1. Introduction
Microtubule actin crosslinking factor 1 (MACF1) is a
spectraplakin protein with a N-terminal actin-binding domain,
plakin domain, an EF-hand calcium-binding domain, with a
spectrin-repeat rod and C-terminal growth-arrest specific 2-related
microtubule-binding domain. These structural domains enable MACF1
to perform its primary function as a crosslinker of microtubules
and actin microfilaments (1-4),
cytoskeletal filamentous proteins involved in vesicular
trafficking, cytoarchitecture, cell division, and cell migration.
In addition to its function as a cytoskeletal crosslinker, it is
widely established that MACF1 plays a role in Wnt signaling, as a
component of the Wnt signaling protein complex (axin1, beta-catenin
and glycogen synthase kinase) that activates Wnt transmembrane
proteins and subsequently induces Wnt transcriptional targets
(5). Because several studies
provide instructive and informative presentations of MACF1
structure and function (6-8),
the focus in the present review is an overview of its role in the
etiology of brain tumors, specifically glioblastomas.
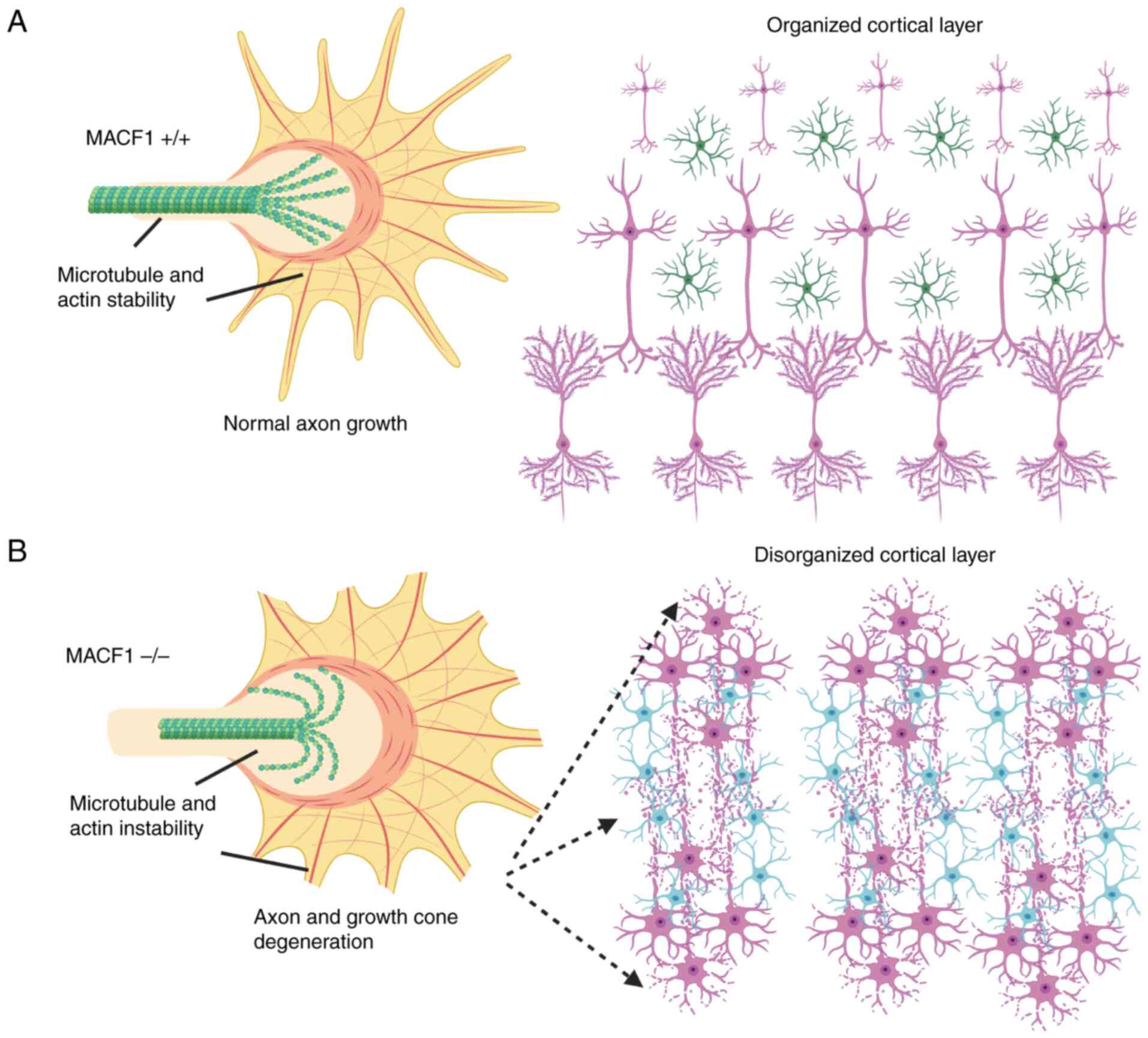
2. The role of MACF1 in the nervous
system
Early seminal work by Goryunov et al
(9) demonstrated that MACF1 has a
significant and prominent role in the mammalian nervous system
using in vivo tissue specific knockout technology. To that
end, A Cre-loxP approach was employed to knockout MACF1 in the
early stages of mouse nervous system development, which
consequently compromised the organizational structure of the
cerebral cortex and neuronal axon migration (9), while a more recent study by Ka et
al (10), showed that MACF1
regulates GABAergic interneuron migration and positioning in the
developing mouse brain using a conditional deletion approach.
Several confirmatory studies, particularly in vitro
knockdown and deletion experiments of MACF1, showed that the most
important nervous system function of this spectraplakin protein is
its crosslinking capacity during axon outgrowth and migration
(11-15).
Additionally, clinical nervous system manifestations of MACF1
alterations were previously reported in a study by Dobyns et
al (16), which described
missense variants and in-frame deletions within the growth-arrest
specific 2-related microtubule-binding domain that resulted in
brain malformations of children. Collectively, these studies
corroborate a defined function of MACF1 during neuron outgrowth and
axon migration as part of nervous system development (Fig. 1). However, despite robust data that
MACF1 contributes to neuronal development and maturation, few
investigations have examined its function in glial cells
(astrocytes, oligodendrocytes and microglia) and non-neuronal cells
that provide support and protection for neurons.
3. Cancer genetic aberrations of MACF1
Several investigations have provided experimental
evidence that show various MACF1 genetic abnormalities (Table I). One of the earliest studies
implicating MACF1 in cancer was described in 2011; alternative
splicing in adenocarcinoma patients was examined using microarray
analyses and reverse transcription polymerase chain reaction
(17). MACF1 was identified as one
of four alternatively spliced transcripts that may contribute to
non-small cell lung cancer (NSCLC) tumorigenesis as a consequence
of exon alterations (17). In
support of studies that evaluated MACF1 exon alterations as an
underlying inducer of adenocarcinoma tumorigenesis, whole exome
sequencing revealed MACF1 mutations in renal cell carcinomas and
endometrial cancer as a genetic driver of tumorigenesis in these
cancers (18,19). Furthermore, a recent study by Tian
et al (2020), identified MACF1 mutations as a correlation of
poor prognosis in patients with breast cancer. With respect to
genetic alterations of MACF1 in brain tumors, specifically
glioblastomas, cancer genome atlas cbioportal (https://www.cbioportal.org/) analyses revealed that 5%
of patient samples consisted of mutations or amplifications
(21-23).
 | Table IMACF1 genetic abnormalities. Genetic
mutations and amplifications of MACF1 have been found in several
solid cancers. |
Table I
MACF1 genetic abnormalities. Genetic
mutations and amplifications of MACF1 have been found in several
solid cancers.
| First author,
year | Cancer type | Results | (Refs.) |
|---|
| Misquitta-Ali et
al, 2011 | Non-small cell lung
cancer | Alternative
transcript splicing | (17) |
| Arai et al,
2014 | Renal cell
carcinoma | Mutations | (18) |
| Chang et al,
2017 | Endometrial
cancer | Mutations | (19) |
| Tian et al,
2022 | Breast cancer | Mutations | (20) |
| Cerami et
al, 2012; Gao et al, 2013; de Bruijn et al,
2023 | Glioblastomas | Mutations,
amplifications | (21-23) |
4. Oncogenic properties and expression of
MACF1
MACF1 has been described to play a significant role
in cancer development, primarily through its influence on cellular
processes such as proliferation, migration and apoptosis.
Specifically, in acute myeloid leukemia (AML), MACF1 overexpression
was associated with poor overall survival and attributed to the
promotion of AML cell proliferation by affecting pro-tumorigenic
downstream targets, Runx2 and the PI3K/Akt signaling pathway
(24). By contrast, silencing
MACF1 in AML cells led to reduced proliferation and provided
evidence of this spectraplakin as a therapeutic target for managing
this type of leukemia (24).
Consistent with these findings, MACF1 expression was also
upregulated in serous ovarian cancer and correlated with shorter
recurrence-free survival and overall survival (25), while in NSCLC cells, particularly
in gefitinib-resistant cells, circ_MACF1 (a circular RNA form of
MACF1) regulates drug sensitivity and cellular behavior through its
interaction with miR-942-5p and TGFBR2(26). This axis influences cell
proliferation, migration, and invasion while promoting apoptosis
and sensitivity to gefitinib, suggesting that targeting circ_MACF1
could overcome resistance to EGFR inhibitors such as gefitinib in
NSCLC. Collectively, these investigations provide a premise for the
role of MACF1 in the etiology and progression of central nervous
system glial-derived tumors. It is also noteworthy that previous
investigations of plakin family members, plectin and desmoplakin,
have been described as biomarkers for glioblastomas (27-29).
5. MACF1 promotes glial cell
transformation
Malignant brain tumors in the central nervous system
are arguably the deadliest types of cancers diagnosed, with
glioblastomas being the most common, with an average median
survival of 12-14 months and a five-year survival rate of ~5%
(30,31). A major contributing factor to the
poor prognosis of these cancers is their complex genetic
heterogeneity that underlies their pathological origin, evolution
and therapeutic resistance. Identification of novel mediators of
disease transformation, progression and therapeutic evasion are
critical to advancing strategies for the clinical management and
treatment of these cancers. It is widely established that the
evolution of glioblastomas from astrocytes and neural progenitor
stem cells are a consequence of genetic mutations, deletions and
amplifications in phosphatase and tensin homolog, neurofibromin1,
p53, epidermal growth factor receptor and platelet-derived growth
factor receptor (32-35).
Genetic alterations in these oncogenes and tumor suppressors that
contribute to glioblastoma initiation and progression are supported
by knockout and genetically engineered mice models (36,37).
Although aberrant genetic abnormalities of receptor
tyrosine kinases, phosphatases and transcription factors have been
attributed to glioblastoma development, cytoskeletal proteins such
as nestin, vimentin and alpha-actinin have also been identified as
contributors to the inception of these tumors based largely on
expression analyses (38-40).
The best characterized of these, nestin, an intermediate filament
expressed in neural progenitor cells, has long been recognized as a
contributing oncogenic element in glioblastomas. Experimentally,
nestin positive neural stem cells have been demonstrated to give
rise to gliomas in murine models when transduced with EGFRvIII
(39). Paralleling expression
patterns of nestin and the previously mentioned cytoskeletal
proteins, Afghani et al (41), also observed that MACF1 expression
was absent in normal brain tissue and low-grade gliomas
(oligodendrogliomas and medulloblastomas) but displayed significant
expression in glioblastomas, which have high recurrence and
mortality rates. These data suggested that MACF1 is a potential
oncoprotein and therapeutic target in high-grade astrocyte derived
gliomas.
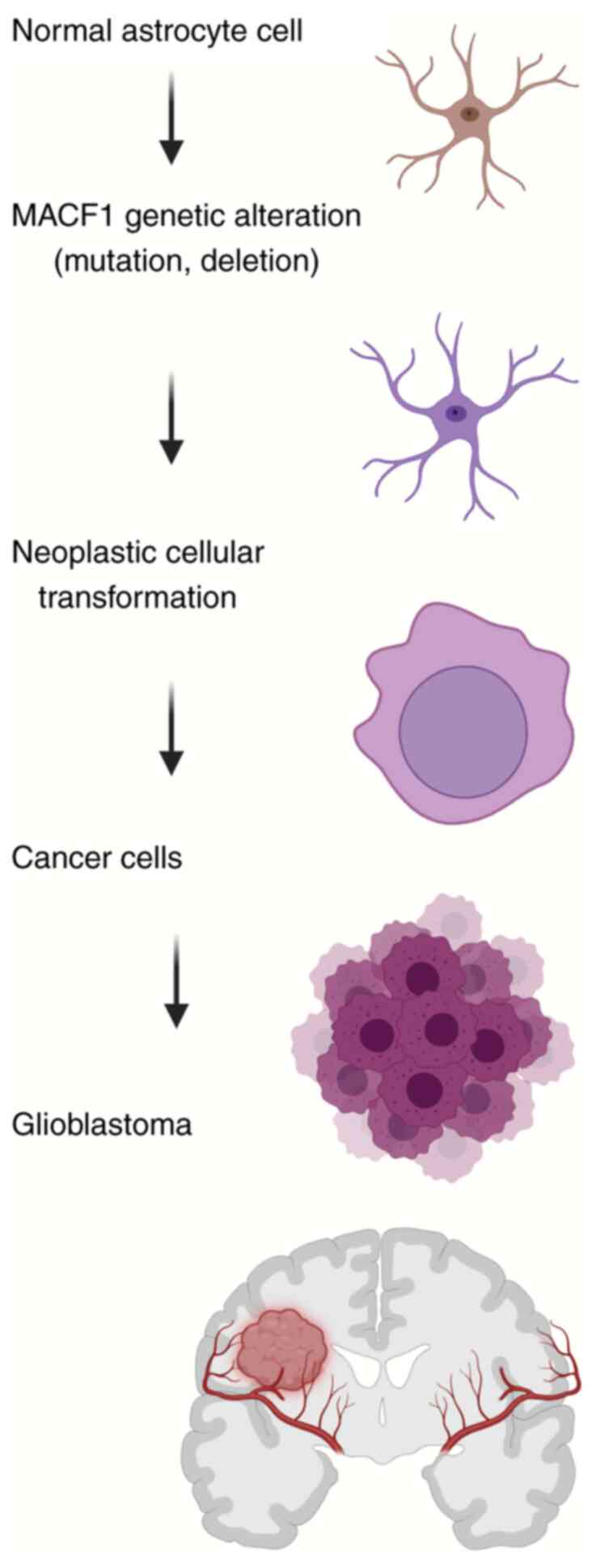
Despite the observation that MACF1 was expressed at
high levels in glioblastomas and that negatively regulating its
function impaired glioblastoma cell proliferation and migration,
the role of this spectraplakin protein as a tumorigenic driver in
cancer and glioblastomas specifically, has not been investigated.
However, a preliminary assessment of MACF1 tumor transformation
properties in normal astrocytes, one of the two cell types, along
with neural progenitor stem cells, considered the cellular origins
of glioblastomas was conducted to evaluate whether MACF1
perpetuates tumorigenic characteristics. To that end, unpublished
data of MACF1 overexpression studies performed in normal
astrocytes, previously demonstrated to express low MACF1 protein
levels, displayed significant increases in cell viability and
anchorage independent growth (42-44),
indicators of the transformed phenotype in normal astrocyte cells
(Fig. 2). These cellular responses
are consistent with the aforementioned oncogenic role of
cytoskeletal nestin in glioblastoma formation.
In addition to primary tumor development, secondary
glioblastomas and disease progression as manifested by tumor
recurrence resulting from normal tissue invasion is a collateral
oncogenic process, also derived from genetic abnormalities as
aforementioned, that leads to poor disease management and high
mortality rates. Further support of the pro-tumorigenic role of
MACF1 in glioblastomas, specifically as it relates to disease
recurrence, was also demonstrated in unpublished experimental
studies (42-44),
which showed that MACF1 overexpression increased astrocyte cell
migration, a prerequisite cell behavior of metastatic invasion.
Taken together, these cellular biological data (42-44)
provide evidence that spectraplakin protein is causally involved in
primary and secondary glioblastoma tumorigenesis and expands the
notion that MACF1 contributes to tumor development due to mutations
and alternative splicing events identified in endometrial cancer,
renal cell carcinomas and lung cancers, respectively (17-19).
6. Wnt-MACF1-mTOR signaling
As previously discussed, early investigations have
established that MACF1 plays a mechanistic role in Wnt-mediated
signaling. Specifically, MACF1 downregulation was demonstrated to
reduce nuclear β-catenin and transcriptional activation of Wnt
responsive genes (5). More
importantly, aberrant regulation of Wnt signaling is also known to
contribute to tumor proliferation and migratory invasion in
malignant brain tumors (45-48),
providing a correlative association that the onco-tumorigenic
impact of MACF1 is related to its interaction with the Wnt
signaling pathway (Fig. 3), a
well-characterized mechanistic mediator of tumor cell survival and
proliferation. As it pertains to central nervous system-derived
cancers such as glioblastomas, the most direct evidence for a
mechanistic role of MACF1 intracellular signaling in these tumors
was provided by studies from Afghani et al (41), which showed that downregulation of
MACF1 reduced Axin and phospho-β-catenin protein levels in
glioblastoma cells (41).
Furthermore, studies by Bonner et al (49) in irradiated glioblastoma cells
revealed that genetic silencing of MACF1 reduced the expression of
ribosomal protein s6, a downstream effector target of mTORC1, and
consequently sensitized these astrocyte-derived cancer cells to
radiation (49). This is
particularly significant given the established roles of both the
Wnt signaling pathway and PI3K-Akt-mTOR signaling axis as
contributors in glioblastoma progression (Fig. 3), invasion and therapeutic
resistance (50-53).
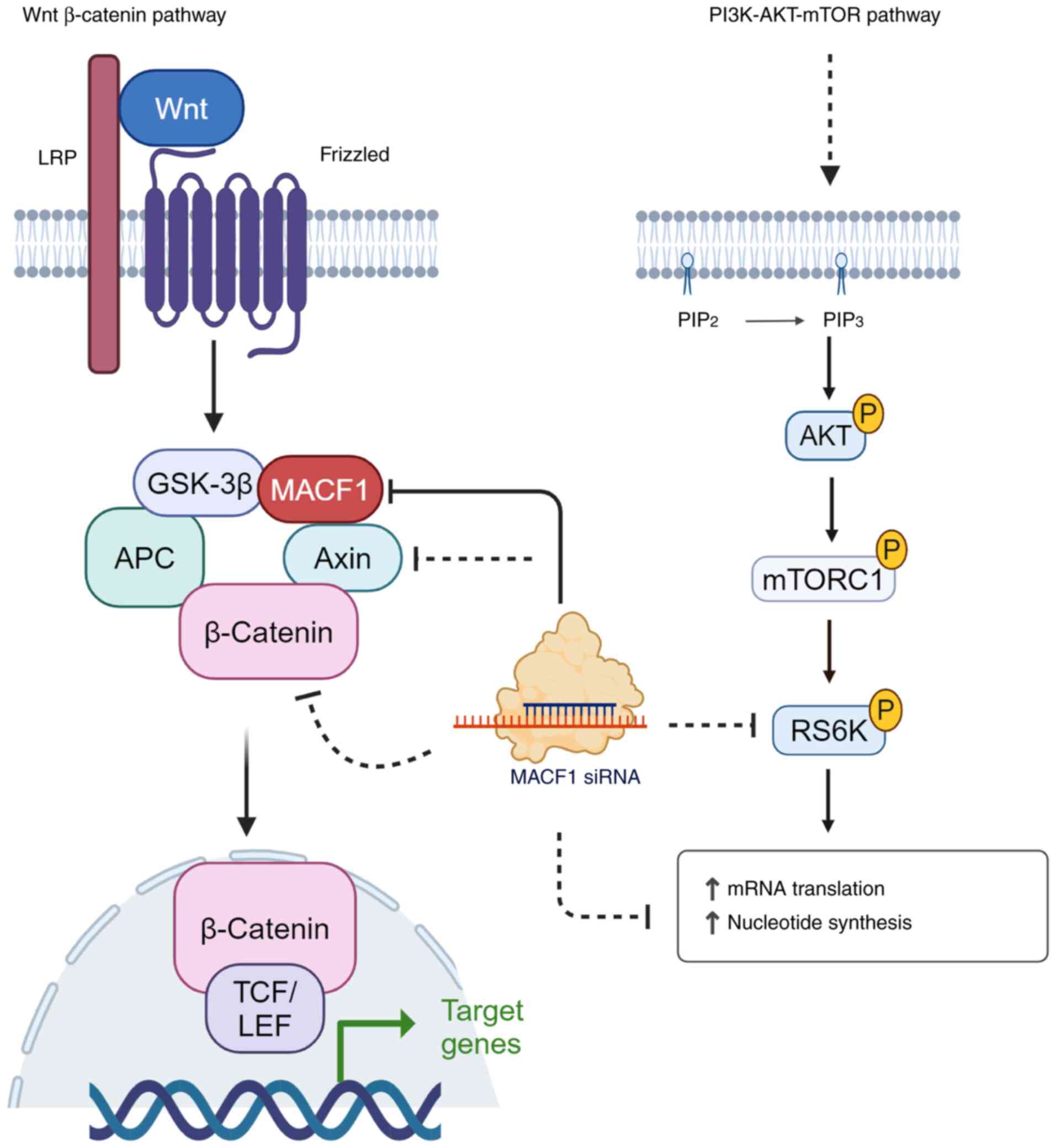 | Figure 3MACF1 is an effector mediator of Wnt
and mTOR signaling. MACF1 has been described as a component of the
Wnt signaling complex (GSK3β, axin, APC and beta-catenin) and
assists with the translocation of these signaling mediators to the
LRP receptor and activation of this signaling cascade.
Subsequently, beta-catenin is released to facilitate its
transcriptional activation function via interaction with TCF/LCF.
Suppression of MACF1 has been demonstrated to reduce axin,
beta-catenin,and s6-ribosomal protein expression levels and
attributed to reducing glioblastoma cell proliferation and
migration. The figure was created using bioRender (https://www.biorender.com/). MACF1, microtubule actin
crosslinking factor; GSK-3, glycogen synthase kinase-3; APC,
adenomatous polyposis coli; LRP, low-density lipoprotein
receptor-related protein; TCF/LEF, T cell factor, lymphocyte
enhancer factor-1. |
More importantly, when MACF1 is genetically
silenced, Wnt signaling mediators and mTOR effector proteins are
functionally impaired (Fig. 3).
Given the breadth of these pro-tumorigenic signaling pathways in
several cancers and glioblastomas in particular, a number of
investigations have examined small molecule inhibitors targeting
these pathways in glioblastomas (54,55).
Although signaling functions of MACF1 have been predominantly
associated with positive regulation of the Wnt signaling pathway,
additional studies in AML, a blood and bone marrow cancer have
provided additional insights on intracellular signaling roles of
MACF1. Specifically, silencing MACF1 function in AML cells was
found to reduce runt-related transcription factor Runx2 expression
and inactivated phosphatidyl inositol 3 kinase signaling (24). Furthermore, co-immunoprecipitation
experiments in AML cells provided evidence that MACF1 interacts
with leucine-rich repeat-containing protein 1(56), while osteogenesis studies revealed
that MACF1 positively regulates the TCF4/miR-335-5p signaling
pathway, consequently influencing bone formation (57). Collectively, these studies provide
evidence that extend the mechanistic function of MACF1 beyond Wnt
pathway.
Collectively, this suggests that MACF1 is a
contributor to treatment resistance of glioblastomas by acting as a
signaling mediator in divergent intracellular signaling cascades.
However, despite the absence of MACF1 in normal human astrocytes
and high expression levels in high-grade astrocytomas (19), as well as the onco-transformation
properties of this spectraplakin protein in glial cells, small
pharmacological inhibitory molecules targeting this cytoskeletal
cross-linker have not yet been identified. Because MACF1 crosslinks
microtubules and actin-filaments have prevalent biophysical roles
in mitotic tumor cell division and migration, developing
pharmacological agents that impair MACF1 provides a singular
therapeutic target that disrupts tumor cell behaviors that lead to
glioblastoma progression and therapeutic evasion.
7. MACF1 as a cancer therapeutic target
Although the aforementioned experimental
investigations provided evidence that genetic alterations of MACF1
were prevalent in several cancers, the role of MACF1 in cancer cell
biology and as a neoplastic target had remained unexamined. To that
end, studies by Afghani et al (41) and Bonner et al (49), were the first to investigate MACF1
as a cancer therapeutic target and demonstrated that inhibiting the
functional expression of MACF1 alone and in combination with
radiation and the clinically used DNA damaging agent, temozolomide,
had antitumorigenic effects on glioblastomas (Table II), astrocyte-derived central
nervous system tumors (57,58).
Additionally, findings in glioblastomas along with those by Wang
et al (58), revealed that
negative regulation of MACF1 impaired glioblastoma cell migration
and melanoma metastasis by decreasing the epithelial to mesenchymal
transition (Table II) and thus
provided evidence of the functional role of MACF1 in metastatic
invasion (57,58).
 | Table IITherapeutic targeting of MACF1.
Singular negative genetic inhibitory targeting of MACF1 and
combinatorial silencing with clinical therapeutic treatment
strategies promote anti-tumorigenic responses. |
Table II
Therapeutic targeting of MACF1.
Singular negative genetic inhibitory targeting of MACF1 and
combinatorial silencing with clinical therapeutic treatment
strategies promote anti-tumorigenic responses.
| First author,
year | Cancer type | Results | (Refs.) |
|---|
| Afghani et
al, 2017 | Glioblastomas | Inhibition of MACF1
impaired glioblastoma progression in patient derived xenograft cell
lines | (41) |
| Kaur et al,
2013 | Glioblastomas | Silencing
MACF1sensitized glioblastoma cells to DNA damaging agents | (48) |
| Wang et al,
2020 | Melanoma | Targeted MACF1
inhibition prevents metastasis | (58) |
To date, therapeutic agents that directly target
MACF1 are not yet available. Given MACF1's role in cellular
processes such as intracellular signaling and cell migration, which
are often dysregulated in cancer, warrants the development and
evaluation of anticancer drugs that target this cytoskeletal
protein. Further rationale to support the feasibility of developing
such drugs includes the role of MACF1 in cytoskeleton dynamics for
maintaining cell shape, polarity and motility, which are important
characteristics of cancer cell invasion and metastasis. It is also
noteworthy that because of MACF1's role in Wnt signaling, which is
often dysregulated in several cancers, inhibiting the function of
this plakin protein represents a novel neoplastic target. However,
a caveat to the druggability of MACF1 is its large size of ~600 kDa
and the numerous structural domains that it contains. Additionally,
engineering molecules that target such a large protein as well as
bioavailability challenges posed by the blood brain barrier to
access astrocytic glioblastomas, provide unique challenges.
8. Conclusion
The development of cancers are a consequence of
combinatorial genetic factors and their expressed products that
underlie intra- and inter-tumor heterogeneity. MACF1 is a potential
novel tumorigenic protein that may contribute to the clinical
etiology and progression of astrocyte-derived cancers such as
glioblastomas that reside in the central nervous system,
specifically the human brain, by perpetuating glial cell
proliferation and invasion. The investigation of MACF1 in cancer
biology, specifically glioblastomas, as a novel oncoprotein that
contributes to the etiology and progression of these central
nervous system-derived tumors warrants continued investigation. To
further establish the pro-tumorigenic role of MACF1 in the
evolution of brain tumors, it is essential to perform oncogenic
analyses of MACF1 in more translational applicable model systems
with diverse genetic backgrounds, such as orthotopic
patient-derived xenograft brain tumor models. The utility of these
model systems would provide further insight and perspective of
MACF1 in the context of oncogenes that drive a plethora of
intracellular signaling mechanisms and regulate tumorigenic cell
behaviors such as the PI3K signaling pathway, one of the most
prevalent in the perpetuation of tumorigenesis, and in the absence
of tumor suppressors during oncogenic transformation. Additionally,
pursuing the development of chemotherapeutic agents that target
MACF1 will broaden clinical approaches beyond therapeutic agents
such as vinca alkaloids that inhibit microtubules used to treat
this disease.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
KB and QQ conceptualized and developed the review
framework and wrote the manuscript. Both authors read and approved
the final version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hu L, Xiao Y, Xiong Z, Zhao F, Yin C,
Zhang Y, Su P, Li D, Chen Z, Ma X, et al: MACF1, versatility in
tissue-specific function and in human disease. Semin Cell Dev Biol.
69:3–8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Applewhite DA, Grode KD, Duncan MC and
Rogers SL: The actin-microtubule cross-linking activity of
Drosophila Short stop is regulated by intramolecular inhibition.
Mol Biol Cell. 24:2885–2893. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Goryunov D and Liem RK: Microtubule-Actin
cross-linking factor 1: Domains, interaction partners, and
tissue-specific functions. Methods Enzymol. 569:331–353.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cusseddu R, Robert A and Côté JF: Strength
through unity: The power of the mega-scaffold MACF1. Front Cell Dev
Biol. 9(641727)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yin C, Zhang Y, Hu L, Tian Y, Chen Z, Li
D, Zhao F, Su P, Ma X, Zhang G, et al: Mechanical unloading reduces
microtubule actin crosslinking factor 1 expression to inhibit
β-catenin signaling and osteoblast proliferation. J Cell Physiol.
233:5405–5419. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bouameur JE, Favre B and Borradori L:
Plakins, a versatile family of cytolinkers: Roles in skin integrity
and in human diseases. J Invest Dermatol. 134:885–894.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hu L, Su P, Li R, Yin C, Zhang Y, Shang P,
Yang T and Qian A: Isoforms, structures, and functions of versatile
spectraplakin MACF1. BMB Rep. 49:37–44. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Quick QA: Microtubule-Actin crosslinking
factor 1 and plakins as therapeutic drug targets. Int J Mol Sci.
19(368)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Goryunov D, He CZ, Lin CS, Leung CL and
Liem RK: Nervous-tissue-specific elimination of microtubule-actin
crosslinking factor 1a results in multiple developmental defects in
the mouse brain. Mol Cell Neurosci. 44:1–14. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ka M, Moffat JJ and Kim WY: MACF1 controls
migration and positioning of cortical GABAergic interneurons in
mice. Cereb Cortex. 27:5525–5538. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Qu Y, Alves-Silva J, Gupta K, Hahn I,
Parkin J, Sánchez-Soriano N and Prokop A: Re-evaluating the
actin-dependence of spectraplakin functions during axon growth and
maintenance. Dev Neurobiol. 82:288–307. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Alves-Silva J, Sánchez-Soriano N, Beaven
R, Klein M, Parkin J, Millard TH, Bellen HJ, Venken KJ, Ballestrem
C, Kammerer RA and Prokop A: Spectraplakins promote
microtubule-mediated axonal growth by functioning as structural
microtubule-associated proteins and EB1-dependent +TIPs (tip
interacting proteins). J Neurosci. 32:9143–9158. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ka M and Kim WY: Microtubule-Actin
crosslinking factor 1 is required for dendritic arborization and
axon outgrowth in the developing brain. Mol Neurobiol.
53:6018–6032. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ka M, Jung EM, Mueller U and Kim WY: MACF1
regulates the migration of pyramidal neurons via microtubule
dynamics and GSK-3 signaling. Dev Biol. 395:4–18. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Moffat JJ, Ka M, Jung EM, Smith AL and Kim
WY: The role of MACF1 in nervous system development and
maintenance. Semin Cell Dev Biol. 69:9–17. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Dobyns WB, Aldinger KA, Ishak GE, Mirzaa
GM, Timms AE, Grout ME, Dremmen MHG, Schot R, Vandervore L, van
Slegtenhorst MA, et al: MACF1 mutations encoding highly conserved
zinc-binding residues of the GAR domain cause defects in neuronal
migration and axon guidance. Am J Hum Genet. 103:1009–1021.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Misquitta-Ali CM, Cheng E, O'Hanlon D, Liu
N, McGlade CJ, Tsao MS and Blencowe BJ: Global profiling and
molecular characterization of alternative splicing events
misregulated in lung cancer. Mol Cell Biol. 31:138–150.
2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Arai E, Sakamoto H, Ichikawa H, Totsuka H,
Chiku S, Gotoh M, Mori T, Nakatani T, Ohnami S, Nakagawa T, et al:
Multilayer-omics analysis of renal cell carcinoma, including the
whole exome, methylome and transcriptome. Int J Cancer.
135:1330–1342. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chang YS, Huang HD, Yeh KT and Chang JG:
Identification of novel mutations in endometrial cancer patients by
whole-exome sequencing. Int J Oncol. 50:1778–1784. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tian Y, Zhu K, Li Y, Ren Z and Wang J:
MACF1 mutations predict poor prognosis: A novel potential
therapeutic target for breast cancer. Am J Transl Res.
14:7670–7688. 2022.PubMed/NCBI
|
|
21
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6(pl1)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
de Bruijn I, Kundra R, Mastrogiacomo B,
Tran TN, Sikina L, Mazor T, Li X, Ochoa A, Zhao G, Lai B, et al:
Analysis and visualization of longitudinal genomic and clinical
data from the AACR project GENIE biopharma collaborative in
cBioPortal. Cancer Res. 83:3861–3867. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang P, Zhang J, Zhang H and Zhang F: The
role of MACF1 on acute myeloid leukemia cell proliferation is
involved in Runx2-targeted PI3K/Akt signaling. Mol Cell Biochem.
478:433–441. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu L, Hu K, Zeng Z, Xu C, Lv J, Lin Z and
Wen B: Expression and clinical significance of microtubule-actin
cross-linking factor 1 in serous ovarian cancer. Recent Pat
Anticancer Drug Discov. 16:66–72. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fan D, Yang Y and Zhang W: A novel
circ_MACF1/miR-942-5p/TGFBR2 axis regulates the functional
behaviors and drug sensitivity in gefitinib-resistant non-small
cell lung cancer cells. BMC Pulm Med. 22(27)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Žugec M, Furlani B, Castañon MJ, Rituper
B, Fischer I, Broggi G, Caltabiano R, Barbagallo GMV, Di Rosa M,
Tibullo D, et al: Plectin plays a role in the migration and volume
regulation of astrocytes: A potential biomarker of glioblastoma. J
Biomed Sci. 31(14)2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kubelt C, Hattermann K, Sebens S, Mehdorn
HM and Held-Feindt J: Epithelial-to-mesenchymal transition in
paired human primary and recurrent glioblastomas. Int J Oncol.
46:2515–2525. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Žugec M, Furlani B, Castañon MJ, Rituper
B, Fischer I, Broggi G, Caltabiano R, Barbagallo GMV, Di Rosa M,
Tibullo D, et al: Plectin plays a role in the migration and volume
regulation of astrocytes: A potential biomarker of glioblastoma. J
Biomed Sci. 31(14)2024.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ostrom QT, Cioffi G, Gittleman H, Patil N,
Waite K, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United States in 2012-2016. Neuro Oncol. 21 (Suppl
5):v1–v100. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ostrom QT, Price M, Neff C, Cioffi G,
Waite KA, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical
report: Primary brain and other central nervous system tumors
diagnosed in the United States in 2016-2020. Neuro Oncol. 25 (12
Suppl 2):iv1–iv99. 2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhang P, Xia Q, Liu L, Li S and Dong L:
Current opinion on molecular characterization for GBM
classification in guiding clinical diagnosis, prognosis, and
therapy. Front Mol Biosci. 7(562798)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Georgescu MM: Translation into clinical
practice of the G1-g7 molecular subgroup classification of
glioblastoma: Comprehensive demographic and molecular pathway
profiling. Cancers (Basel). 16(361)2024.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lazzarini E, Silvestris DA, Benvenuto G,
Osti D, Fattore L, Paterra R, Finocchiaro G, Malatesta P, Daga A,
Gallotti AL, et al: Genome-wide profiling of patient-derived
glioblastoma stem-like cells reveals recurrent genetic and
transcriptomic signatures associated with brain tumors. J
Neurooncol. 163:47–59. 2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ludwig K and Kornblum HI: Molecular
markers in glioma. J Neurooncol. 134:505–512. 2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Robertson FL, Marqués-Torrejón MA,
Morrison GM and Pollard SM: Experimental models and tools to tackle
glioblastoma. Dis Model Mech. 12(dmm040386)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Miyai M, Tomita H, Soeda A, Yano H, Iwama
T and Hara A: Current trends in mouse models of glioblastoma. J
Neurooncol. 135:423–432. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ciechomska IA, Wojnicki K, Wojtas B,
Szadkowska P, Poleszak K, Kaza B, Jaskula K, Dawidczyk W, Czepko R,
Banach M, et al: Exploring novel therapeutic opportunities for
glioblastoma using patient-derived cell cultures. Cancers (Basel).
15(1562)2023.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen Z, Herting CJ, Ross JL, Gabanic B,
Vallcorba MP, Szulzewsky F, Wojciechowicz ML, Cimino PJ,
Ezhilarasan R, Sulman EP, et al: Genetic driver mutations
introduced in identical cell-of-origin in murine glioblastoma
reveal distinct immune landscapes but similar response to
checkpoint blockade. Glia. 68:2148–2166. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Quick Q and Skalli O: Alpha-actinin 1 and
alpha-actinin 4: Contrasting roles in the survival, motility, and
RhoA signaling of astrocytoma cells. Exp Cell Res. 316:1137–1147.
2010.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Afghani N, Mehta T, Wang J, Tang N, Skalli
O and Quick QA: Microtubule actin cross-linking factor 1, a novel
target in glioblastoma. Int J Oncol. 50:310–316. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Quick Q and Bonner K: Immunoblot, cell
viability, and transformation bar graphs. 2023 https://doi.org/10.6084/m9.figshare.24391903
(unpublished data).
|
|
43
|
Quick Q and Bonner K: Cell motility bar
graph and images. 2023 https://doi.org/10.6084/m9.figshare.24392593
(unpublished data).
|
|
44
|
Quick Q and Bonner K: Methods. 2023
https://doi.org/10.6084/m9.figshare.24393145
(unpublished data).
|
|
45
|
Li GF, Cheng YY, Li BJ, Zhang C, Zhang XX,
Su J, Wang C, Chang L, Zhang DZ, Tan CL and Wang N: miR-375
inhibits the proliferation and invasion of glioblastoma by
regulating Wnt5a. Neoplasma. 66:350–356. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Precilla DS, Kuduvalli SS, Purushothaman
M, Marimuthu P, Muralidharan AR and Anitha TS: Wnt/β-catenin
antagonists: Exploring new avenues to trigger old drugs in
alleviating glioblastoma multiforme. Curr Mol Pharmacol.
15:338–360. 2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
De Robertis A, Valensin S, Rossi M, Tunici
P, Verani M, De Rosa A, Giordano C, Varrone M, Nencini A, Pratelli
C, et al: Identification and characterization of a small-molecule
inhibitor of Wnt signaling in glioblastoma cells. Mol Cancer Ther.
12:1180–1189. 2013.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kaur N, Chettiar S, Rathod S, Rath P,
Muzumdar D, Shaikh ML and Shiras A: Wnt3a mediated activation of
Wnt/β-catenin signaling promotes tumor progression in glioblastoma.
Mol Cell Neurosci. 54:44–57. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Bonner K, Borlay D, Kutten O and Quick QA:
Inhibition of the spectraplakin protein microtubule actin
crosslinking factor 1 sensitizes glioblastomas to radiation. Brain
Tumor Res Treat. 8:43–52. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Langhans J, Schneele L, Trenkler N, von
Bandemer H, Nonnenmacher L, Karpel-Massler G, Siegelin MD, Zhou S,
Halatsch ME, Debatin KM and Westhoff MA: The effects of
PI3K-mediated signaling on glioblastoma cell behaviour.
Oncogenesis. 6(398)2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Li X, Wu C, Chen N, Gu H, Yen A, Cao L,
Wang E and Wang L: PI3K/Akt/mTOR signaling pathway and targeted
therapy for glioblastoma. Oncotarget. 7:33440–33450.
2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Suwala AK, Koch K, Rios DH, Aretz P,
Uhlmann C, Ogorek I, Felsberg J, Reifenberger G, Köhrer K, Deenen
R, et al: Inhibition of Wnt/beta-catenin signaling downregulates
expression of aldehyde dehydrogenase isoform 3A1 (ALDH3A1) to
reduce resistance against temozolomide in glioblastoma in vitro.
Oncotarget. 9:22703–22716. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Von Achenbach C, Weller M, Kaulich K,
Gramatzki D, Zacher A, Fabbro D, Reifenberger G and Szabó E:
Synergistic growth inhibition mediated by dual PI3K/mTOR pathway
targeting and genetic or direct pharmacological AKT inhibition in
human glioblastoma models. J Neurochem. 153:510–524.
2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Salphati L, Alicke B, Heffron TP,
Shahidi-Latham S, Nishimura M, Cao T, Carano RA, Cheong J, Greve J,
Koeppen H, et al: Brain distribution and efficacy of the brain
penetrant PI3K inhibitor GDC-0084 in orthotopic mouse models of
human glioblastoma. Drug Metab Dispos. 44:1881–1889.
2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Guo T, Wu C, Zhang J, Yu J, Li G, Jiang H,
Zhang X, Yu R and Liu X: Dual blockade of EGFR and PI3K signaling
pathways offers a therapeutic strategy for glioblastoma. Cell
Commun Signal. 21(363)2023.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wang Y, Tong H, Wang J, Hu L and Huang Z:
LRRC1 knockdown downregulates MACF1 to inhibit the malignant
progression of acute myeloid leukemia by inactivating
β-catenin/c-Myc signaling. J Mol Histol. 55:37–50. 2024.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhang K, Qiu W, Li H, Li J, Wang P, Chen
Z, Lin X and Qian A: MACF1 overexpression in BMSCs alleviates
senile osteoporosis in mice through TCF4/miR-335-5p signaling
pathway. J Orthop Translat. 39:177–190. 2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wang X, Jian X, Dou J, Wei Z and Zhao F:
Decreasing microtubule actin cross-linking factor 1 inhibits
melanoma metastasis by decreasing epithelial to mesenchymal
transition. Cancer Manag Res. 12:663–673. 2020.PubMed/NCBI View Article : Google Scholar
|