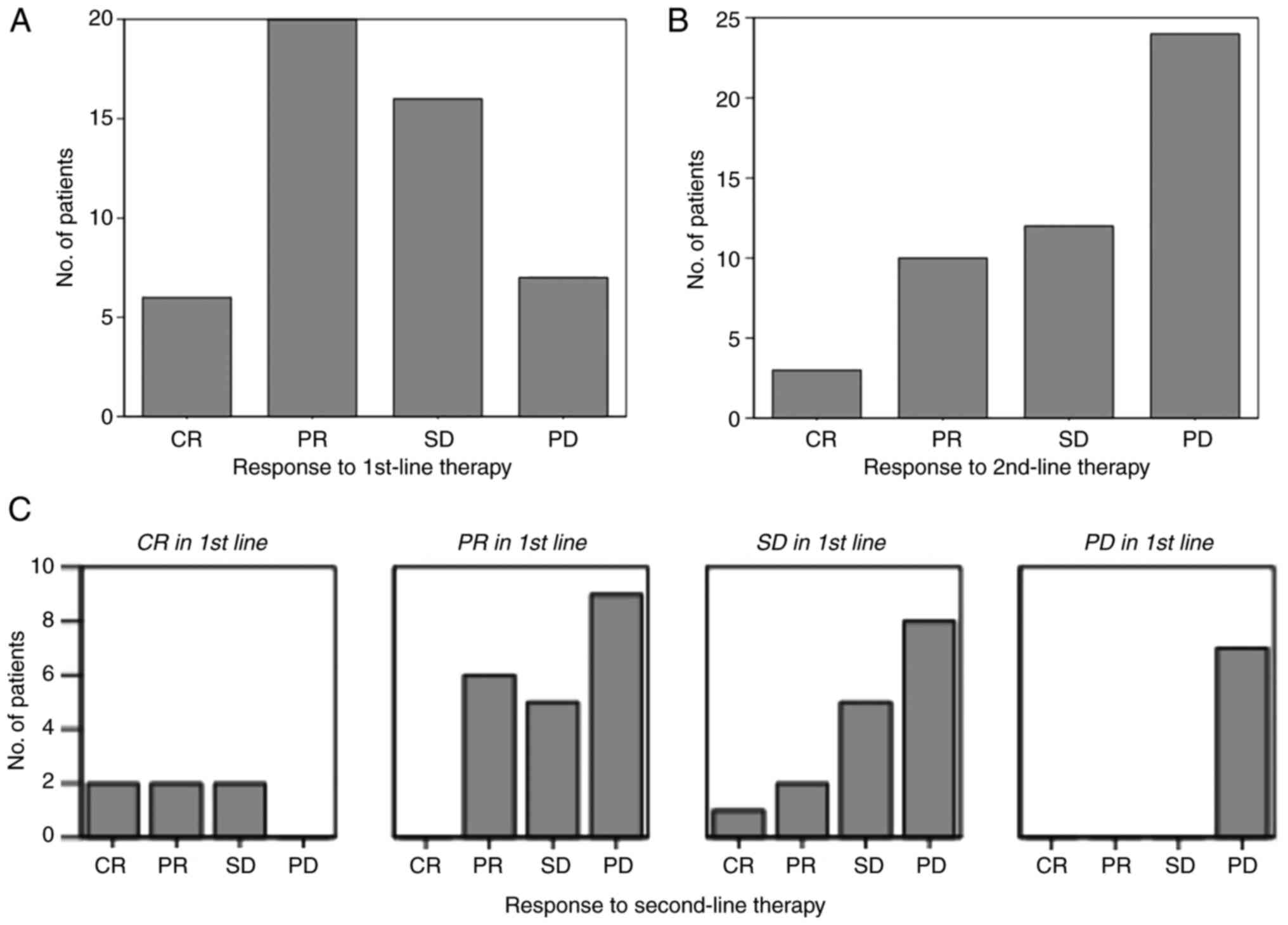
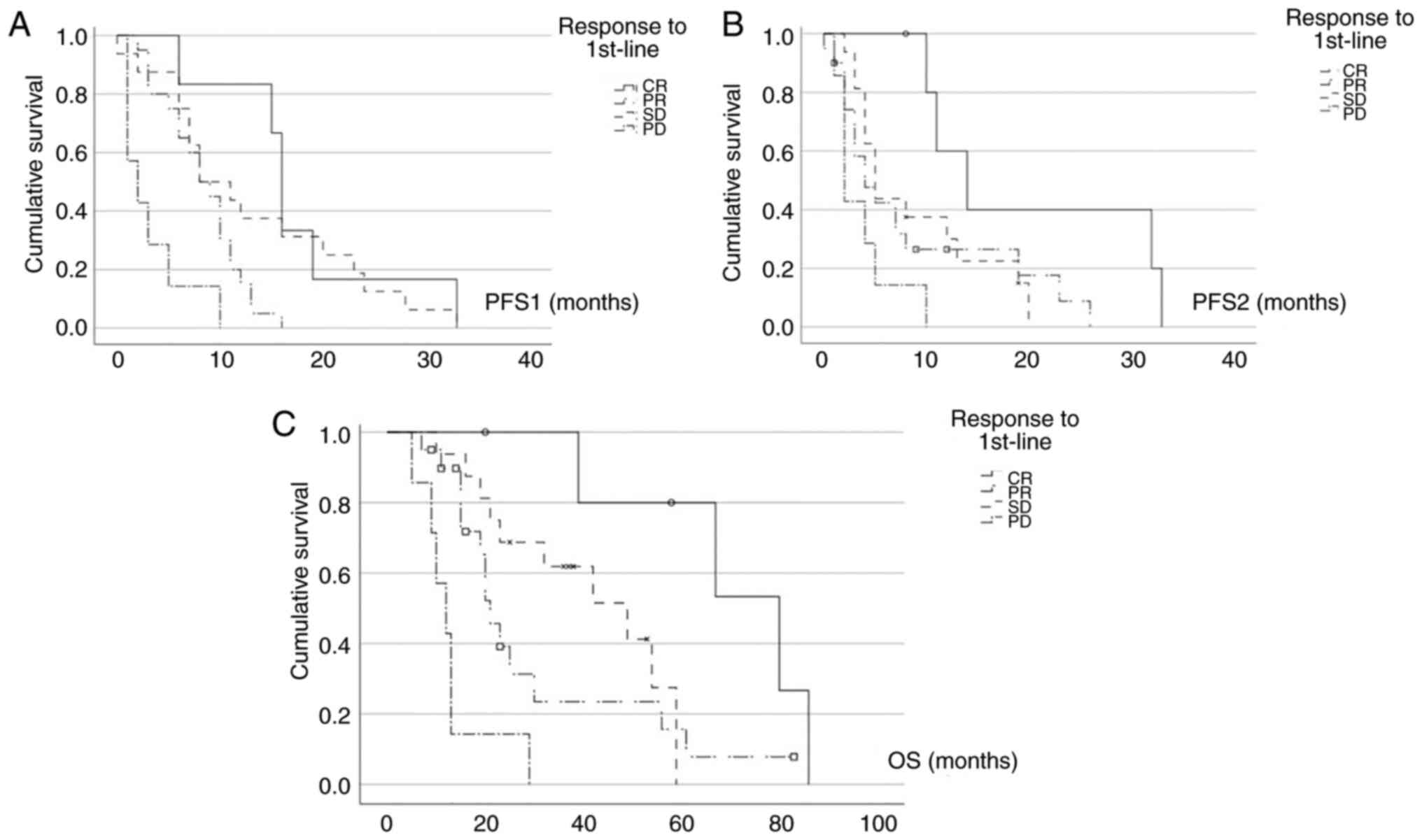
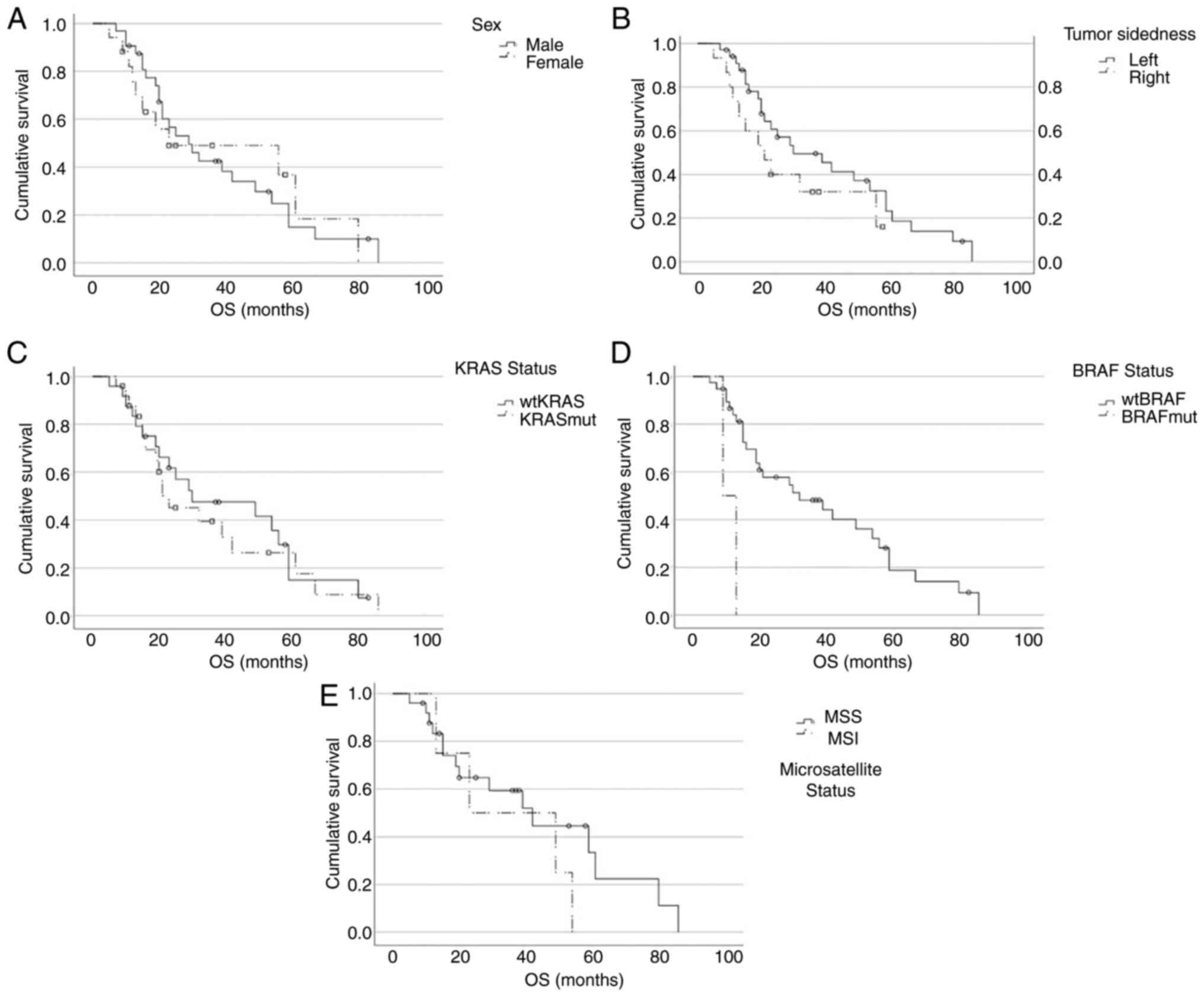
|
1
|
De Falco V, Napolitano S, Roselló S,
Huerta M, Cervantes A, Ciardiello F and Troiani T: How we treat
metastatic colorectal cancer. ESMO Open. 4 (Suppl
2)(e000813)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tharin Z, Blanc J, Alaoui IC, Bertaut A
and Ghiringhelli F: Influence of first line chemotherapy strategy
depending on primary tumor location in metastatic colorectal
cancer. J Gastrointest Oncol. 12:1509–1517. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ogura T, Kakuta M, Yatsuoka T, Nishimura
Y, Sakamoto H, Yamaguchi K, Tanabe M, Tanaka Y and Akagi K:
Clinico-pathological characteristics and prognostic impact of
colorectal cancers with NRAS mutations. Oncol Rep. 32:50–56.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Network NCC. NCCN Clinical Practice
Guidelines in Oncology (NCCN Guidelines): Colon Cancer. In.
2022.
|
|
5
|
Tejpar S, Stintzing S, Ciardiello F,
Tabernero J, Van Cutsem E, Beier F, Esser R, Lenz HJ and Heinemann
V: Prognostic and predictive relevance of primary tumor location in
patients with RAS wild-type metastatic colorectal cancer:
Retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA
Oncol. 3:194–201. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Antoniotti C, Borelli B, Rossini D,
Pietrantonio F, Morano F, Salvatore L, Lonardi S, Marmorino F,
Tamberi S, Corallo S, et al: AtezoTRIBE: A randomised phase II
study of FOLFOXIRI plus bevacizumab alone or in combination with
atezolizumab as initial therapy for patients with unresectable
metastatic colorectal cancer. BMC Cancer. 20(683)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vodenkova S, Buchler T, Cervena K,
Veskrnova V, Vodicka P and Vymetalkova V: 5-fluorouracil and other
fluoropyrimidines in colorectal cancer: Past, present and future.
Pharmacol Ther. 206(107447)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Culy CR, Clemett D and Wiseman LR:
Oxaliplatin. A review of its pharmacological properties and
clinical efficacy in metastatic colorectal cancer and its potential
in other malignancies. Drugs. 60:895–924. 2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fujita KI, Kubota Y, Ishida H and Sasaki
Y: Irinotecan, a key chemotherapeutic drug for metastatic
colorectal cancer. World J Gastroenterol. 21:12234–12248.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cervantes A, Adam R, Roselló S, Arnold D,
Normanno N, Taïeb J, Seligmann J, De Baere T, Osterlund P, Yoshino
T, et al: Metastatic colorectal cancer: ESMO clinical practice
guideline for diagnosis, treatment and follow-up. Ann Oncol.
34:10–32. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Aparicio J, Virgili Manrique AC, Capdevila
J, Muñoz Boza F, Galván P, Richart P, Oliveres H, Páez D, Hernando
J, Serrano S, et al: Randomized phase II trial of
FOLFIRI-panitumumab compared with FOLFIRI alone in patients with
RAS wild-type circulating tumor DNA metastatic colorectal cancer
beyond progression to first-line FOLFOX-panitumumab: the BEYOND
study (GEMCAD 17-01). Clin Transl Oncol. 24:2155–2165.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dariya B, Aliya S, Merchant N, Alam A and
Nagaraju GP: Colorectal cancer biology, diagnosis, and therapeutic
approaches. Crit Rev Oncog. 25:71–94. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bukowski K, Kciuk M and Kontek R:
Mechanisms of multidrug resistance in cancer chemotherapy. Int J
Mol Sci. 21(3233)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
organization for research and treatment of cancer, national cancer
institute of the united states, national cancer institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tirumani SH, Kim KW, Nishino M, Howard SA,
Krajewski KM, Jagannathan JP, Cleary JM, Ramaiya NH and Shinagare
AB: Update on the role of imaging in management of metastatic
colorectal cancer. Radiographics. 34:1908–1928. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Campos Gudiño R, McManus KJ and
Hombach-Klonisch S: Aberrant HMGA2 expression sustains genome
instability that promotes metastasis and therapeutic resistance in
colorectal cancer. Cancers (Basel). 15(1735)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hammond WA, Swaika A and Mody K:
Pharmacologic resistance in colorectal cancer: A review. Ther Adv
Med Oncol. 8:57–84. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yu H, Lim HH, Tjokro NO, Sathiyanathan P,
Natarajan S, Chew TW, Klonisch T, Goodman SD, Surana U and Dröge P:
Chaperoning HMGA2 protein protects stalled replication forks in
stem and cancer cells. Cell Rep. 6:684–697. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Summer H, Li O, Bao Q, Zhan L, Peter S,
Sathiyanathan P, Henderson D, Klonisch T, Goodman SD and Dröge P:
HMGA2 exhibits dRP/AP site cleavage activity and protects cancer
cells from DNA-damage-induced cytotoxicity during chemotherapy.
Nucleic Acids Res. 37:4371–4384. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Deng X, Kong F, Li S, Jiang H, Dong L, Xu
X, Zhang X, Yuan H, Xu Y, Chu Y, et al: A KLF4/PiHL/EZH2/HMGA2
regulatory axis and its function in promoting
oxaliplatin-resistance of colorectal cancer. Cell Death Dis.
12(485)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Peter S, Yu H, Ivanyi-Nagy R and Dröge P:
Cell-based high-throughput compound screening reveals functional
interaction between oncofetal HMGA2 and topoisomerase I. Nucleic
Acids Res. 44(e162)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Benson AB, Venook AP, Al-Hawary MM, Arain
MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, et
al: Colon cancer, version 2.2021, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 19:329–359. 2021.PubMed/NCBI View Article : Google Scholar
|