Introduction
Colorectal cancer (CRC) is the third most frequently
diagnosed cancer worldwide and the second leading cause of
cancer-associated deaths. Unfortunately, 20% of patients show
distant metastases at diagnosis and about 35% will develop
metastases during the course of the disease (1). In 75-90% of advanced cases, it is not
possible to resect all lesions and thus treatment with palliative
chemo-immunotherapy is applied (2). Although current first- and
second-line therapies consider genetic characteristics, like
KRAS/NRAS/BRAF mutations, microsatellite instability (MSI), and
other factors, including tumour location, age, and co-morbidities
(3), the therapeutic mainstay
consists of 5-Fluorouracil (5-FU)-based chemotherapy.
Generally, 5-FU or its prodrug capecitabine is
applied with or without irinotecan and/or oxaliplatin, plus
targeted immunotherapy, like the anti-vascular endothelial growth
factor (VEGF) antibody bevacizumab or the anti-epidermal growth
factor receptor (EGFR) agents cetuximab or panitumumab (4). Anti-EGFR antibodies are less
effective in right-sided CRC and not effective in RAS-mutated CRC
(5,6).
5-FU is an antimetabolite, which, due to its
structural similarity to the ribonucleic acid uracil, gets
incorporated into RNA instead of uracil and leads to the inhibition
of DNA biosynthesis and cell growth. It also inhibits the
thymidylate synthase, an enzyme important for pyrimidine synthesis.
5-FU is often combined with Leucovorin® (folinic acid),
a chemo-protectant that blocks the side effects of 5-FU and is used
for the potentiation of the 5-FU tumouricidal effects. It increases
patients' survival and response rates (7). Oxaliplatin is a platinum compound and
causes inhibition of DNA synthesis by crosslinking DNA (8). Irinotecan is an analogue of
camptothecin, which is converted to its active metabolite in the
body. Irinotecan inhibits topoisomerase 1, causes inhibition of DNA
synthesis and DNA double strand breaks, and ultimately leads to
cell cycle arrest and cell death (9).
The combination-chemotherapy regimens FOLFOX
(folinic acid, 5-FU and oxaliplatin; CAPOX when oral capecitabine
is applied) and FOLFIRI (folinic acid, 5-FU and irinotecan; CAPIRI
when oral capecitabine is applied) show equal efficacy, however,
have a different toxicity profile, which can guide decision-making
(10).
The triplet FOLFOXFIRI can be used in certain cases,
e.g. for fit patients without co-morbidities or for patients
harbouring a BRAF mutation (10).
In mCRC patients, current second-line treatment
depends mainly on the prior first-line treatment. Patients, who
have received an oxaliplatin-containing doublet combination
therapy, are switched to an irinotecan-containing doublet, and vice
versa. Again, in second-line treatment, anti-EGFR monoclonal
antibodies (mAbs) and bevacizumab can be added to the chemotherapy.
Aflibercept is an alternative anti-VEGF agent, which can be applied
in second-line therapy (10).
Anti-VEGF agents can be effective, although the patient progressed
during first-line treatment. Until recently, anti-EGFR antibodies
were considered only useful in one line of treatment. New treatment
options suggest that reintroduction or continuing use of anti-EGFR
therapy can be beneficial, if no KRAS mutations have occurred
during the initial treatment (7,11).
However, tumours can be resistant against
chemotherapeutic agents, either prior to treatment start (‘innate
chemoresistance’) or during the course of treatment (‘acquired
chemoresistance’). Often tumours are not only resistant to one
drug, but also to other or similar agents (‘multidrug-resistance’)
(7,12). Many mechanisms, such as elevated
metabolism, enhanced drug efflux, increased DNA repair capacity,
growth factors, or genetic and epigenetic factors are involved in
the process of chemoresistance, but this process is yet not fully
understood (13).
This study thus aimed to investigate the role of
multidrug-resistance in a real-world setting: this study explored,
if under current first- and second-line protocols for mCRC,
response to first-line therapy can predict response to second-line
therapy. The null-hypothesis of this study is that the rates of
remissions in second-line chemo-immunotherapy do not differ between
patients with CRC stage IV, who had a response in first-line
chemo-immunotherapy, and patients, who had no response in
first-line chemo-immunotherapy.
Patients and methods
Study design
This study is a retrospective cohort study analysing
patients' data from the data management system of the University
Hospital Krems, and the Oncology Information System (OIS) of Lower
Austria. All participants received treatment for CRC stage IV at
the Department of Oncology of the University Hospital Krems.
The study was approved by the Commission for
Scientific Integrity and Ethics at the Karl Landsteiner University
of Health Sciences in September 2022 (EK No. 1046/2022) and was
conducted according to the Declaration of Helsinki. Due to the
retrospective nature of this study informed consent was waived as
approved by the Commission for Scientific Integrity and Ethics at
the Karl Landsteiner University of Health Sciences.
Study population and statistical
analyses
Between 01.01.2015 and 31.12.2021, 125 patients were
diagnosed with advanced CRC and received treatment at the
University Hospital Krems. After screening all eligible patients
against the predefined inclusion and exclusion criteria, 49
patients could be included in this study. These patients were
analysed according to their response to treatment for mCRC.
Inclusion criteria were patients with histologically
proven CRC stage IV, who underwent palliative treatment with
chemo-immunotherapy consisting of either 5-FU or the 5-FU
derivative capecitabine with or without oxaliplatin and/or
irinotecan, respectively, combined with the mAbs bevacizumab,
cetuximab or panitumumab, between 01.01.2015 and 31.12.2021 at the
University Hospital Krems. Exclusion criteria were patients, who
were cured by surgery following initial or adjuvant
chemo-immunotherapy and not experiencing a recurrence of the
disease, other cancer types or stages and patients under the age of
18.
Treatment responses to first- and second-line
therapy were confirmed according to the Response Evaluation
Criteria in Solid Tumors (RECIST), which comprise complete response
(CR), partial response (PR), stable disease (SD) and progressive
disease (PD) based on computer tomography (CT) or magnetic
resonance imaging (MRI) re-staging examinations after initiation of
chemo-immunotherapy. These criteria were established in 2000 by an
international collaboration including the European Organisation for
Research and Treatment of Cancer (EORTC), National Cancer Institute
of the United States, and the National Cancer Institute of Canada
Clinical Trials Group (14), later
updated in 2009(15) and have
meanwhile evolved as standard treatment response criteria in the
majority of clinical trials for solid tumours (16).
All data were analysed and presented in a
pseudonymized form, as every patient received initially a
pseudonymized identification number (001, 002…).
The following variables were analysed and used for
statistical evaluation: The descriptive parameters age (in years,
at begin of treatment), sex (female, male), tumour sidedness (left,
right), KRAS status (wild-type, mutant), NRAS status (wild-type,
mutant), BRAF status (wild-type, mutant) as well as microsatellite
status (MSS, MSI).
Measured outcome parameters included response to
first-line treatment (CR, PR, SD, PD), response to second-line
treatment (CR, PR, SD, PD), progression free survival of first-line
treatment (PFS1), progression free survival of second-line
treatment (PFS2) and overall survival (OS).
After analysing the above variables, patients were
assigned to different groups based on their remission status upon
first-line treatment (CR, PR, SD, PD) to compare their response
rates to second-line treatment. Data were analysed according to an
intent-to-treat approach. P<0.05 was considered to indicate a
statistically significant difference. Moreover, Kaplan-Meier curves
of progression free and overall survival were plotted and analysed
using the log-rank test. This was done for stratification based on
therapy-response to first- or second-line therapy or on patient or
tumour specific factors. All statistical analyses were done with
IBM SPSS Statistics 29.0 and Microsoft Excel for Microsoft 365
(Version 2303).
Results
Patient population
In total, 49 patients were included in this study:
17 females (34.7%) and 32 (65.3%) males. The age span was 38 to 78
years, with a median age at therapy start of 63 years. All patients
were treated at the Department of Internal Medicine 2 of the
University Hospital Krems for CRC stage IV. The gender distribution
was shifted towards male sex in the patient population. Table I shows an overview of the patients'
characteristics including gender, age, tumour sidedness as well as
mutational and microsatellite status.
 | Table ICharacteristics of the patient
population. |
Table I
Characteristics of the patient
population.
| Characteristic | Value |
|---|
| Median age, years
(range) | 63 (38-78) |
| Sex, n (%) | |
|
Female | 17 (34.7%) |
|
Male | 32 (65.3%) |
| Tumour sidedness, n
(%) | |
|
Left | 34 (69.4%) |
|
Right | 15 (30.6%) |
| KRAS mutation, n
(%) | |
|
Positive | 25 (51.0%) |
|
Negative | 24 (49.0%) |
| NRAS mutation, n
(%) | |
|
Positive | 4 (8.2%) |
|
Negative | 38 (77.5%) |
|
Not
determined | 7 (14.3%) |
| BRAF mutation, n
(%) | |
|
Positive | 2 (4.1%) |
|
Negative | 38 (77.5%) |
|
Not
determined | 9 (18.4%) |
| Microsatellite
status, n (%) | |
|
MSI | 4 (8.2%) |
|
MSS | 25 (51.0%) |
|
Not
determined | 20 (40.8%) |
Patients' tumour specimens were tested for various
genetic mutations and alterations: all tumour samples were tested
for KRAS mutations, here, 25 (51.0%) were positive. Only 2 (4.1%)
samples displayed a BRAF mutation, one patient with a right-sided
primary tumour, the other with left-sided CRC. Moreover, 4 (8.2%)
tumour samples had an NRAS mutation and 4 patients (8.2%) harboured
MSI. These tests, however, were not performed in all tumour patient
samples (Table I), as in the first
years of the observation period, these tests have not been
routinely established. Two patients harboured two mutations at the
same time: one patient had KRAS and NRAS alterations, the other
NRAS and BRAF.
Furthermore, the location of the tumour of each
patient was evaluated. 34 (69.4%) patients had a primary tumour on
the left side of the colon, while only 15 (30.6%) participants were
diagnosed with right-sided CRC. Tumors located in the lower parts
of the large intestine, the sigmoid colon and rectum, predominated
in our study cohort. 10 (66.7%) of the patients with right-sided
CRC were women, the other 5 (33.4%) were men. KRAS mutations were
nearly equally distributed between these two groups with 18 (52.9%)
of 34 patients with a left-sided tumour, and 7 (46.7%) of 15
patients with a right-sided tumour. Considering MSI, 2 of 4
patients had right-sided CRC and the other 2 had left-sided
CRC.
Response
All included patients received anti-tumour therapy
in a first- and second-line setting. Table II shows a general overview of the
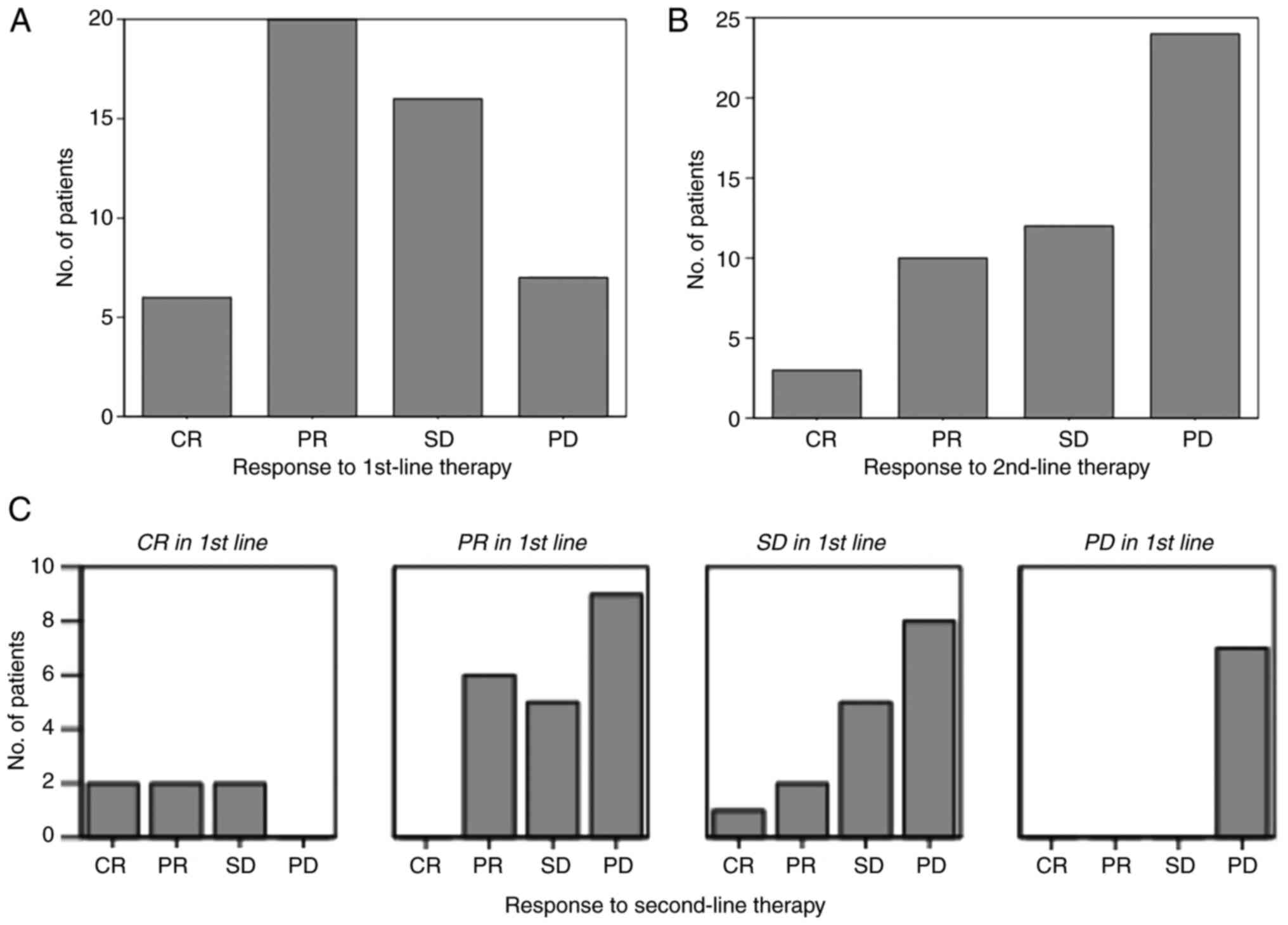
response and survival of patients after each line of therapy. After
first-line therapy 6 patients (12.2%) achieved a complete response,
while the majority reached either a partial response (20; 40.8%) or
stable disease (16; 32.7%). Overall response rate (ORR) was thus
53%. Conversely, in 7 (14.3%) patients the disease showed a
progressive behaviour despite first-line treatment (Fig. 1A).
 | Table IIResponse and survival after first- and
second-line therapy (overall cohort). |
Table II
Response and survival after first- and
second-line therapy (overall cohort).
| Variable | N (%) |
|---|
| Response
first-line | |
|
CR | 6 (12.2) |
|
PR | 20 (40.8) |
|
SD | 16 (32.7) |
|
PD | 7 (14.3) |
| Response
second-line | |
|
CR | 3 (6.1) |
|
PR | 10 (20.4) |
|
SD | 12 (24.5) |
|
PD | 24 (49.0) |
| Progress after
second-line | |
|
Yes | 43 (87.8) |
|
No | 6 (12.2) |
| Living status at the
end of the observation period | |
|
Alive | 13 (26.5) |
|
Dead | 36 (73.5) |
In second-line treatment, nearly half of the
patients (24; 49%) had progressive disease. 10 (20.4%) participants
achieved a partial response and 12 (24.5%) stable disease. Only 3
(6.1%) patients reached a complete response; ORR was 26.5%, as can
be seen in Fig. 1B. The 2 patients
with a BRAF mutation had progressive disease in both therapy
lines.
When grouped by first-line therapy response,
Fig. 1C clearly displays that all
patients included in this study with a complete response after
first-line therapy have achieved at least ‘stable disease’ in
second-line therapy (ORR=66.6%). On the contrary, patients with
progressive disease after first-line treatment did also not respond
to second-line therapy (ORR=0%).
Survival analyses
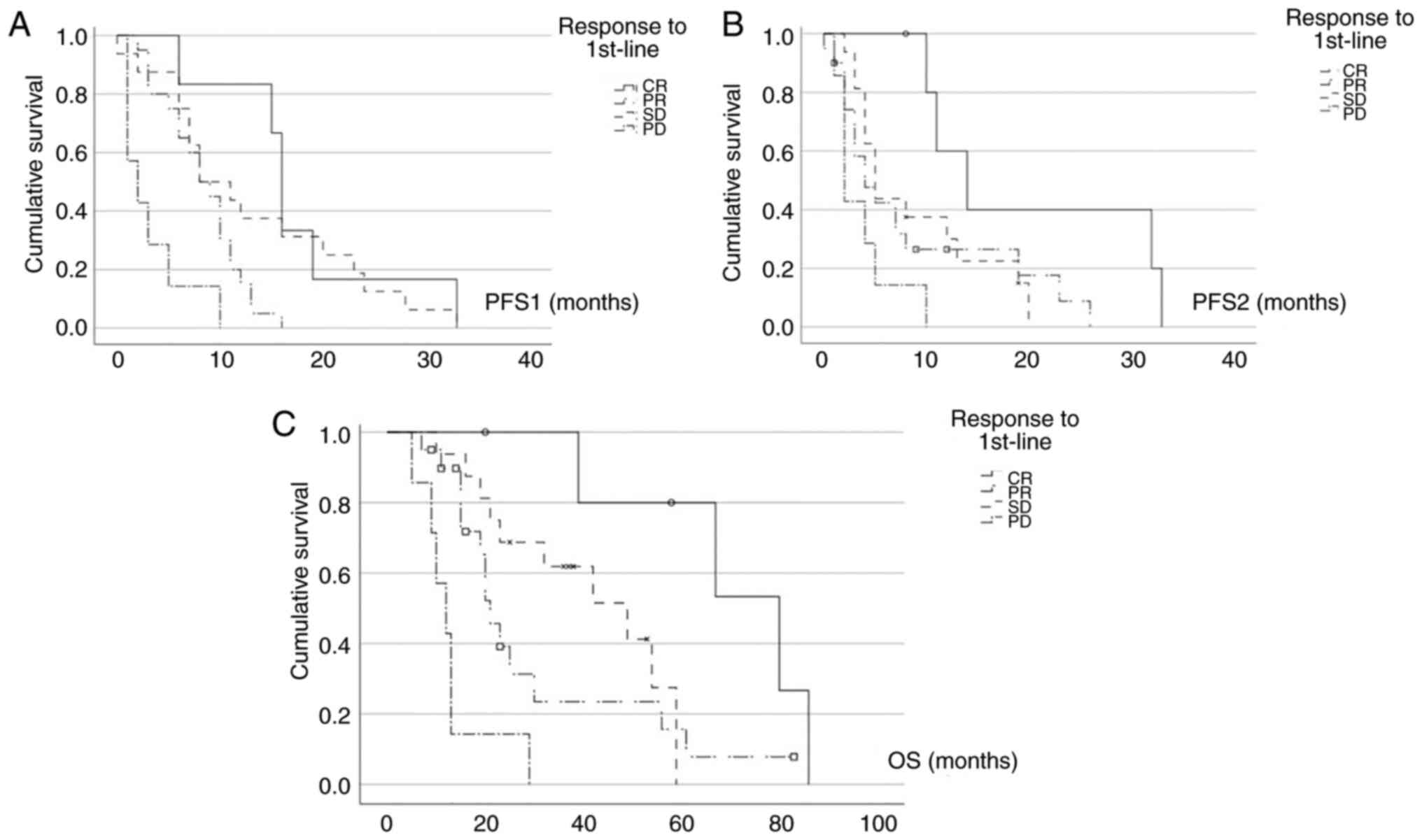
Next, we performed survival analyses. With regard to
progression-free survival, Fig. 2A
shows the PFS curves of first-line therapy (PFS1) based on the
response to first-line therapy. Patients, who achieved a CR in
first-line treatment had the longest PFS1, with a median PFS1 of 16
months [m; 95% confidence interval (CI): 14.9-17.1m] which was
statistically significantly longer than for patients with PR
(median PFS1=8m, 95% CI: 5.1-10.9; P=0.002) and compared to
patients with PD (median PFS1=2m, 95% CI: 0-4.6; P<0.001). Also,
patients with a PR in first-line therapy had statistically
significantly longer PFS1 than patients with PD (P=0.003). This
could also be observed for patients with SD (median PFS1=8m, 95%
CI: 2.8-13.2) compared to patients with PD (P=0.001).
We also performed progression-free survival analyses
of second-line treatment (PFS2) based on the response to first-line
treatment. The Kaplan-Meier curves of Fig. 2B clearly depict, that patients with
a CR in first-line treatment also had the longest PFS2 of all
included patients (median PFS2=14m, 95% CI: 7.6-20.4). This is
highly statistically significant in comparison to patients, who had
progressive disease in first-line therapy (median PFS2=2m, 95% CI:
1.1-2.9; P<0.001). Again, patients with a PR in first-line
therapy had longer PFS2 (median PFS2=4m, 95% CI: 1.2-6.8) than
patients with PD in first-line therapy, although not statistically
significant (P=0.143). For patients with SD compared to patients
with PD in first-line therapy, PFS2 was also longer and
statistically significant (median PFS2=5m, 95% CI: 3.7-6.3;
P=0.019).
With regard to overall survival, Fig. 2C displays, that patients with a CR
after first-line therapy had the longest OS, with a median OS of 80
months (95% CI: 46-114). On the contrary, patients with PD had the
shortest OS, with only median 12 months (95% CI: 6.9-17.1).
Here, response to first-line therapy stratifies the
groups clearly with statistically significant differences for
patients with CR in first-line compared to patients with PD
(P=0.019), patients with PR in first-line (median OS=21m, 95% CI:
17.3-24.7) in comparison to patients with PD (P=0.003) and patients
with SD (median OS=49m, 95% CI: 25.6-72.4) compared to patients
with PD (P<0.001).
Survival analyses depending on patient
characteristics
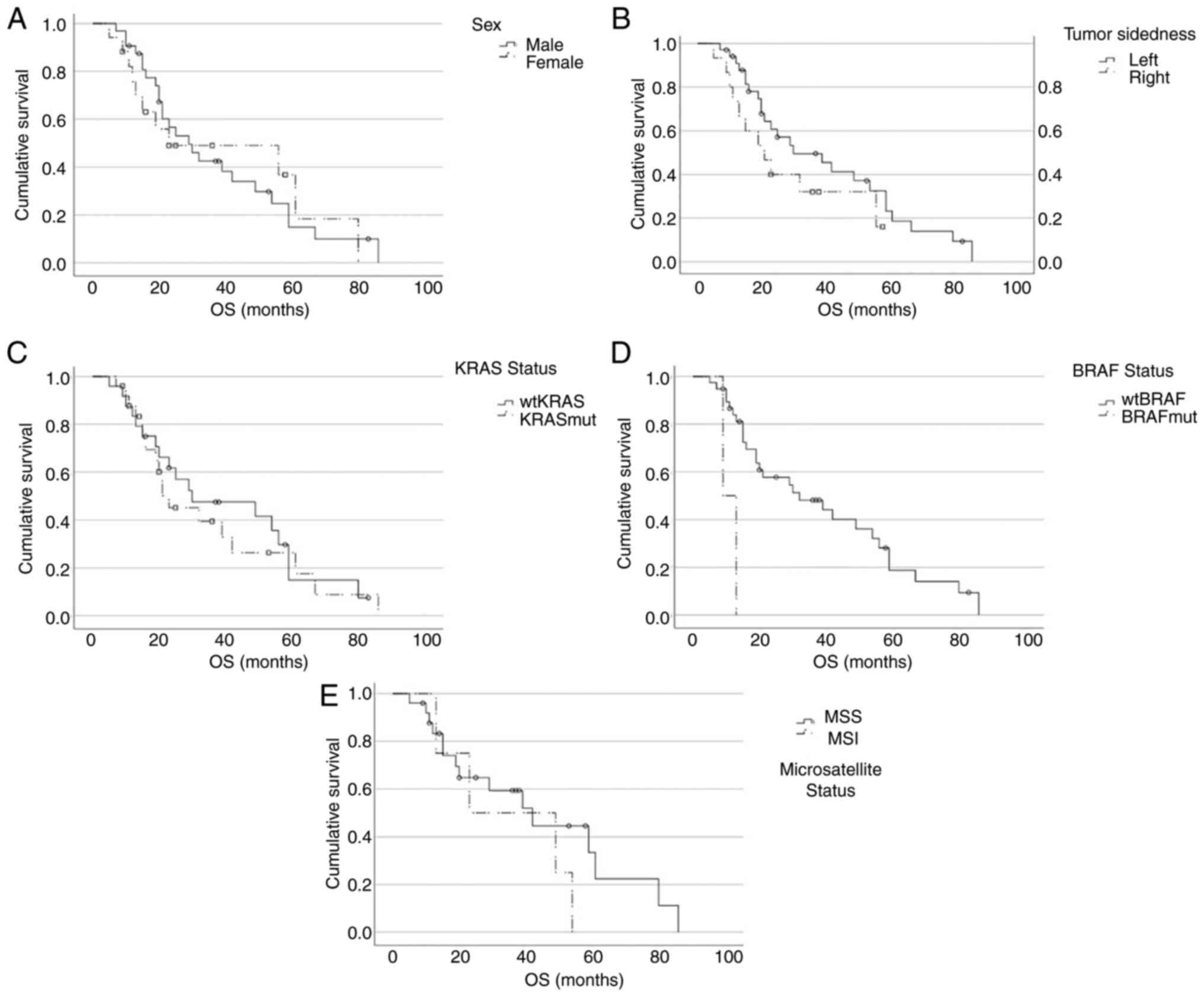
We further looked at survival parameters based on
patients and tumour characteristics. Here, stratification was not
done based on therapy-response to first- or second-line therapy as
in Fig. 2 but on patient or tumour
specific factors (Fig. 3).
Fig. 3A illustrates that sex had
no impact on OS in our study cohort (female sex: median OS=23m, 95%
CI: 0-71.2; male sex: median OS=29m, 95% CI: 17.5-40.5; P=0.938).
Fig. 3B shows that also tumour
sidedness had no statistically significant impact on OS in our
patient groups, although a trend towards longer OS could be
observed for patients with left sided primary tumours (left side:
median OS=30m, 95% CI: 10.4-49.6; right side: median OS=21m, 95%
CI: 10.9-31.1; P=0.176).
Furthermore, patients with wild type KRAS (wtKRAS)
had a slightly longer OS compared to patients with a KRAS mutation
(KRASmut), but again not statistically significant (wtKRAS: median
OS=30m, 95% CI: 0-62.2; KRASmut: median OS=23m, 95% CI: 10.7-35.3;
P=0.697), which is depicted in Fig.
3C.
Fig. 3D shows that
patients with a BRAF mutation (BRAFmut) had significantly lower OS
compared to patients without a BRAF mutation (wtBRAF: median
OS=32m, 95% CI: 9.7-54.3; BRAFmut: median OS=9m, 95% CI: not
calculable, due to low patient number of n=2; P=0.003).
Considering MSI, here again no significant
difference could be observed with regards to OS (MSS: median
OS=42m, 95%CI: 21.3-62.7; MSI: median OS=23m, 95% CI: 0-58.3;
P=0.320; Fig. 3E).
These results clearly reject the Null-hypothesis,
that the rates of remission in second-line chemo-immunotherapy do
not differ between patients with CRC stage IV, who had a remission
in first-line chemo-immunotherapy, and patients, who had no
remission in first-line chemo-immunotherapy.
Discussion
Cancer research has aimed for decades to better
understand carcinogenesis, tumour biology and host-tumour
interactions in order to find more specific and less toxic
therapies. For mCRC, several important biological factors such as
tumour sidedness, KRAS/NRAS/BRAF mutations, or microsatellite
instability have been described and therapy algorithms were
developed in order to optimally treat these patient subgroups.
Nonetheless, for the majority of patients, first-
and second line-therapy of mCRC is usually based on combination
chemotherapy with a 5-FU backbone, if not for BRAF-mutated or
MSI-high tumours. Thus, therapy-resistance could be an important
factor, based on the high similarity of first- and second-line
treatment. We therefore investigated this factor in a retrospective
cohort study and could clearly demonstrate that patients, who do
not respond to first-line treatment have little benefit of
second-line treatment. In our cohort none of the patients, who had
PD to first-line therapy responded to second-line therapy (ORR=0%).
This dramatic finding has to be further investigated in larger
studies and the true percentage of ORR is for sure not zero;
however, it clearly demonstrates the limited benefit of current
treatment strategies for these patients and the high need for
different therapy approaches.
Unfortunately, clinical data on response to
second-line treatment in mCRC patients not responding to first-line
treatment is scarce. To the best of our knowledge, we could not
find one single randomized clinical trial (RCT) nor prospective
analysis addressing specifically this question. There are numerous
RCTs evaluating in general the efficacy and toxicity of second-line
systemic therapy in mCRC patients, where disease progressed,
recurred or did not respond to first-line systemic therapy.
Due to the retrospective nature of this study,
mechanistic aspects of therapy-resistance could unfortunately not
be investigated, but it seems most likely, that innate
chemoresistance plays a major role. Biological factors for chemo-
or multidrug-resistance comprise numerous mechanisms, such as
elevated metabolism, enhanced drug efflux, increased DNA repair
capacity, growth factors, or genetic and epigenetic factors
(13,17,18).
Antimetabolites, such as 5-FU cause base lesions, which promote
replication fork stalling in proliferating cancer cells. Here,
resistance can be acquired by e.g. stabilization of these stalled
replication forks (19). Another
mechanism for 5-FU resistance is enhancement of poly [ADP-ribose]
polymerase 1 (PARP1) activity, increasing the base excision repair
capacity in cancer cells (20). As
PARP-inhibitors are broadly applied in clinical oncology with a
favourable toxicity profile, these substances could be interesting
candidates for combination strategies in order to overcome
chemo-resistance.
For oxaliplatin, which induces intra-strand
dinucleotide DNA adducts that have to be repaired again by
nucleotide excision repair mechanisms, it could be shown, that
upregulation of the high-mobility group A 2 gene (HMGA2) could
induce oxaliplatin-resistance (21).
Eventually, for irinotecan, it could be shown, that
also its mode of action (trapping the topoisomerase I, leading to
replication fork stalling and collapse and cytotoxic double strand
breaks), could be counterfeit by HGMA2, as HGMA2 can inhibit
topoisomerase I trapping (17,22).
This preclinical evidence of cellular mechanisms,
especially of HGMA2 conveying resistance to two important drugs for
chemotherapy of mCRC cells, should underline the high importance of
multidrug-resistance.
To date, clinical research data on chemo-resistance
in CRC is unfortunately still scarce.
Due to the retrospective nature of this study, also
our data has numerous limitations. As mentioned before, in the
first years of the observation period, molecular biologic testing
for BRAF and MSI was not routinely established and therefore
especially data on these patient groups have many missing
variables. Thus, the OS curves in Fig.
3 stratify only for patient or tumour characteristics but not
in conjunction with first- or second-line therapy responses in
these respective subgroups, as these subgroups are too small for
robust interpretation. In future studies, special emphasis should
be put on these patient groups in order to evaluate the effect of
stratified therapies compared to 5-FU based second-line
chemo-immunotherapy.
Moreover, retrospective studies always harbour the
risk of selection bias and the influence of confounding
variables.
In the future, patients with PD to first-line
therapy should be tested thoroughly by gaining novel tumour
specimens, which have to be comprehensively tested, including for
known factors of multidrug-resistance. Ideally, detected
alterations should be compared to the original specimen harboured
during primary diagnosis in order to differentiate between acquired
and innate chemoresistance. These patients should be regarded as
‘functional high risk’ and thus primarily treated within clinical
trials. Based on the comprehensive testing results, targeted
therapies or immunotherapies should be preferred to conventional
chemotherapies. If the comprehensive profiling lacks specific
targets or biomarkers for response to immunotherapy, clinical
trials that specifically address chemo-resistance should be
performed in this patient group.
For another proportion of these patients, especially
frail ones and patients that suffered from severe toxicities of
first-line therapy, BSC might be an option in order to avoid
further side effects of futile therapies. So far, the current
National Comprehensive Cancer Network (NCCN)-guidelines recommend
BSC as second line option for patients with PD after first line and
no improvement to functional status, or after failure to
second-line treatment (23).
On the long run, however, novel and different
therapy approaches have to be developed in order to benefit also
this precarious patient group. This is most effectively done in
randomized clinical trials and thus future studies should focus
especially on this patient group, because of their high medical
need.
Besides its retrospective nature, one big limitation
of this study is its small sample size due to the design as
single-centre study. Moreover, not all patients could be included,
because they had to receive at least two lines of
chemo-immunotherapy to evaluate PFS1, PFS2 and OS. Furthermore, not
all patients, which were included in the study, were tested for all
genetic markers, thus leading to missing data. Because of the small
sample size, also subgroup analyses could not be performed. Future
studies should be large enough to do subgroup analyses in order to
rule out confounding factors such as co-morbidities, treatment
response time, and other clinical characteristics.
In conclusion, this study demonstrates that, with
current treatment strategies applying 5-FU-based
chemo-immunotherapy in first-as well as second-line treatment of
mCRC patients, response to first-line therapy is a strong predictor
for response in second-line and OS. By only exchanging the
chemotherapeutic combination partner and additive antibody, the
negative factor of not responding to first-line therapy, most
likely caused by multidrug-resistance, could not be overcome in
this study population.
These findings have to be confirmed in larger
studies, but raise the need for more basic research in CRC and on
multidrug-resistance in order to gain novel treatment options,
especially for patients not responding to first-line 5-FU-based
chemo-immunotherapy.
Acknowledgements
Not applicable.
Funding
Funding: The authors appreciate the contribution of NÖ
Landesgesundheitsagentur, the legal entity of University Hospitals
in Lower Austria, for providing the organizational framework to
conduct this research. They would also like to acknowledge the
support by the Open Access Publishing Fund of Karl Landsteiner
University of Health Sciences, Krems, Austria. This research did
not receive any specific grant from funding agencies in the public,
commercial, or not-for-profit sectors.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
This work is based on the bachelor's thesis of HP,
submitted in July 2023, at the Karl Landsteiner University of
Health Sciences to acquire the academic degree Bachelor of Health
Sciences. JS and MP conceived and designed the study. JS and HP
acquired the data. JS and HP confirm the authenticity of all the
raw data. JS, HP, GK and MP analysed and interpreted the data. JS
and HP drafted the article. JS, HP, GK and MP revised the
manuscript critically for important intellectual content. JS, HP,
GK and MP read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The study was approved by the Commission for
Scientific Integrity and Ethics at the Karl Landsteiner University
of Health Sciences in September 2022 (EK No. 1046/2022) and was
conducted according to The Declaration of Helsinki. Due to the
retrospective nature of the present study the need for informed
consent was waived; this was as approved by the Commission for
Scientific Integrity and Ethics at the Karl Landsteiner University
of Health Sciences.
Patient consent for publication
Not applicable.
Competing interests
JS declares honorarium payments from Abbvie, Amgen,
Gilead, Janssen, Kite, Merck, Merck Sharp & Dohme, Miltenyi,
Novartis, Pfizer, Roche and Servier as an invited speaker or expert
consulting, which are not relevant for this study. MP declares
financial support from Roche for research projects, also not
relevant for this study. The other authors declare that they have
no competing interests.
References
|
1
|
De Falco V, Napolitano S, Roselló S,
Huerta M, Cervantes A, Ciardiello F and Troiani T: How we treat
metastatic colorectal cancer. ESMO Open. 4 (Suppl
2)(e000813)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tharin Z, Blanc J, Alaoui IC, Bertaut A
and Ghiringhelli F: Influence of first line chemotherapy strategy
depending on primary tumor location in metastatic colorectal
cancer. J Gastrointest Oncol. 12:1509–1517. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ogura T, Kakuta M, Yatsuoka T, Nishimura
Y, Sakamoto H, Yamaguchi K, Tanabe M, Tanaka Y and Akagi K:
Clinico-pathological characteristics and prognostic impact of
colorectal cancers with NRAS mutations. Oncol Rep. 32:50–56.
2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Network NCC. NCCN Clinical Practice
Guidelines in Oncology (NCCN Guidelines): Colon Cancer. In.
2022.
|
|
5
|
Tejpar S, Stintzing S, Ciardiello F,
Tabernero J, Van Cutsem E, Beier F, Esser R, Lenz HJ and Heinemann
V: Prognostic and predictive relevance of primary tumor location in
patients with RAS wild-type metastatic colorectal cancer:
Retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA
Oncol. 3:194–201. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Antoniotti C, Borelli B, Rossini D,
Pietrantonio F, Morano F, Salvatore L, Lonardi S, Marmorino F,
Tamberi S, Corallo S, et al: AtezoTRIBE: A randomised phase II
study of FOLFOXIRI plus bevacizumab alone or in combination with
atezolizumab as initial therapy for patients with unresectable
metastatic colorectal cancer. BMC Cancer. 20(683)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vodenkova S, Buchler T, Cervena K,
Veskrnova V, Vodicka P and Vymetalkova V: 5-fluorouracil and other
fluoropyrimidines in colorectal cancer: Past, present and future.
Pharmacol Ther. 206(107447)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Culy CR, Clemett D and Wiseman LR:
Oxaliplatin. A review of its pharmacological properties and
clinical efficacy in metastatic colorectal cancer and its potential
in other malignancies. Drugs. 60:895–924. 2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fujita KI, Kubota Y, Ishida H and Sasaki
Y: Irinotecan, a key chemotherapeutic drug for metastatic
colorectal cancer. World J Gastroenterol. 21:12234–12248.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Cervantes A, Adam R, Roselló S, Arnold D,
Normanno N, Taïeb J, Seligmann J, De Baere T, Osterlund P, Yoshino
T, et al: Metastatic colorectal cancer: ESMO clinical practice
guideline for diagnosis, treatment and follow-up. Ann Oncol.
34:10–32. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Aparicio J, Virgili Manrique AC, Capdevila
J, Muñoz Boza F, Galván P, Richart P, Oliveres H, Páez D, Hernando
J, Serrano S, et al: Randomized phase II trial of
FOLFIRI-panitumumab compared with FOLFIRI alone in patients with
RAS wild-type circulating tumor DNA metastatic colorectal cancer
beyond progression to first-line FOLFOX-panitumumab: the BEYOND
study (GEMCAD 17-01). Clin Transl Oncol. 24:2155–2165.
2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dariya B, Aliya S, Merchant N, Alam A and
Nagaraju GP: Colorectal cancer biology, diagnosis, and therapeutic
approaches. Crit Rev Oncog. 25:71–94. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bukowski K, Kciuk M and Kontek R:
Mechanisms of multidrug resistance in cancer chemotherapy. Int J
Mol Sci. 21(3233)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van
Oosterom AT, Christian MC and Gwyther SG: New guidelines to
evaluate the response to treatment in solid tumors. European
organization for research and treatment of cancer, national cancer
institute of the united states, national cancer institute of
Canada. J Natl Cancer Inst. 92:205–216. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Tirumani SH, Kim KW, Nishino M, Howard SA,
Krajewski KM, Jagannathan JP, Cleary JM, Ramaiya NH and Shinagare
AB: Update on the role of imaging in management of metastatic
colorectal cancer. Radiographics. 34:1908–1928. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Campos Gudiño R, McManus KJ and
Hombach-Klonisch S: Aberrant HMGA2 expression sustains genome
instability that promotes metastasis and therapeutic resistance in
colorectal cancer. Cancers (Basel). 15(1735)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hammond WA, Swaika A and Mody K:
Pharmacologic resistance in colorectal cancer: A review. Ther Adv
Med Oncol. 8:57–84. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yu H, Lim HH, Tjokro NO, Sathiyanathan P,
Natarajan S, Chew TW, Klonisch T, Goodman SD, Surana U and Dröge P:
Chaperoning HMGA2 protein protects stalled replication forks in
stem and cancer cells. Cell Rep. 6:684–697. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Summer H, Li O, Bao Q, Zhan L, Peter S,
Sathiyanathan P, Henderson D, Klonisch T, Goodman SD and Dröge P:
HMGA2 exhibits dRP/AP site cleavage activity and protects cancer
cells from DNA-damage-induced cytotoxicity during chemotherapy.
Nucleic Acids Res. 37:4371–4384. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Deng X, Kong F, Li S, Jiang H, Dong L, Xu
X, Zhang X, Yuan H, Xu Y, Chu Y, et al: A KLF4/PiHL/EZH2/HMGA2
regulatory axis and its function in promoting
oxaliplatin-resistance of colorectal cancer. Cell Death Dis.
12(485)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Peter S, Yu H, Ivanyi-Nagy R and Dröge P:
Cell-based high-throughput compound screening reveals functional
interaction between oncofetal HMGA2 and topoisomerase I. Nucleic
Acids Res. 44(e162)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Benson AB, Venook AP, Al-Hawary MM, Arain
MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Farkas L, et
al: Colon cancer, version 2.2021, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 19:329–359. 2021.PubMed/NCBI View Article : Google Scholar
|