrition | Stop | Relieved |
| 4(11) | Male | 72 | Gallbladder
carcinoma | Sintilimab,
anlotinib | 2 weeks after
receiving 1 dose of sintimab | TEN | Methylprednisolone
immunoglobulin albumin encapsulation tapering of glucocortico and
oral nystatin | Stop | Relieved |
| 5(12) | Male | 38 | Non-small lung
adenocarcinoma | Sintilimab | After the fourth
cycle | Lichenoid
mucocutaneous reactions | Gargling with a
dexamethasone sodium phosphate solution | Continue | Oral mucosa lesions
reap peared regularly but the skin lesions did not |
| 6(13) | Male | 71 | Advanced Non-small
lung adenocarcinoma | Sintilimab | 1 week later after
the fifth cycle | Lichenoid
dermatitis | Traditional chinse
medine | Patient asked
stop | Relieved |
| 7(14) | Male | 70 | Colorectal
cancer | Sintilimab,
fruquintinib | After 5 months | Bullous
pemphigoid | Oral
methylprednisolone | Patient asked
continue | Relieved |
| 8(15) | Male | 55 | Gallbladder
neuroendocrine carcinoma | Sintilimab, etoposide
and cisplatin | 39 days after the
second cycle | Refractory
pruritus | Naloxone | Not mentioned | Relieved |
Gastric cancer remains one of the most common types
of cancer and still exhibits the 3rd highest mortality rate among
all cancers (16). Due to its
molecular and phenotypic diversity, the main treatment approach for
early-stage gastric cancer is endoscopic resection. However, since
the majority of patients with gastric cancer are diagnosed in the
middle or late stages of the disease, non-early operable gastric
cancer is commonly treated with surgery. Emerging evidence has
suggested that perioperative and adjuvant therapies can improve the
survival rate of patients with gastric cancer (17,18).
In China, immunotherapy combined with chemotherapy has been
approved as a first-line treatment strategy for advanced gastric
cancer (19). A previous study
demonstrated that the adoption of the S-1 plus oxaliplatin and
Tigio (SOX) regimen combined with a PD-1 inhibitor could improve
the pathological response rate in patients with locally advanced
gastric cancer (20). Sintilimab,
an immune drug independently developed in China, has demonstrated
significant efficacy in treating several types of malignant tumors
(21). However, while
immunotherapy has notably improved patient prognosis, it has also
been associated with immune-related adverse events (irAEs).
Skin toxicities are the most commonly reported irAEs
associated with ICIs (22). A wide
range of dermatological manifestations, varying in severity, can
occur in patients treated with ICIs, including vitiligo, lichenoid
dermatitis, psoriasis, bullous pemphigoid, granulomatous diseases,
drug rash with eosinophilia and systemic symptoms, and
Stevens-Johnson and Sweet syndromes (23,24).
Sintilimab-induced severe adverse skin reactions are rare. However,
they are associated with high mortality rates (25). Skin-related adverse reactions to
immunotherapy, such as rashes or dermatitis, typically occur during
the first or second cycle of treatment. In the present case report,
however, the rash appeared after the third cycle of sintilimab and
subsequently spread throughout the body. The patient experienced
difficulty in breathing when the rash occurred. The male patient
had no prior history of skin-related conditions and the gastric
cancer itself could not have caused a severe rash. Additionally, no
adverse skin reactions were observed when the patient was
previously treated with SOX. The multiple rashes on the patient's
body subsided completely after treatment with corticosteroids.
Based on the aforementioned findings, the physician suggested that
the rashes in this case were directly associated with the use of
sintilimab.
According to the ‘Management of
Immunotherapy-Related Toxicities Version 1.2022, National
Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in
Oncology’ published by the NCCN (26), the treatment approaches for
immune-related skin toxicity include both systemic and topical
therapy. For grade I adverse skin reactions, immunotherapy can be
continued, while topical emollient and moderate potency steroids
can be applied to the affected areas. Oral antihistamines can be
used to treat itching, while a medium-potency topical steroid can
be applied to the rash area. For grade II adverse skin reactions,
in addition to the aforementioned treatments, if the patient does
not respond to a topical emollient within one week of application,
treatment with 0.5 mg/kg/day prednisone and dermatologist
consultation should be considered. When the patient's skin adverse
reactions reach grade III or IV, treatment of the affected areas
with high potency topical steroids and prednisone/IV
methylprednisolone (0.5-1 mg/kg/day; increase dose up to 2
mg/kg/day if no improvement), urgent dermatology consultation and
possible inpatient care should be considered. In the present case
report, the patient's adverse skin reactions reached grade III and
he was therefore treated with intravenous methylprednisolone,
topical steroids and oral antihistamines. Immunotherapy with ICIs
should be held, and treatment should be discontinued (27). Therefore, the patient discontinued
sintilimab treatment, according to the NCCN and American Society of
Clinical Oncology guidelines (27).
For patients needing long-term steroids, especially
the elderly, diabetic or immunocompromised, it is vital to
implement proactive strategies to manage toxicity. Immunotherapy
should only be resumed once the toxicity has been reduced to a mild
level. Patients must also be informed of the risk of recurring
immune-related toxicities. For patients who have clearly benefited
from immunotherapy, it may be unnecessary to continue, as the
toxicity risks could outweigh the benefits (26-28).
In the present case, the patient experienced skin
adverse reactions without any accompanying organ toxicity. A
literature review similarly revealed no reports of organ toxicity
in cases involving adverse skin reactions. However, in clinical
practice, it is common to encounter patients with immune-related
multi-organ toxicities. For instance, there was a case (Chen et
al, unpublished data) of immune-related hepatitis, colitis,
pneumonitis, and hypothyroidism occurring together, but without any
skin side reaction involvement. Furthermore, a recent case report
highlights cases of multi-organ toxicities induced by immunotherapy
(29).
In the present case study, the patient's eczema
dermatitis completely subsided after hormone therapy and the
patient achieved pCR after surgery. This is a rare finding compared
with the previous reported cases. Based on the aforementioned
finding it was hypothesized that the patients who experienced irAEs
could achieve improved outcomes. However, this finding warrants
further investigation.
The early diagnosis and reasonable management of
patients with irAEs are very crucial. Therefore, early detection,
active intervention and dynamic follow-up are of great importance.
The early identification and timely treatment of these adverse
events could serve a significant role in improving prognosis and
response to immunotherapy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Nanjing Health
Science and Technology Development Special Fund Project (grant no.
YKK21232).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
YB contributed to manuscript writing, literature
search and acquisition of data. HC undertook the treatment and
monitoring of the patient, while he was also involved in study
conception and design. YD, HS, HF and YY contributed to manuscript
drafting, aggregation of materials and data analysis. YB was
involved in manuscript revision and reviewing for intellectual
content, and interpretation of data. YB and HC confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present case report was performed according to
the guidelines of the Declaration of Helsinki and approved
(approval no. JNZ-2024-N18, 16 May 2024) by the Institutional
Ethics Committee of the Nanjing Jiangning Hospital of Traditional
Chinese Medicine (Nanjing, China).
Patient consent for publication
The patient himself and his son Written informed
consent for the publication of this case report and accompanying
images was provided by the patient and his son.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Banday AH and Abdalla M: Immune checkpoint
inhibitors: Recent clinical advances and future prospects. Curr Med
Chem. 30:3215–3237. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hoy SM: Sintilimab: First global approval.
Drugs. 79:341–346. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L,
Shu Y, Li J, Zhao J, Pan H, et al: Sintilimab plus chemotherapy for
unresectable gastric or gastroesophageal junction cancer: The
ORIENT-16 randomized clinical trial. JAMA. 330:2064–2074.
2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang L, Wu Q, Chi H and Yang G: Letter to
the editor for the article ‘Efficacy and safety of neoadjuvant
sintilimab in combination with FLOT chemotherapy in patients with
HER2-negative locally advanced gastric or gastroesophageal junction
adenocarcinoma: An investigator-initiated, single-arm, open-label,
phase II study’. Int J Surg. 110:6026–6027. 2024.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ramos-Casals M, Brahmer JR, Callahan MK,
Flores-Chavez A, Keegan N, Khamashta MA, Lambotte O, Mariette X,
Prat A and Suarez-Almazor ME: Immune-related adverse events of
checkpoint inhibitors. Nat Rev Dis Primers. 6(38)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ruggiero R, Fraenza F, Scavone C, di Mauro
G, Piscitelli R, Mascolo A, Ferrajolo C, Rafaniello C, Sportiello
L, Rossi F and Capuano A: Immune checkpoint inhibitors and
immune-related adverse drug reactions: Data from Italian
pharmacovigilance database. Front Pharmacol. 11(830)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu S, Wang Z, Ge Y and Zhao Y: Prognostic
significance of an innovative staging system based on the
logarithmic odds of positive lymph nodes for resectable
gastroesophageal cancer after neoadjuvant chemoradiation: A
population-based study with external validation of data. J Transl
Med. 22(801)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yan J, Ma N, Qiao WL, Liu KQ, Liu DW, Wang
Y, Qiao TT, Hao XQ and Zheng MD: Adverse skin reactions induced by
sintilimab in advanced lung squamous carcinoma: A case report and
review of the literature. Ann Transl Med. 10(1411)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Li G, Gong S, Wang N and Yao X: Toxic
epidermal necrolysis induced by sintilimab in a patient with
advanced non-small cell lung cancer and comorbid pulmonary
tuberculosis: A case report. Front Immunol.
13(989966)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang W, Xu X, Xia D, Wang H, Jiang J and
Yang G: Toxic epidermal necrolysis associated with
chemoimmunotherapy for lymphoma: Case report and literature review.
Immunotherapy. 14:275–282. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhao Y, Cao Y, Wang X and Qian T:
Treatment of PD-1 inhibitor-associated toxic epidermal necrolysis:
A case report and brief review. Onco Targets Ther. 15:345–351.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhou S, Zhang Z, Feng X, Zhao C and Jiang
L: Lichenoid mucocutaneous reactions associated with sintilimab
therapy in a non-small cell lung adenocarcinoma patient: Case
report and review. Front Pharmacol. 14(1276788)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu Y, Tang J, Yu LY and Jiang Q:
Successful treatment of immune-related lichenoid dermatitis by
Weiling decoction in a patient with non-small cell lung cancer: A
case report and review of literature. Explore (NY). 19:730–735.
2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang T, Shao Q, Xiao C and Liu L: Case
report: Bullous pemphigoid associated with sintilimab therapy for
pMMR/MSS colorectal cancer. Front Oncol. 13(1124730)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen L, Cao X, Luo X and Jiang T:
Refractory pruritus caused by sintilimab and its clinical
management: A case report. Heliyon. 10(e34107)2024.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rawla P and Barsouk A: Epidemiology of
gastric cancer: Global trends, risk factors and prevention. Prz
Gastroenterol. 14:26–38. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zeng Y and Jin RU: Molecular pathogenesis,
targeted therapies, and future perspectives for gastric cancer.
Semin Cancer Biol. 86:566–582. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang G, Huang Y, Zhou L, Yang H, Lin H,
Zhou S, Tan Z and Qian J: Immunotherapy and targeted therapy as
first-line treatment for advanced gastric cancer. Crit Rev Oncol
Hematol. 198(104197)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Huang X, Fang J, Huang L, Chen H, Chen H,
Chai T, Ye Z, Chen H, Xu Q, Du Y and Yu P: SOX combined with
sintilimab versus SOX alone in the perioperative management of
locally advanced gastric cancer: A propensity score-matched
analysis. Gastric Cancer. 26:1040–1050. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jia R, Li Y, Xu N, Jiang HP, Zhao CH, Liu
RR, Shi Y, Zhang YY, Wang SY, Zhou H and Xu JM: Sintilimab in
patients with previously treated metastatic neuroendocrine
neoplasms. Oncologist. 27:e625–e632. 2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wongvibulsin S, Pahalyants V, Kalinich M,
Murphy W, Yu KH, Wang F, Chen ST, Reynolds K, Kwatra SG and Semenov
YR: Epidemiology and risk factors for the development of cutaneous
toxicities in patients treated with immune-checkpoint inhibitors: A
United States population-level analysis. J Am Acad Dermatol.
86:563–572. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Weber JS, Dummer R, de Pril V, Lebbé C and
Hodi FS: Patterns of onset and resolution of immune-related adverse
events of special interest with ipilimumab: Detailed safety
analysis from a phase 3 trial in patients with advanced melanoma.
Cancer. 119:1675–1682. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Teng YS and Yu S: Molecular mechanisms of
cutaneous immune-related adverse events (irAEs) induced by immune
checkpoint inhibitors. Curr Oncol. 30:6805–6819. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ye Z, Yang W, Xuan B, Li X, He J, Si H and
Ma W: Efficacy and safety evaluation of sintilimab for cancer
treatment: A systematic review and meta-analysis of randomized
controlled trials. Front Pharmacol. 13(895187)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Thompson JA, Schneider BJ, Brahmer J,
Achufusi A, Armand P, Berkenstock MK, Bhatia S, Budde LE, Chokshi
S, Davies M, et al: Management of immunotherapy-related toxicities,
version 1.2022, NCCN clinical practice guidelines in oncology. J
Natl Compr Canc Netw. 20:387–405. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Schneider BJ, Naidoo J, Santomasso BD,
Lacchetti C, Adkins S, Anadkat M, Atkins MB, Brassil KJ, Caterino
JM, Chau I, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: ASCO
guideline update. J Clin Oncol. 39:4073–4126. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhou Q, Qin Z, Wu G, Yan P, Wang Q, Qu J,
Jiang J and Ye D: Sintilimab-induced myocarditis suspected in a
patient with esophageal cancer and followed septic shock: Case
report and literature review. Front Oncol.
14(1465395)2024.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ni CX, Zhao Y, Qian H, Fu H, Yan YY, Qiu
YS, Zhou CC, Huang F, Shen FM, Li DJ and Xu Q: Long survival in a
pancreatic carcinoma patient with multi-organ toxicities after
sintilimab treatment: A case report. Front Pharmacol.
14(1121122)2023.PubMed/NCBI View Article : Google Scholar
|