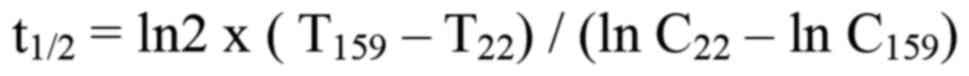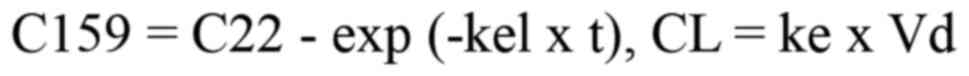Introduction
Docetaxel is a cytotoxic anticancer drug that
promotes and stabilizes microtubule polymerization and inhibits
cell division. It is used to treat many types of cancer, including
breast, non-small cell lung, esophageal, and ovarian cancers
(1). Docetaxel is taken up into
liver cells by organic anion transporting peptides (OATP) 1B1 and
OATP1B3 and is metabolized and inactivated mainly by cytochrome
P450 3A4 (CYP3A4) (1). Docetaxel
and its metabolites in liver cells are excreted into the bile
mainly by ABCB1 and ABCC2(1).
Therefore, concomitant use of drugs that affect the activity of
these transporters or CYP3A4 may pose a significant risk of
affecting the therapeutic effect of docetaxel.
Here, we report a case of breast cancer that was
diagnosed and treated with docetaxel during rifampicin and
clarithromycin treatment for nontuberculous mycobacteriosis (NTM).
The recommended treatment for NTM is a combination of two or three
antimicrobial agents selected according to the species and the
susceptibility of the organism (2). The patient in this case required
rifampicin and clarithromycin combination therapy for NTM and
docetaxel, a key drug for breast cancer. Rifampicin is a CYP3A4
inducer and OATP inhibitor (3-6),
and clarithromycin is a CYP3A4 inhibitor and ABCB1 inhibitor
(3,7,8).
Drug interactions involving rifampicin and clarithromycin were
expected to cause unusual pharmacokinetics of docetaxel, which
could lead to unexpected adverse drug reactions (ADRs) or decreased
effectiveness of treatment.
We measured the blood concentration of docetaxel to
ensure the efficacy and safety of docetaxel administration, and to
investigate the effects of rifampicin and clarithromycin
combination therapy on the pharmacokinetics of docetaxel.
Case report
A 58-year-old woman presented to Gunma University
Hospital (Maebashi, Japan) with a mass in the left breast in 2016.
She was 168 cm tall and weighed 52 kg at presentation, and her only
complaint was awareness of the left breast mass. Based on needle
biopsy findings, the patient was diagnosed with advanced cancer of
the left breast (stage IV, scirrhous carcinoma; estrogen receptor:
negative, progesterone receptor: negative, HER2: 3+). Laboratory
values for renal and hepatic function at admission were all within
normal limits: total bilirubin, 0.9 mg/dl; alanine transaminase, 19
U/l; and serum creatinine: 0.71 mg/dl. The patient had NTM and had
been taking clarithromycin tablets 200 mg twice daily, rifampicin
capsules 450 mg once daily, and ethambutol tablets 500 mg once
daily for 1 year, as well as famotidine tablets 10 mg twice daily
and loxoprofen tablets 60 mg for pain.
A triweekly regimen of docetaxel, trastuzumab, and
pertuzumab was initiated on an outpatient basis to treat the breast
cancer. The first course consisted of docetaxel (75
mg/m2), trastuzumab (8 mg/kg), and pertuzumab (840 mg)
on day 1. From the second course, the pertuzumab dose was reduced
to 420 mg according to the planned regimen. For the first 3 days of
each course, 8 mg of dexamethasone was administered intravenously
for prophylaxis against nausea. After consultation with the
respiratory physician and breast surgeon, it was decided that
clarithromycin tablets, rifampicin capsules, and ethambutol tablets
for the treatment of NTM would be continued during treatment with
anticancer agents. Because the effects of drug interactions
involving clarithromycin and rifampicin on the pharmacokinetics of
docetaxel are not known, pegfilgrastim 3.6 mg was administered on
day 2 of the first course to account for the risk of neutropenia.
The patient contracted influenza 7 days after the first dose of
docetaxel, so a 7-day rest period was added after the end of the
course. After the second course, anticancer agents and
pegfilgrastim 3.6 mg were administered as scheduled. Neutropenia
was not observed during the entire treatment course, and there were
no signs of infection, except for the aforementioned infection with
influenza virus and NTM. There were no grade 3 or higher ADRs
according to the Common Terminology Criteria for Adverse Events
(Table I) (9), and the cancer disappeared on imaging
after the fourth courses, indicating a complete response. The
patient was then switched to maintenance therapy with trastuzumab
and pertuzumab. After 5 years of maintenance therapy, there were no
signs of recurrence.
 | Table ICTCAE grade of ADRs at the start of
each course. |
Table I
CTCAE grade of ADRs at the start of
each course.
| | ADR (CTCAE
grade) |
|---|
| Course |
Neutropeniaa | Alopecia | Dysgeusia | Dysesthesia |
|---|
| 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 2 | 1 | 0 |
| 3 | 0 | 2 | 1 | 0 |
| 4 | 0 | 2 | 1 | 1 |
| 5 | 0 | 2 | 0 | 1 |
| 6 | 0 | 2 | 0 | 1 |
| 7 | 0 | 2 | 0 | 1 |
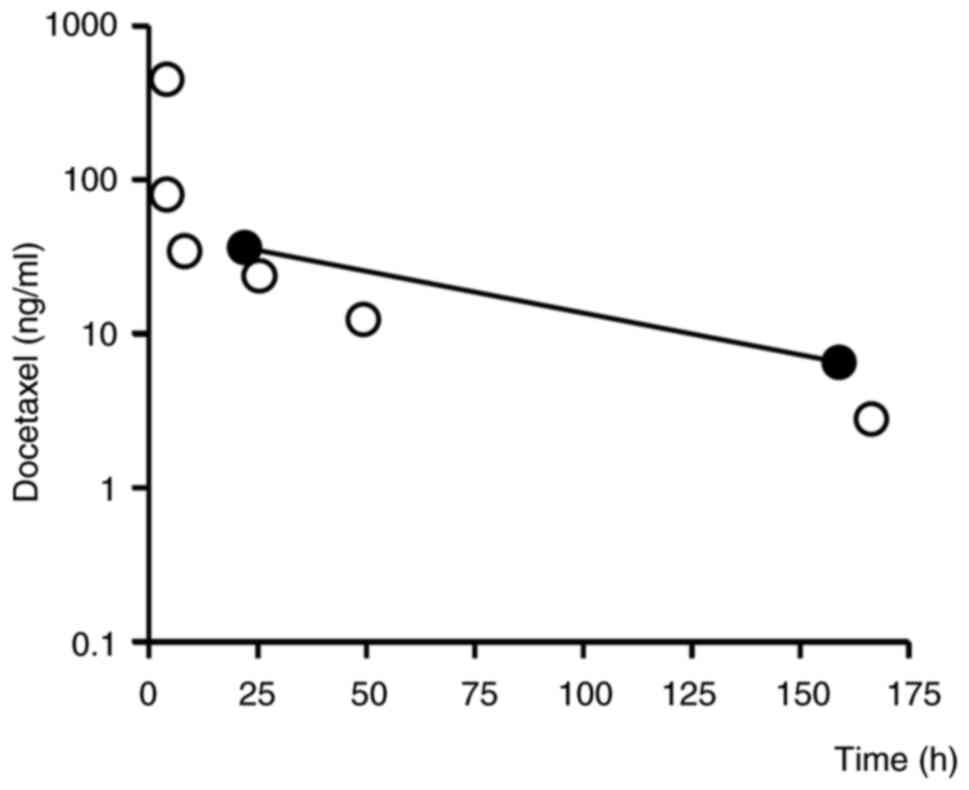
Blood samples were taken 22 h after the first course
of docetaxel administration, and blood levels of docetaxel,
rifampicin, and clarithromycin were measured by ultra
high-performance liquid chromatography-tandem mass spectrometry
(ACQUITY UPLC-Xevo TQ MS). The blood concentrations of docetaxel at
22 and 159 h after its administration (i.e., in the elimination
phase) were 36.1 and 6.5 ng/ml (Fig.
1), respectively, which are higher than previously reported
(10). The half-life of docetaxel
was calculated to be 55.4 h based on the blood concentration values
at 22 and 159 h using the following formula:
where T22 and T159 are the
times at 22 and 159 h after administration and C22 and
C159 are the corresponding docetaxel concentrations. The
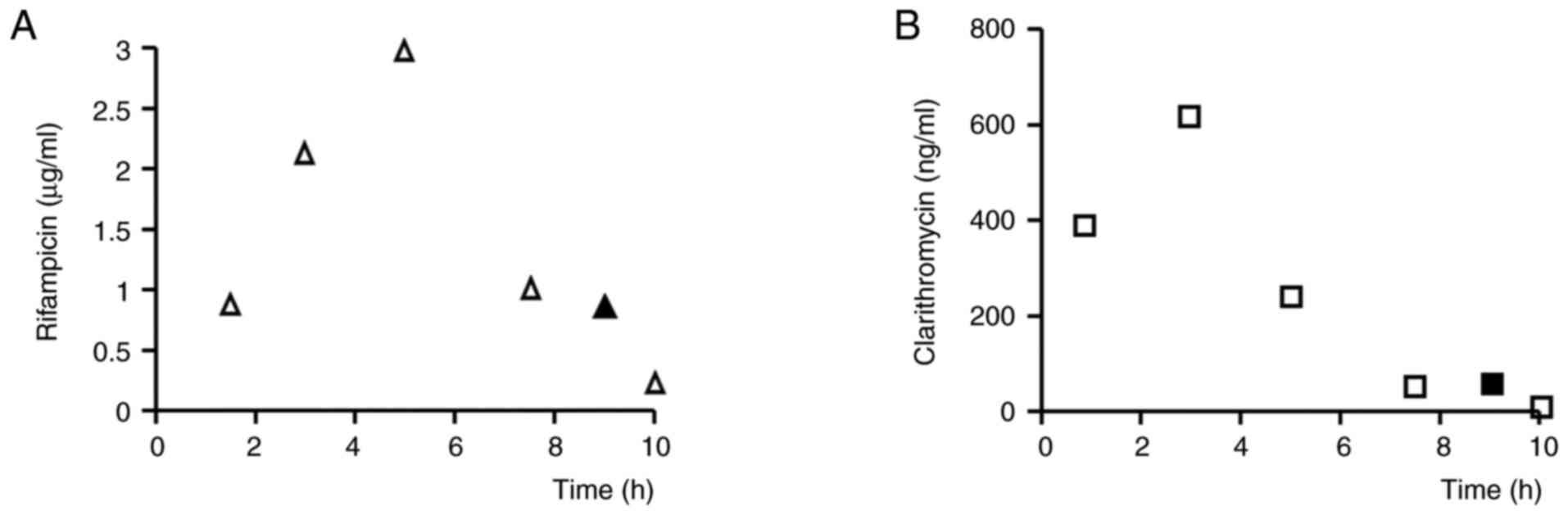
blood levels of rifampicin and clarithromycin were 0.75 µg/ml and
62 ng/ml, respectively, at 22 h after docetaxel administration,
which was 9 h after oral administration of rifampicin and
clarithromycin (Fig. 2).
Discussion
Although the prevalence of NTM is low at
approximately 6 per 100,000, the number of affected patients is
reported to be increasing worldwide (11,12),
and there is a possibility of that patients receiving rifampicin
and clarithromycin will require administration of anticancer drugs
as in the present case. Therefore, it is necessary to accumulate
more information regarding the concomitant use of rifampicin and
clarithromycin.
The measured blood concentration of docetaxel
following administration of rifampicin and clarithromycin in our
patient was clearly higher than that in typical patients who
receive the same dose of docetaxel. The elimination half-life of
docetaxel was also clearly longer and was calculated from blood
concentrations at two time points to be 55.7 h, about three times
longer than previously reported values (10). The distribution volume of docetaxel
was 74 l/m2 (13), and
the patient's body surface area was calculated to be 1.58
m2 using the Du Bois equation. Assuming that elimination
follows a linear one-compartment model, the clearance of docetaxel
was calculated from C22 and C159 using the following formula, and
was found to be 1.45 l/h.
The docetaxel clearance in patients (n=40) who
received docetaxel 75 mg/m2, as this patient did, was
30.0±14.2 l/h (mean ± SD) (10).
The calculated clearance in this patient was more than 2 SD lower
than the mean value in previously reported patients, and was
considered to be significantly lower than the clearance in the
general patient population.
More than 95% of the total metabolic clearance of
docetaxel (22 l/h/m2) is via hepatic metabolism
(13), so its metabolism may be
affected by variations in hepatic clearance. Since the clearance
reduction when docetaxel is combined with ketoconazole is reported
to be 49% (14) and the CYP3A4
inhibitory activity of ketoconazole is considered to be 100%, the
contribution of CYP3A4 to docetaxel metabolism is considered to be
49%. Akiyama et al (15)
reported that the metabolism of midazolam, a substrate of CYP3A4,
is enhanced in NTM patients receiving rifampicin and
clarithromycin. CYP3A4 induction by rifampicin is due to
transcriptional promotion via activation of the pregnane X
receptor, and according to a report by Hisaka et al
(16), it increases the clearance
of midazolam about 8-fold. In contrast, CYP3A4 is inhibited by
clarithromycin via an irreversible reaction that occurs when an
intermediate metabolite of clarithromycin forms a complex with
CYP3A4. This is called mechanism-based inhibition (MBI). Hisaka
et al (16) reported that
clarithromycin inhibits the clearance of midazolam to 20% of the
original value. Since docetaxel and midazolam may differ in their
reactivity to various enzymes, a definite answer cannot be given.
However, based on the information about midazolam, it is possible
to envision that the clearance increases about 8-fold due to the
increase in the expression level of CYP3A4 caused by the action of
rifampicin, but that the activity is inhibited to 20% of the
original value due to MBI by clarithromycin. As a result, it is
thought that the contribution of the increase in expression level
by rifampicin is large, and the activity of CYP3A4 becomes about
1.6-fold larger. Further basic research is needed to examine this
hypothesis. However, in our patient, factors other than CYP3A4 may
have had a significant influence, since the increase in blood
levels cannot be explained by CYP3A4-mediated drug-drug
interactions alone.
OATP1B3 and ABCB1 are transporters that can affect
the pharmacokinetics of docetaxel. Hu et al (17) reported a 2- to 3-fold increase in
the area under the curve (AUC) of docetaxel by OATP1B knockout (KO)
in an in vivo study using mice. Similarly, Iusuf et al
(18) reported a 3-fold increase
in the AUC of docetaxel in a study using OATP KO mice. Furthermore,
clinical trials have found an increase in the AUC of docetaxel when
sorafenib, an OATP1B1 inhibitor, was combined with docetaxel
(19). When ritonavir, an
inhibitor of both CYP3A4 and ABCB1, was concomitantly administered
with docetaxel, the clearance of docetaxel decreased by more than
90% (20). These results suggest
that metabolism by CYP3A4 and transport by OATP1B3 and ABCB1 have a
major influence on docetaxel elimination. Like docetaxel,
atorvastatin is a substrate for OATPs and CYP3A4, and concomitant
use of rifampicin induces CYP3A4. Therefore, considering only the
metabolism by CYP3A4, the AUC of atorvastatin would be expected to
decrease by 80-90% when the two are combined (16). Clinically, however, the concomitant
use of rifampicin has been reported to increase the AUC of
atorvastatin in a dose-dependent manner, with the increase being as
much as 10-fold (4,5). Moreover, although the effects of
clarithromycin on the pharmacokinetics of docetaxel have not been
reported, a clinical study evaluating the effect of clarithromycin
on the pharmacokinetics of the ABCB1 substrates dabigatran and
rivaroxaban found that clarithromycin increased the AUC of both
drugs by approximately 2-fold (7).
Therefore, a concern is that, in addition to inhibiting CYP3A4,
clarithromycin may inhibit ABCB1 at clinical doses, potentially
leading to a large decrease in docetaxel clearance.
Although the contributions of OATPs and ABCB1 to the
hepatic clearance of docetaxel have not been determined, our
findings suggest that the inhibitory effect of rifampicin on OATPs
and that of clarithromycin on ABCB1 possibly reduced hepatic
clearance.
Overall, CYP3A4 activity is likely to be elevated in
patients concomitantly receiving rifampicin and clarithromycin.
However, for docetaxel, which is transported by OATPs and ABCB1,
the inhibition of both transporters by rifampicin and
clarithromycin greatly reduces systemic clearance. In the same way
that the metabolism of atorvastatin, a substrate of OATP and 3A4,
is rate-limiting due to hepatic uptake by OATP (21), the rate-limiting factor for the
metabolism of docetaxel is OATP rather than CYP, and OATP may have
a greater influence on clearance. The blood levels of rifampicin
and clarithromycin measured in our patient were similar to those
reported for patients with NTM (22), indicating that her exposure to
rifampicin and clarithromycin was typical NTM patients treated with
these drugs.
A limitation of this study is that the metabolic
capacity of docetaxel in this patient was not determined in the
absence of rifampicin and clarithromycin, and the possibility that
the patient's basal metabolic capacity is atypical cannot be ruled
out. The attending physician determined that rifampicin and
clarithromycin could not be discontinued as treatment for NTM, so
all of this patient's docetaxel treatments were administered with
the concomitant use of rifampicin and clarithromycin. Therefore, it
is important to evaluate the effects of rifampicin and
clarithromycin combination therapy on the pharmacokinetics of
docetaxel in a larger number of similar cases, and basic research
data are also needed in order to support pharmacokinetic studies.
Furthermore, the mechanism of this drug-drug interaction could not
be understood from the data in this case. Therefore, in the future,
it is hoped that basic research will be conducted on the
pharmacokinetics of docetaxel when used in combination with
rifampicin and clarithromycin. Specifically, studies are needed to
clarify the differences in the influence of docetaxel as a
substrate for CYP3A4, OATP, and ABCB1 depending on the presence or
absence of rifampicin and clarithromycin, and in vivo
studies are needed to comprehensively evaluate these effects.
In conclusion, this case demonstrated that the
elimination of docetaxel is likely to be delayed in cases with
blood concentrations of rifampicin and clarithromycin commonly seen
in the treatment of NTM. In cases of cancer treated with docetaxel
where combination therapy with clarithromycin and rifampicin is
also required, it is desirable to monitor the blood concentration
of docetaxel, given that the blood concentration of docetaxel in
individual patients may vary widely due to complex drug
interactions.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HY, TA, YI, SO, DN, KO, JH and KY participated in
the design of the study and reviewed the results. JH, HY, TA, YI
and SO made the diagnosis, and discussed this case and carried out
the acquisition of data. HY, TA and DN analyzed serum
concentrations. HY, TA, DN and KY were responsible for the
calculation of pharmacokinetic parameters. HY, TA and KY drafted
the manuscript. All authors helped to draft the manuscript, and
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent as obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nieuweboer AJ, de Morrée ES, de Graan AJ,
Sparreboom A, de Wit R and Mathijssen RH: Inter-patient variability
in docetaxel pharmacokinetics: A review. Cancer Treat Rev.
41:605–613. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Daley CL, Iaccarino JM, Lange C, Cambau E,
Wallace RJ Jr, Andrejak C, Böttger EC, Brozek J, Griffith DE,
Guglielmetti L, et al: Treatment of Nontuberculous Mycobacterial
Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical
Practice Guideline. Clin Infect Dis. 71:e1–e36. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhou SF: Drugs behave as substrates,
inhibitors and inducers of human cytochrome P450 3A4. Curr Drug
Metab. 9:310–322. 2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mori D, Kimoto E, Rago B, Kondo Y,
King-Ahmad A, Ramanathan R, Wood LS, Johnson JG, Le VH, Vourvahis
M, et al: Dose-Dependent Inhibition of OATP1B by Rifampicin in
Healthy Volunteers: Comprehensive Evaluation of Candidate
Biomarkers and OATP1B Probe Drugs. Clin Pharmacol Ther.
107:1004–1013. 2020.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Maeda K: Organic anion transporting
polypeptide (OATP)1B1 and OATP1B3 as important regulators of the
pharmacokinetics of substrate drugs. Biol Pharm Bull. 38:155–168.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bolleddula J, Gopalakrishnan S, Hu P, Dong
J and Venkatakrishnan K: Alternatives to rifampicin: A review and
perspectives on the choice of strong CYP3A inducers for clinical
drug-drug interaction studies. Clin Transl Sci. 15:2075–2095.
2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gouin-Thibault I, Delavenne X, Blanchard
A, Siguret V, Salem JE, Narjoz C, Gaussem P, Beaune P,
Funck-Brentano C, Azizi M, et al: Interindividual variability in
dabigatran and rivaroxaban exposure: Contribution of ABCB1 genetic
polymorphisms and interaction with clarithromycin. J Thromb
Haemost. 15:273–283. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gessner A, König J and Fromm MF: Clinical
aspects of transporter-mediated drug-drug interactions. Clin
Pharmacol Ther. 105:1386–1394. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Common Terminology Criteria for Adverse
Events (CTCAE) v5.0. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf,
2017.
|
|
10
|
Baker SD, Li J, ten Tije AJ, Figg WD,
Graveland W, Verweij J and Sparreboom A: Relationship of systemic
exposure to unbound docetaxel and neutropenia. Clin Pharmacol Ther.
77:43–53. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sharma SK and Upadhyay V: Epidemiology,
diagnosis & treatment of non-tuberculous mycobacterial
diseases. Indian J Med Res. 152:185–226. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Namkoong H, Kurashima A, Morimoto K,
Hoshino Y, Hasegawa N, Ato M and Mitarai S: Epidemiology of
pulmonary nontuberculous mycobacterial disease, Japan. Emerg Infect
Dis. 22:1116–1117. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Clarke SJ and Rivory LP: Clinical
pharmacokinetics of docetaxel. Clin Pharmacokinet. 36:99–114.
1999.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Engels FK, Ten Tije AJ, Baker SD, Lee CK,
Loos WJ, Vulto AG, Verweij J and Sparreboom A: Effect of cytochrome
P450 3A4 inhibition on the pharmacokinetics of docetaxel. Clin
Pharmacol Ther. 75:448–454. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Akiyama N, Inui N, Mori K, Nakamura Y,
Hayakawa H, Tanaka S, Uchida S, Namiki N, Watanabe H and Suda T:
Effect of rifampicin and clarithromycin on the CYP3A activity in
patients with Mycobacterium avium complex. J Thorac Dis.
11:3814–3821. 2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hisaka A, Ohno Y, Yamamoto T and Suzuki H:
Prediction of pharmacokinetic drug-drug interaction caused by
changes in cytochrome P450 activity using in vivo information.
Pharmacol Ther. 125:230–248. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hu S, Mathijssen RHJ, de Bruijn P, Baker
SD and Sparreboom A: Inhibition of OATP1B1 by tyrosine kinase
inhibitors: In vitro-in vivo correlations. Br J Cancer.
117(e3)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Iusuf D, Hendrikx JJ, van Esch A, van de
Steeg E, Wagenaar E, Rosing H, Beijnen JH and Schinkel AH: Human
OATP1B1, OATP1B3 and OATP1A2 can mediate the in vivo uptake and
clearance of docetaxel. Int J Cancer. 136:225–233. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Awada A, Hendlisz A, Christensen O, Lathia
CD, Bartholomeus S, Lebrun F, de Valeriola D, Brendel E, Radtke M,
Delaunoit T, et al: Phase I trial to investigate the safety,
pharmacokinetics and efficacy of sorafenib combined with docetaxel
in patients with advanced refractory solid tumours. Eur J Cancer.
48:465–474. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Koolen SL, Oostendorp RL, Beijnen JH,
Schellens JH and Huitema AD: Population pharmacokinetics of
intravenously and orally administered docetaxel with or without
co-administration of ritonavir in patients with advanced cancer. Br
J Clin Pharmacol. 69:465–474. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yoshikado T, Yoshida K, Kotani N, Nakada
T, Asaumi R, Toshimoto K, Maeda K, Kusuhara H and Sugiyama Y:
Quantitative analyses of hepatic OATP-mediated interactions between
statins and inhibitors using PBPK modeling with a parameter
optimization method. Clin Pharmacol Ther. 100:513–523.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Alffenaar JW, Nienhuis WA, de Velde F,
Zuur AT, Wessels AM, Almeida D, Grosset J, Adjei O, Uges DR and van
der Werf TS: Pharmacokinetics of rifampin and clarithromycin in
patients treated for Mycobacterium ulcerans infection. Antimicrob
Agents Chemother. 54:3878–3883. 2010.PubMed/NCBI View Article : Google Scholar
|