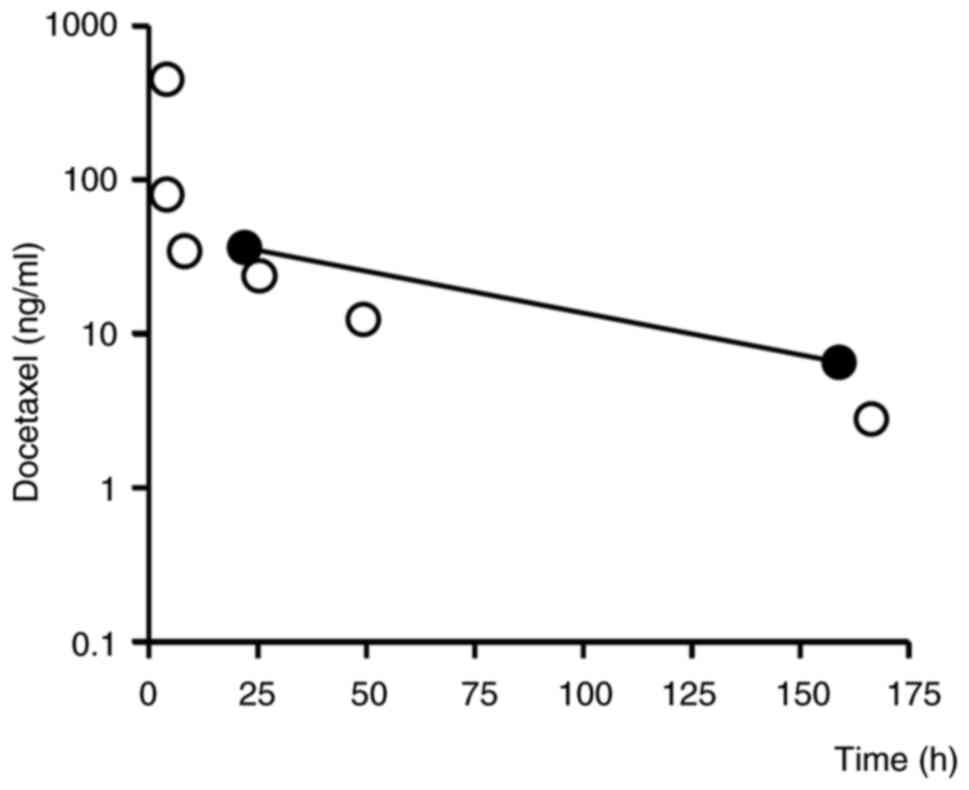
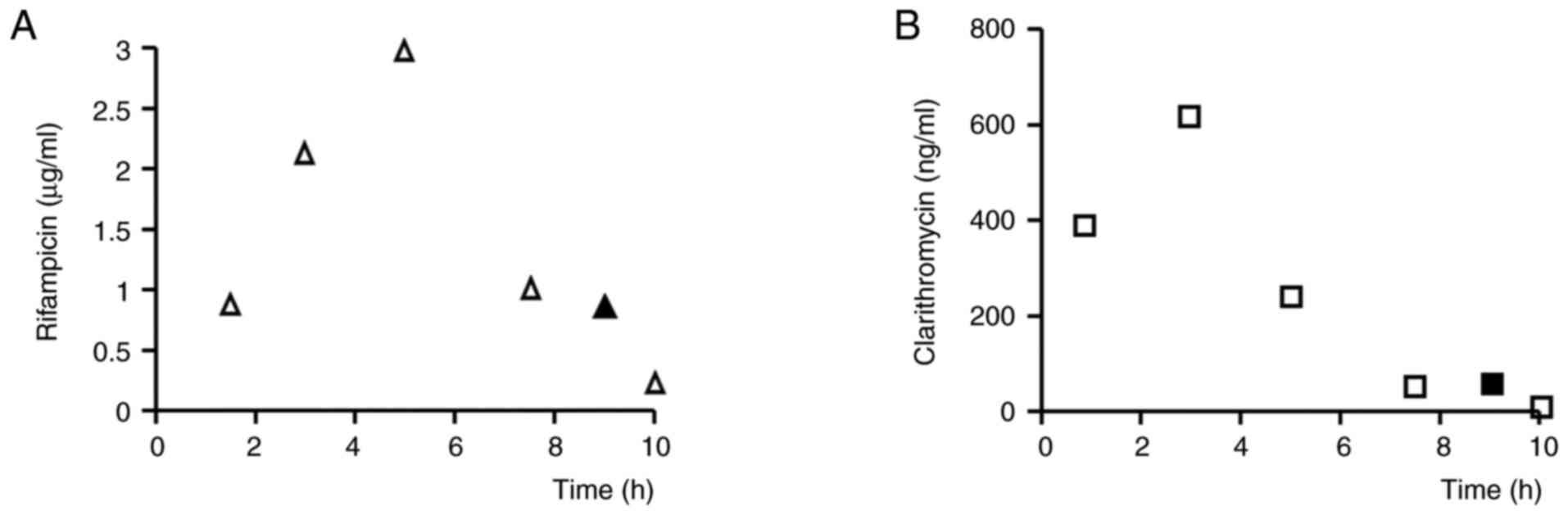
|
1
|
Nieuweboer AJ, de Morrée ES, de Graan AJ,
Sparreboom A, de Wit R and Mathijssen RH: Inter-patient variability
in docetaxel pharmacokinetics: A review. Cancer Treat Rev.
41:605–613. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Daley CL, Iaccarino JM, Lange C, Cambau E,
Wallace RJ Jr, Andrejak C, Böttger EC, Brozek J, Griffith DE,
Guglielmetti L, et al: Treatment of Nontuberculous Mycobacterial
Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical
Practice Guideline. Clin Infect Dis. 71:e1–e36. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhou SF: Drugs behave as substrates,
inhibitors and inducers of human cytochrome P450 3A4. Curr Drug
Metab. 9:310–322. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mori D, Kimoto E, Rago B, Kondo Y,
King-Ahmad A, Ramanathan R, Wood LS, Johnson JG, Le VH, Vourvahis
M, et al: Dose-Dependent Inhibition of OATP1B by Rifampicin in
Healthy Volunteers: Comprehensive Evaluation of Candidate
Biomarkers and OATP1B Probe Drugs. Clin Pharmacol Ther.
107:1004–1013. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Maeda K: Organic anion transporting
polypeptide (OATP)1B1 and OATP1B3 as important regulators of the
pharmacokinetics of substrate drugs. Biol Pharm Bull. 38:155–168.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bolleddula J, Gopalakrishnan S, Hu P, Dong
J and Venkatakrishnan K: Alternatives to rifampicin: A review and
perspectives on the choice of strong CYP3A inducers for clinical
drug-drug interaction studies. Clin Transl Sci. 15:2075–2095.
2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gouin-Thibault I, Delavenne X, Blanchard
A, Siguret V, Salem JE, Narjoz C, Gaussem P, Beaune P,
Funck-Brentano C, Azizi M, et al: Interindividual variability in
dabigatran and rivaroxaban exposure: Contribution of ABCB1 genetic
polymorphisms and interaction with clarithromycin. J Thromb
Haemost. 15:273–283. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gessner A, König J and Fromm MF: Clinical
aspects of transporter-mediated drug-drug interactions. Clin
Pharmacol Ther. 105:1386–1394. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Common Terminology Criteria for Adverse
Events (CTCAE) v5.0. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf,
2017.
|
|
10
|
Baker SD, Li J, ten Tije AJ, Figg WD,
Graveland W, Verweij J and Sparreboom A: Relationship of systemic
exposure to unbound docetaxel and neutropenia. Clin Pharmacol Ther.
77:43–53. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sharma SK and Upadhyay V: Epidemiology,
diagnosis & treatment of non-tuberculous mycobacterial
diseases. Indian J Med Res. 152:185–226. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Namkoong H, Kurashima A, Morimoto K,
Hoshino Y, Hasegawa N, Ato M and Mitarai S: Epidemiology of
pulmonary nontuberculous mycobacterial disease, Japan. Emerg Infect
Dis. 22:1116–1117. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Clarke SJ and Rivory LP: Clinical
pharmacokinetics of docetaxel. Clin Pharmacokinet. 36:99–114.
1999.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Engels FK, Ten Tije AJ, Baker SD, Lee CK,
Loos WJ, Vulto AG, Verweij J and Sparreboom A: Effect of cytochrome
P450 3A4 inhibition on the pharmacokinetics of docetaxel. Clin
Pharmacol Ther. 75:448–454. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Akiyama N, Inui N, Mori K, Nakamura Y,
Hayakawa H, Tanaka S, Uchida S, Namiki N, Watanabe H and Suda T:
Effect of rifampicin and clarithromycin on the CYP3A activity in
patients with Mycobacterium avium complex. J Thorac Dis.
11:3814–3821. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hisaka A, Ohno Y, Yamamoto T and Suzuki H:
Prediction of pharmacokinetic drug-drug interaction caused by
changes in cytochrome P450 activity using in vivo information.
Pharmacol Ther. 125:230–248. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hu S, Mathijssen RHJ, de Bruijn P, Baker
SD and Sparreboom A: Inhibition of OATP1B1 by tyrosine kinase
inhibitors: In vitro-in vivo correlations. Br J Cancer.
117(e3)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Iusuf D, Hendrikx JJ, van Esch A, van de
Steeg E, Wagenaar E, Rosing H, Beijnen JH and Schinkel AH: Human
OATP1B1, OATP1B3 and OATP1A2 can mediate the in vivo uptake and
clearance of docetaxel. Int J Cancer. 136:225–233. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Awada A, Hendlisz A, Christensen O, Lathia
CD, Bartholomeus S, Lebrun F, de Valeriola D, Brendel E, Radtke M,
Delaunoit T, et al: Phase I trial to investigate the safety,
pharmacokinetics and efficacy of sorafenib combined with docetaxel
in patients with advanced refractory solid tumours. Eur J Cancer.
48:465–474. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Koolen SL, Oostendorp RL, Beijnen JH,
Schellens JH and Huitema AD: Population pharmacokinetics of
intravenously and orally administered docetaxel with or without
co-administration of ritonavir in patients with advanced cancer. Br
J Clin Pharmacol. 69:465–474. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yoshikado T, Yoshida K, Kotani N, Nakada
T, Asaumi R, Toshimoto K, Maeda K, Kusuhara H and Sugiyama Y:
Quantitative analyses of hepatic OATP-mediated interactions between
statins and inhibitors using PBPK modeling with a parameter
optimization method. Clin Pharmacol Ther. 100:513–523.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Alffenaar JW, Nienhuis WA, de Velde F,
Zuur AT, Wessels AM, Almeida D, Grosset J, Adjei O, Uges DR and van
der Werf TS: Pharmacokinetics of rifampin and clarithromycin in
patients treated for Mycobacterium ulcerans infection. Antimicrob
Agents Chemother. 54:3878–3883. 2010.PubMed/NCBI View Article : Google Scholar
|