Introduction
Gastric cancer is one of the most common malignant
tumors worldwide, and mortality and morbidity rates are continuing
to increase. Notably, the incidence of gastric cancer ranks fifth
among malignant tumors, and the mortality rate is third highest,
following lung and liver cancer (1-7).
Radical surgery combined with adjuvant chemoradiotherapy, targeted
therapy and immunotherapy has demonstrated potential in the
treatment of gastric cancer; however, the five-year survival rate
of patients remains low. The five-year survival rate of patients
with advanced gastric cancer is <20% (8).
Gastric cancer exhibits high levels of
heterogeneity, and is associated with a poor prognosis and low
rates of survival. At present, targeted therapies for advanced
gastric cancer include anti-human epidermal growth factor receptor
2 and vascular endothelial growth factor receptor 2, and research
is focused on the use of CLDN18.2 as a potential treatment option.
Notably, first-line treatment includes immunotherapy, and
peri-operative clinical trials are ongoing. However, immunotherapy
exhibits numerous limitations, including inconsistent treatment
responses, drug resistance and treatment-associated adverse events
(9-11).
Thus, further investigations are required to optimize current
immunotherapeutic regimens and improve the effectiveness and safety
of immunotherapy in the treatment of gastric cancer.
Tumor recurrence and metastasis are the main causes
of death following radical gastrectomy. Novel, non-invasive,
inexpensive treatment options using serum tumor markers are
required for early detection and intervention, following the
recurrence or metastasis of gastric cancer (12-15).
Carcinoembryonic antigen (CEA) is a glycoprotein
located in the gastrointestinal mucosal epithelia, while
carbohydrate antigen (CA) is expressed at the carbohydrate sites of
high-molecular weight mucins. Notably, both CEA and CA bind to the
integrin family, mediating calcium-independent intercellular
adhesion. The mechanisms underlying CEA and CA are comparable to
the mechanisms underlying tumor invasion and metastasis (16,17).
Alpha-fetoprotein (AFP) is a glycoprotein belonging to the albumin
family. AFP is closely associated with the occurrence and
development of liver cancer and a variety of tumors, such as
gastric, pancreatic, lung and colorectal cancer. AFP is used as a
positive detection index for a variety of tumors (18), and results of a previous study
revealed that AFP is significantly elevated in patients with
AFP-positive gastric cancer (19).
Notably, both CA19-9 and CA242 are key markers of gastric cancer
(20), and these proteins, along
with CEA and AFP, are routinely measured following radical
gastrectomy.
Numerous previous studies assessed pre-operative
serum tumor marker levels as risk factors for recurrence or
metastasis (21,22); however, these studies did not focus
on post-operative serum tumor marker levels. Notably, few studies
investigated the association between post-operative positive tumor
markers and recurrence or metastasis (23,24).
Thus, early detection and timely intervention may improve the
survival rate of patients with recurrence or metastasis of gastric
cancer, following radical resection.
The present study aimed to determine the predictive
value of CEA, AFP, CA19-9 and CA242 for recurrence/metastasis of
gastric cancer following radical resection, to aid in early
intervention and the treatment of patients. Receiver operating
characteristic (ROC) curve, area under the curve (AUC) and
univariate and multivariate analyses were used for the present
study.
Materials and methods
Patients
The present study was approved (approval no.
20240651) by the Ethical Institutional Review Committee of The
Second Affiliated Hospital of Zhejiang University School of
Medicine (Zhejiang, China). Patient informed consent was waived by
the ethics committee as the present study is retrospective.
Patients with stage I-III gastric cancer admitted to The Second
Affiliated Hospital of Zhejiang University School of Medicine from
January 2016 to September 2018 were enrolled in the present study.
Patients were included in the present study according to the
following criteria: i) Gastric cancer confirmed via gastroscopic
pathology or surgical pathology; ii) a history of radical surgical
resection, open or laparoscopic distal gastrectomy (DG), proximal
gastrectomy (PG) or total gastrectomy (TG); iii) serum CEA, AFP,
CA19-9 and CA242 levels detected prior to surgery; and iv) complete
medical records. Patients were excluded from the present study
according to the following criteria: i) The presence of gastric
stump cancer; ii) the presence of severe infection; iii) the
presence of other malignant tumors; iv) pregnancy or breastfeeding;
v) no history of radical resection; vi) a history of neoadjuvant
therapy; and vii) incomplete follow-up data.
According to the inclusion and exclusion criteria, a
total of 368 patients with gastric cancer were enrolled in the
present study, including 236 men and 132 women (age, 25-87 years).
According to the TNM staging criteria of the Union of International
Cancer Control and the American Joint Committee on Cancer, there
were 152 patients with stage I disease, 115 patients with stage II
disease and 101 patients with stage III disease. Patients included
in the present study were evaluated for CEA, AFP, CA19-9 and CA242
levels at least once within 1 month prior to gastrectomy, and
levels were evaluated 6 to 12 months following surgery. CEA, AFP,
CA19-9 and CA242 levels of >5 ng/ml, >20 ng/ml, >37 U/ml
and 20 U/ml, respectively, were considered positive prior to
surgery (24-27).
Post-operative serum CEA, AFP, CA19-9 and CA242 levels that
exceeded the healthy range were considered positive. Recurrence and
metastasis were evaluated using computed tomography (CT), magnetic
resonance imaging (MRI), ultrasound (US), positron Emission
Tomography-CT (PET-CT) and puncture biopsy pathology.
Clinical features, such as age, pathological stage
and post-operative adjuvant therapy were evaluated in 368 patients
with gastric cancer. Following radical gastrectomy, all patients
were followed up via outpatient or telephone follow-up for a period
of 5 years, and follow-up ended in November 2023. Regular abdominal
B-ultrasonography, CT, MRI, tumor marker, routine blood and PET-CT
examinations were performed, and death or the end of follow-up were
considered the end point of the investigation. The recurrence and
metastasis of all patients with gastric cancer were analyzed, and
patients were divided into recurrence/metastasis and
non-recurrence/metastasis groups. Clinical characteristics, such as
sex, age, depth of invasion, and CEA, AFP, CA19-9 and CA242 levels
were compared between the two groups.
Statistical analysis
All data were statistically analyzed using GraphPad
Prism (version, 9.1.1; GraphPad Software, Inc.). Data are presented
as the number of cases and rate (%). Measurement data with normal
distribution and homogeneity of variance are presented as the mean
± standard deviation (x̄ ± s). Univariate and multivariate analyses
were performed using GraphPad Prism, and Fisher's exact test and
multiple logistic regression were used, respectively. A receiver
operating characteristic (ROC) curve was used to calculate the area
under the curve (AUC). Levels of sensitivity and specificity were
used to evaluate the predictive value of CEA, AFP, CA19-9 and CA242
in the post-operative recurrence or metastasis of gastric cancer.
ROC curves were calculated using the R package (version 1.18.5).
Kaplan-Meier survival (followed by a log-rank test) was analyzed
using the R package survival (version 3.5; https://github.com/therneau/survival). P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical characteristics of patients
with gastric cancer
The median age of 368 patients with gastric cancer
was 60 years. Among them, 236 patients were male (64%) and 132
patients were female (36%). According to the tumor differentiation
grade, 19 cases were high, 16 cases were high-moderate, 70 cases
were moderate, 89 cases were moderate-low and 174 cases were low
grade. Open surgery was performed in 64% of patients, and 26% of
patients underwent TG. Notably, lymph node dissection was D2 in 99%
of the patients. In total, 152 patients exhibited stage I disease
(41%), 115 patients exhibited stage II disease (31%) and 101
patients exhibited stage III disease (28%). In addition, 63% of
patients underwent post-operative adjuvant therapy. In total, 34
(9%) patients were positive for CEA, 4 (1%) patients were positive
for AFP, 39 (11%) patients were positive for CA19-9 and 23 (6%)
patients were positive for CA242. At the end of follow-up, 68/368
patients exhibited recurrence/metastasis, and 300/368 patients did
not experience recurrence/metastasis, with a recurrence/metastasis
rate of 18% (Table I).
 | Table IClinicopathological characteristics of
368 patients. |
Table I
Clinicopathological characteristics of
368 patients.
| Characteristics | Value (%) |
|---|
| Median age
(Range) | 60 (25-87) |
| Sex | |
|
Male | 236(64) |
|
Female | 132(36) |
| Tumor grade | |
|
High | 19(5) |
|
High-moderate | 16(4) |
|
Moderate | 70(19) |
|
Moderate-low | 89(24) |
|
Low | 174(48) |
| Approach of
operation | |
|
Open | 234(64) |
|
Laparoscopic | 134(36) |
| Type of
gastrectomy | |
|
Distal
gastrectomy, proximal gastrectomy | 273(74) |
|
Total
gastrectomy | 95(26) |
| Lymph node
dissection | |
|
D1,
D1+ | 2(1) |
|
D2 | 366(99) |
| Disease
stagea | |
|
I | 152(41) |
|
II | 115(31) |
|
III | 101(28) |
| Adjuvant
chemotherapy | |
|
Yes | 231(63) |
|
No | 137(27) |
| Preoperative
carcinoembryonic antigen | |
|
Positive | 34(9) |
|
Negative | 334(91) |
| Preoperative
alpha-fetoprotein | |
|
Positive | 4(1) |
|
Negative | 364(99) |
| Preoperative
carbohydrate antigen 19-9 | |
|
Positive | 39(11) |
|
Negative | 329(89) |
| Preoperative
carbohydrate antigen 242 | |
|
Positive | 23(6) |
|
Negative | 345(94) |
|
Recurrence/Metastasis | |
|
Yes | 68(18) |
|
No | 300(82) |
Univariate and multivariate analysis
of post-operative recurrence/metastasis in patients with gastric
cancer
All patients with gastric cancer were divided into
recurrence/metastasis (n=68) and non-recurrence/metastasis groups
(n=300). Results of the univariate analysis revealed no significant
difference in age, sex, lymph node dissection, pre-operative CEA
levels and pre-operative AFP levels between patients with
recurrence/metastasis and those with non-recurrence/metastasis.
Notably, age ≥70, open surgery, TG, disease stage III,
pre-operative CA19-9 positivity and pre-operative CA242 positivity
were risk factors for recurrence/metastasis (Table II). Results of the multivariate
logistic regression analysis revealed that approach of operation
was a risk factor for recurrence/metastasis. In addition, disease
stage II and III were risk factors for recurrence/metastasis,
compared with disease stage I (Table
III).
 | Table IIResults of univariate analysis. |
Table II
Results of univariate analysis.
| | Value (%) | |
|---|
|
Characteristics | Patients with
recurrence/metastasis(n=68) | Patients without
recurrence/metastasis (n=300) | P-value |
|---|
| Age, years | | | 0.0138 |
|
≥70 | 20(30) | 47(16) | |
|
<70 | 48(70) | 253(84) | |
| Sex | | | 0.1613 |
|
Male | 49(72) | 187(62) | |
|
Female | 19(28) | 113(38) | |
| Operative
approach | | | <0.0001 |
|
Open | 61(90) | 127(42) | |
|
Laparoscopic | 7(10) | 173(58) | |
| Type of
gastrectomy | | | 0.0309 |
|
Distal
gastrectomy, proximal gastrectomy | 43(63) | 230(77) | |
|
Total
gastrectomy | 25(37) | 70(23) | |
| LN dissection | | | 0.4996 |
|
D1,
D1+ | 0 (0) | 2(1) | |
|
D2 | 68(100) | 298(99) | |
| Disease stage | | | <0.0001 |
|
I | 4(6) | 148(50) | |
|
II | 23(34) | 92(30) | |
|
III | 41(60) | 60(20) | |
| Preoperative
carcinoembryonic antigen | | | 0.2440 |
|
Positive | 9(13) | 25(8) | |
|
Negative | 59(87) | 275(92) | |
| Preoperative
alpha-fetoprotein | | | 0.1570 |
|
Positive | 2(3) | 2(1) | |
|
Negative | 66(97) | 298(99) | |
| Preoperative
CA19-9 | | | 0.0482 |
|
Positive | 12(18) | 27(9) | |
|
Negative | 56(82) | 273(91) | |
| Preoperative
CA242 | | | 0.0215 |
|
Positive | 9(13) | 14(5) | |
|
Negative | 59(87) | 286(95) | |
 | Table IIIResults of multivariate analysis. |
Table III
Results of multivariate analysis.
|
Characteristics | Odds ratio | 95% confidence
interval | P-value |
|---|
| Age, ≥70 | 1.727 | 0.7984-3.699 | 0.1603 |
| Sex | 0.7742 | 0.3862-1.516 | 0.4612 |
| Approach of
operation | 0.3657 | 0.1389-0.8556 | 0.0280 |
| Disease stage
II | 8.132 | 2.166-40.51 | 0.0044 |
| Disease stage
III | 22.84 | 6.167-114.9 | <0.0001 |
| Adjuvant
chemotherapy | 0.8417 | 0.3300-2.157 | 0.7170 |
| Preoperative
carcinoembryonic antigen | 1.010 | 0.9728-1.048 | 0.5842 |
| Preoperative
alpha-fetoprotein | 0.9997 | 0.9729-1.001 | 0.9035 |
| Preoperative
CA19-9 | 1.001 | 0.9970-1.005 | 0.5659 |
| Preoperative
CA242 | 1.000 | 0.9780-1.023 | 0.9819 |
Predictive value of post-operative
CEA, AFP, CA19-9 and CA242 levels for the recurrence/metastasis of
gastric cancer
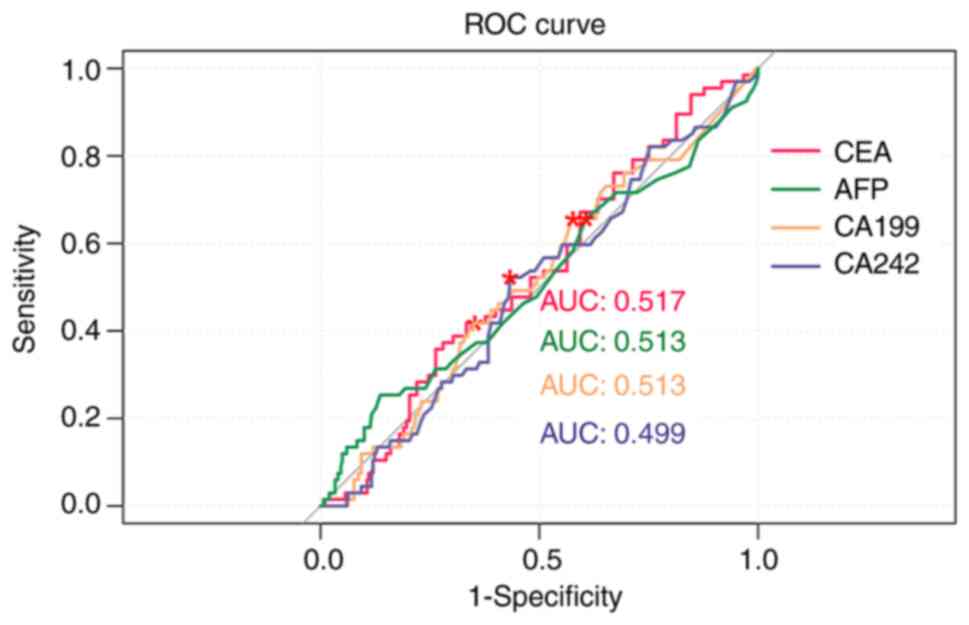
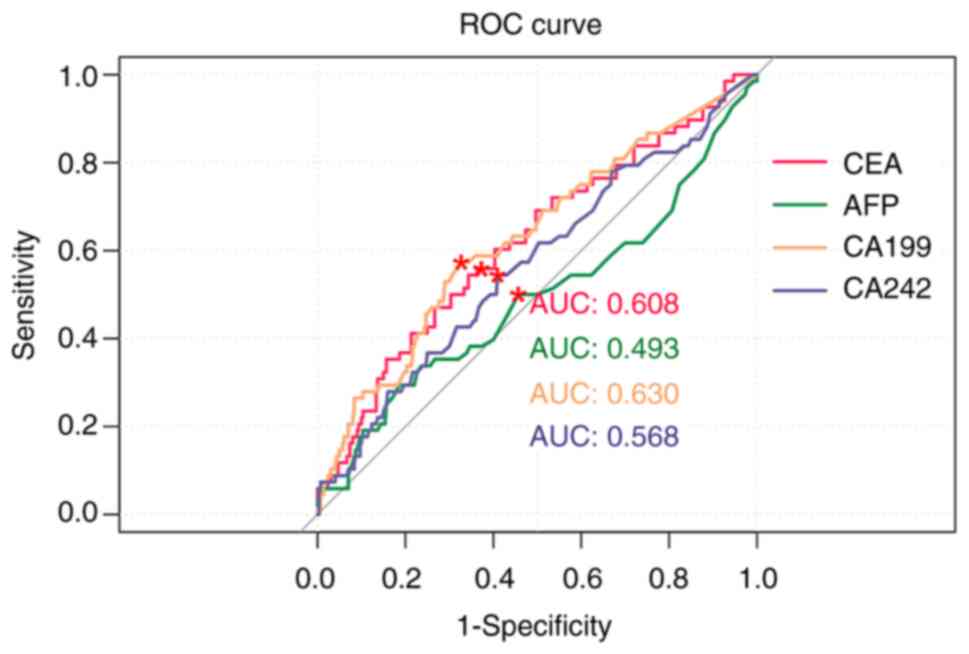
Results of the ROC curve analysis revealed that the
AUC values of pre-operative CEA, AFP, CA19-9 and CA242 levels in
the prediction of recurrence/metastasis were 0.517, 0.513, 0.513
and 0.499, respectively (Fig. 1
and Table IV). The AUC values of
post-operative CEA, AFP, CA19-9 and CA242 levels in the prediction
of recurrence/metastasis were 0.608, 0.493, 0.630 and 0.568,
respectively (Fig. 2 and Table IV). Notably, the AUC values of
post-operative CA19-9 levels were higher than CEA, AFP and
CA242.
 | Table IVEvaluation value of CEA, AFP, CA19-9
and CA242 in postoperative recurrence/metastasis of gastric
cancer. |
Table IV
Evaluation value of CEA, AFP, CA19-9
and CA242 in postoperative recurrence/metastasis of gastric
cancer.
| | Area under the
curve | Specificity | Sensitivity |
|---|
| Preoperative
CEA | 0.517 | 0.647 | 0.418 |
| Preoperative
AFP | 0.513 | 0.393 | 0.657 |
| Preoperative
CA19-9 | 0.513 | 0.423 | 0.657 |
| Preoperative
CA242 | 0.499 | 0.567 | 0.522 |
| Postoperative
CEA | 0.608 | 0.627 | 0.559 |
| Postoperative
AFP | 0.493 | 0.543 | 0.500 |
| Postoperative
CA19-9 | 0.630 | 0.673 | 0.574 |
| Postoperative
CA242 | 0.568 | 0.590 | 0.544 |
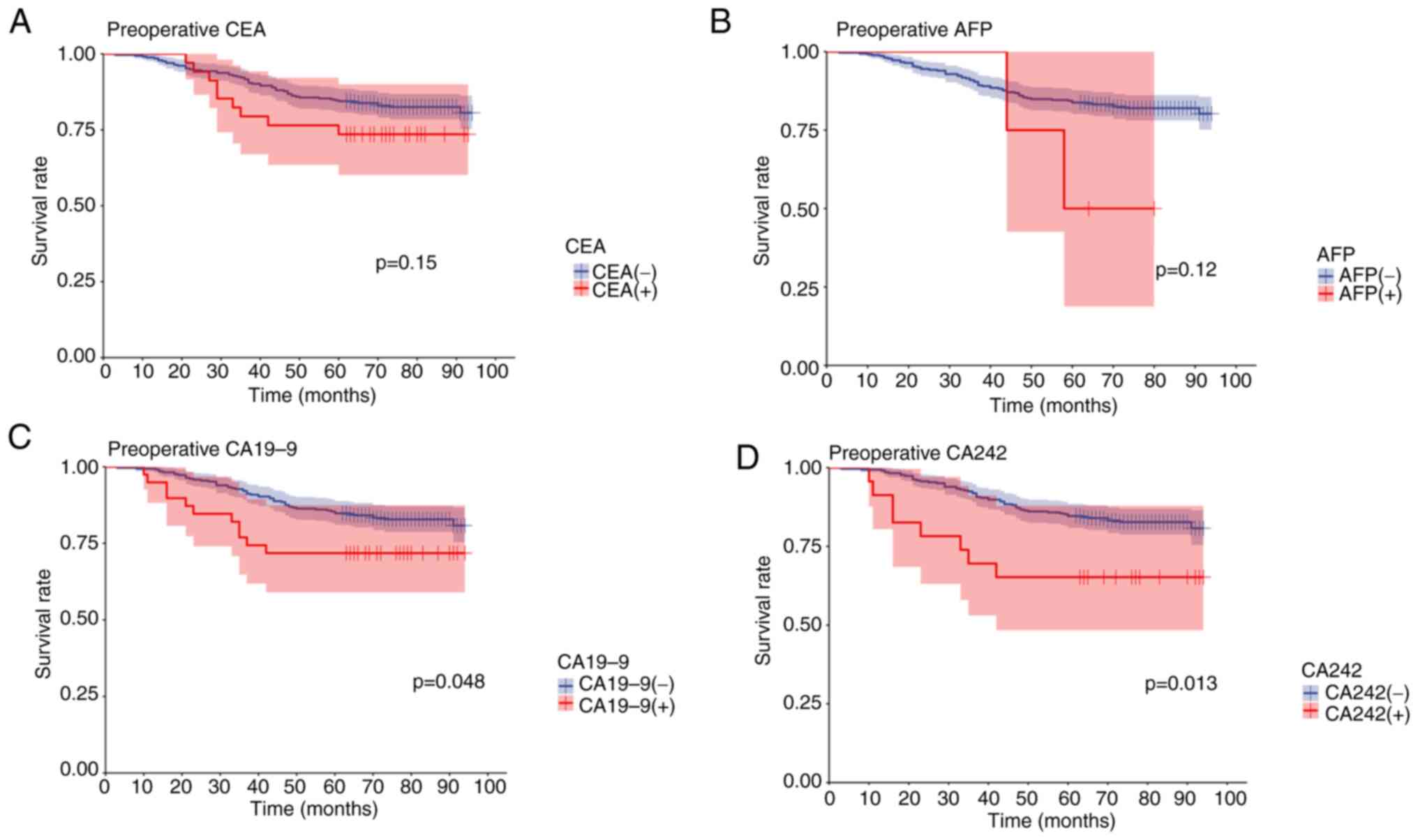
Comparison of cumulative survival rate
between patients with positive or negative CEA, AFP, CA19-9 and
CA242 levels
Cumulative survival was compared between patients
with positive pre-operative serum CEA, AFP, CA19-9 and CA242 levels
and patients with negative pre-operative serum CEA, AFP, CA19-9 and
CA242 levels. Results of the Kaplan-Meier survival analysis
revealed that patients with negative pre-operative CEA, AFP, CA19-9
and CA242 levels exhibited a higher five-year survival rate than
patients with positive pre-operative CEA, AFP, CA19-9 and CA242
levels. In addition, patients with positive pre-operative serum
CEA, AFP, CA19-9 and CA242 levels exhibited a significantly worse
prognosis than those with negative CEA, AFP, CA19-9 and CA242
levels. Notably, the differences between positive and negative
pre-operative CA19-9 and CA242 levels were statistically
significant (Fig. 3). The results
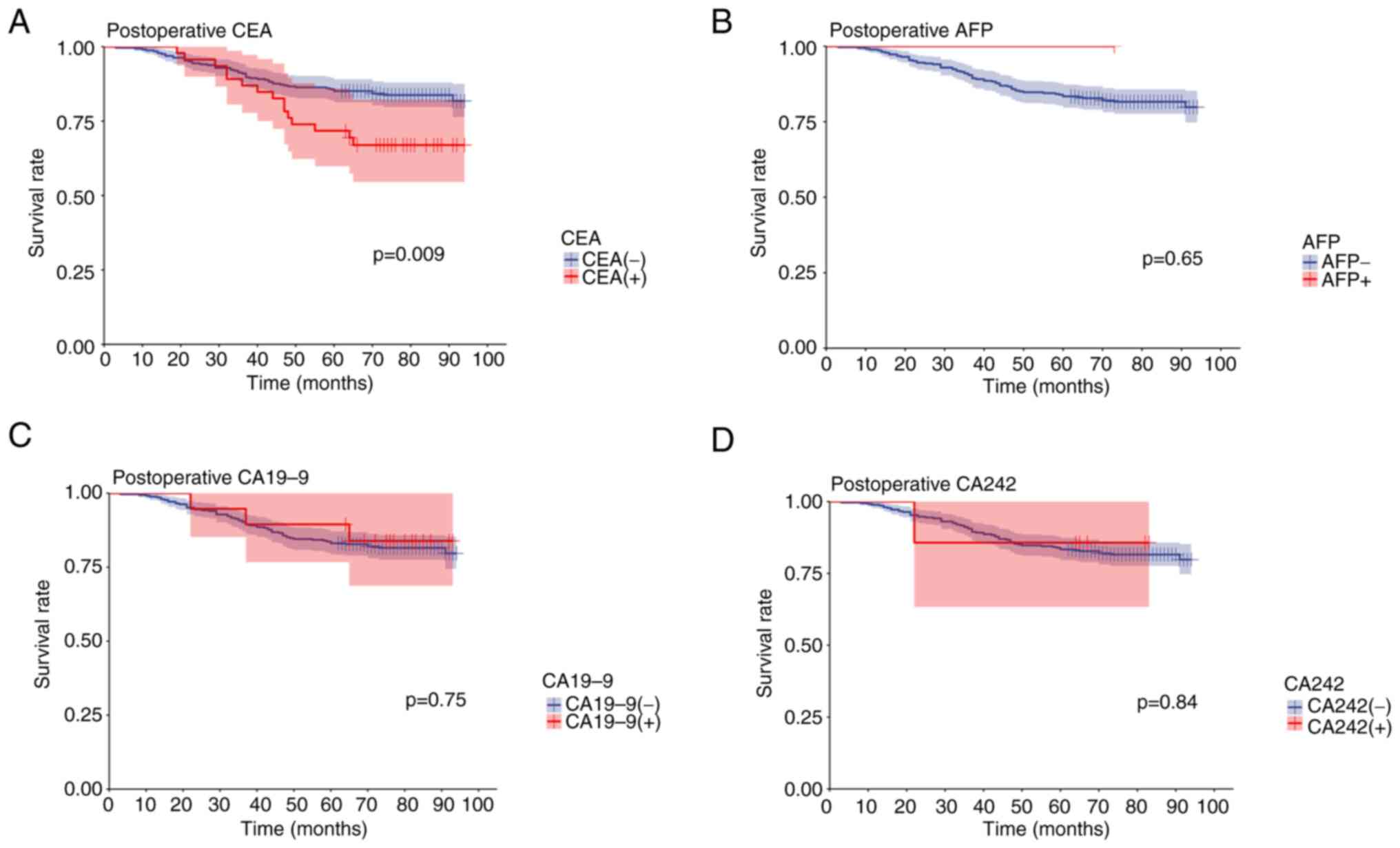
of the present study also revealed that patients with positive
post-operative serum CEA, AFP, CA19-9 and CA242 levels exhibited a
poorer five-year survival rate than patients with negative
post-operative serum CEA, AFP, CA19-9 and CA242 levels. Notably,
these patients also exhibited a significantly worse prognosis.
However, there was no statistically significant difference between
positive and negative post-operative AFP, CA19-9 or CA242 levels.
The results of the present study revealed that the difference
between positive and negative post-operative CEA levels were
statistically significant (Fig.
4).
Discussion
Serum tumor markers are substances that are
synthesized by cells, and these are increased when the body
responds to tumor cells. Levels of serum tumor markers are low in
healthy tissues; however, these are significantly increased in the
serum of patients with tumors.
The results of the present study revealed that serum
CEA, AFP, CA19-9 and CA242 levels in patients with
recurrence/metastasis were significantly higher than those in
patients with non-recurrence/metastasis, suggesting that serum CEA,
AFP, CA19-9 and CA242 may be associated with post-operative
recurrence/metastasis in patients with gastric cancer. In addition,
results of the univariate analysis demonstrated that open surgery,
age ≥70, total gastrectomy, disease stage III, pre-operative CA19-9
positivity and pre-operative CA242 positivity were risk factors for
recurrence/metastasis. However, these factors were not
statistically significant between patients who were positive and
negative for pre-operative CEA and AFP. Notably, there were no
statistically significant differences in sex and lymph node
dissection between patients with recurrence/metastasis and those
with non-recurrence/metastasis. Of the 368 patients who underwent
radical gastrectomy, only two patients underwent D1 or
D1+ lymph node dissection, and the remaining patients
underwent D2 lymph node dissection.
The results of the multivariate logistic analysis
revealed that approach of operation was a risk factor for
recurrence/metastasis. Compared with disease stage I, disease
stages II and III were also risk factors for recurrence/metastasis
of gastric cancer. Notably, these results were consistent with
those of previous studies and results obtained in clinical practice
(14,28,29).
ROC curve analysis was also used to further
determine the predictive value of CEA, AFP, CA19-9 and CA242 levels
in post-operative recurrence/metastasis of gastric cancer. The
results of the present study revealed that the AUC values of
pre-operative CEA, AFP, CA19-9 and CA242 levels were 0.517, 0.513,
0.513 and 0.499, respectively. These results suggested that
pre-operative CEA levels exhibited a high predictive value for
post-operative recurrence/metastasis in patients with gastric
cancer. The AUC values of post-operative CEA, AFP, CA19-9 and CA242
levels were 0.608, 0.493, 0.630 and 0.568, respectively. These
results suggested that CA19-9 may exhibit the highest potential in
predicting post-operative recurrence/metastasis of gastric cancer.
Notably, these results were comparable with those of previous
studies (30,31).
Cumulative survival was also compared between
patients with negative pre-operative and post-operative serum CEA,
AFP, CA19-9 and CA242 levels, and those with positive levels of
these markers. The results of the present study indicated that
patients with positive pre- or post-operative CEA, AFP, CA19-9 and
CA242 levels exhibited a poorer five-year survival rate than
patients with negative levels of these markers. Moreover, prognosis
was significantly worse in patients with positive pre- and
post-operative serum CEA, AFP, CA19-9 and CA242 levels, compared
with patients with negative levels of these markers. Previous
studies revealed that elevated serum CEA and CA19-9 levels are
associated with the prognosis of patients with gastric cancer
(14,24,32).
In the present study, elevated serum CA242 levels were also
associated with the prognosis of patients with gastric cancer.
The present study provides a novel theoretical basis
for the use CEA, AFP, CA19-9 and CA242 as tumor markers for the
prediction of tumor recurrence and metastasis following radical
gastrectomy. Thus, these serum tumor markers may exhibit potential
in predicting tumor recurrence and metastasis following surgery,
when combined with ctDNA. Further investigations should focus on
the optimization of evaluating CEA, AFP, CA19-9 and CA242 in
combination, to determine the optimal combination of tumor markers
for the prediction of recurrence/metastasis of gastric cancer
following radical surgery.
Collectively, the results of the present study
indicated that CEA, AFP, CA19-9 and CA242 exhibited potential in
the prediction of recurrence/metastasis following radical
gastrectomy in patients with gastric cancer. Notably, CA19-9 and
CA242 may exhibit the highest potential in predicting
recurrence/metastasis. In addition, patients enrolled in the
present study had undergone radical gastrectomy, and the majority
of patients with elevated AFP levels presented with advanced stages
of disease. Thus, patients with elevated AFP levels may have
presented with liver metastases that could not be surgically
resected, and were therefore not included in the present study.
Thus, the present study included fewer patients with positive AFP
levels, which may have led to bias.
The AUC value in the ROC curve in the present study
is close to 0.5, which means that the model is weak in
distinguishing between positive and negative samples. Although the
results were statistically significant, the actual predictive power
of the model was weak and may not have sufficient clinical
significance. The importance of the results should not be
overstated in the absence of sufficient differentiation. Future
studies need to optimize the model and improve its prediction
accuracy.
The present study used a retrospective design, which
does introduce data selection bias and other potential confounding
factors. Retrospective design limits the ability of causal
inference and may affect the reliability of the results. Future
studies need to consider multivariate analyses that include more
potential confounding factors. Another limitation is our relatively
small sample size, which may affect the statistical power of the
study and the external validity of the results. Potential errors
that can result from a small sample size. In future studies, the
authors consider conducting a prospective cohort study with a
larger sample size to validate our findings.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Zhejiang
Co-construction Project (grant no. WKJ-ZJ-2310), the Zhejiang
Health Commission Project (grant no. 2022KY790), the Natural
Science foundation of Zhejiang (grant no. LY19H160042) and the
National natural science foundation of China (grant no.
81301889).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FL, KeC, GC and JC contributed to study conception
and design. FL, SX, KaiC and MK prepared materials, and performed
data collection and analysis. FL wrote the manuscript. All authors
commented on the manuscript. GC and JC confirm the authenticity of
all the raw data, supervised the research and revised the
manuscript. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
20240651) by the Institutional Review Committee of the Second
Affiliated Hospital of Zhejiang University School of Medicine
(Hangzhou, China) and was conducted in strict accordance with the
principles of the Declaration of Helsinki. Patient informed consent
was waived by the ethics committee as the present study is
retrospective.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rocken C: Predictive biomarkers in gastric
cancer. J Cancer Res Clin Oncol. 149:467–481. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Luan F, Li X, Cheng X, Huangfu L, Han J,
Guo T, Du H, Wen X and Ji J: TNFRSF11B activates Wnt/β-catenin
signaling and promotes gastric cancer progression. Int J Biol Sci.
16:1956–1971. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ajani JA, D'Amico TA, Bentrem DJ, Chao J,
Cooke D, Corvera C, Das P, Enzinger PC, Enzler T, Fanta P, et al:
Gastric cancer, version 2.2022, NCCN clinical practice guidelines
in oncology. J Natl Compr Canc Netw. 20:167–192. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rihawi K, Ricci AD, Rizzo A, Brocchi S,
Marasco G, Pastore LV, Llimpe FLR, Golfieri R and Renzulli M:
Tumor-associated macrophages and inflammatory microenvironment in
gastric cancer: Novel translational implications. Int J Mol Sci.
22(3805)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ricci AD, Rizzo A and Brandi G: DNA damage
response alterations in gastric cancer: Knocking down a new wall.
Future Oncol. 17:865–868. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zong L, Abe M, Seto Y and Ji J: The
challenge of screening for early gastric cancer in China. Lancet.
388(2606)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Guven DC, Erul E, Kaygusuz Y, Akagunduz B,
Kilickap S, De Luca R and Rizzo A: Immune checkpoint
inhibitor-related hearing loss: A systematic review and analysis of
individual patient data. Support Care Cancer.
31(624)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rizzo A, Santoni M, Mollica V, Logullo F,
Rosellini M, Marchetti A, Faloppi L, Battelli N and Massari F:
Peripheral neuropathy and headache in cancer patients treated with
immunotherapy and immuno-oncology combinations: The MOUSEION-02
study. Expert Opin Drug Metab Toxicol. 17:1455–1466.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hadfield MJ, Mistry H, Pelcovits A, Bansal
R, Andrea S, Chergui A, Ramphal K, Austin M and Khurshid H: Risk
factors for immunotherapy-related adverse events (IrAE) in patients
treated with immune checkpoint inhibitors. Am J Clin Oncol.
46:183–184. 2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fujiya K, Tokunaga M, Makuuchi R,
Nishiwaki N, Omori H, Takagi W, Hirata F, Hikage M, Tanizawa Y,
Bando E, et al: Early detection of nonperitoneal recurrence may
contribute to survival benefit after curative gastrectomy for
gastric cancer. Gastric Cancer. 20 (Suppl 1):S141–S149.
2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Park JS, Choe EA, Park S, Nam CM, Hyung
WJ, Noh SH, Lee S, Kim HS, Jung M, Chung HC and Rha SY: Detection
of asymptomatic recurrence improves survival of gastric cancer
patients. Cancer Med. 10:3249–3260. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shibata C, Nakano T, Yasumoto A, Mitamura
A, Sawada K, Ogawa H, Miura T, Ise I, Takami K, Yamamoto K and
Katayose Y: Comparison of CEA and CA19-9 as a predictive factor for
recurrence after curative gastrectomy in gastric cancer. BMC Surg.
22(213)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zheng CY, Wu J, Chen CS, Huang ZN, Tang
YH, Qiu WW, He QC, Lin GS, Chen QY, Lu J, et al: A scoring model
for predicting early recurrence of gastric cancer with normal
preoperative tumor markers: A multicenter study. Eur J Surg Oncol.
49(107094)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Passos I, Stefanidou E,
Meditskou-Eythymiadou S, Mironidou-Tzouveleki M, Manaki V, Magra V,
Laskou S, Mantalovas S, Pantea S, Kesisoglou I and Sapalidis K: A
review of the significance in measuring preoperative and
postoperative carcinoembryonic antigen (CEA) values in patients
with medullary thyroid carcinoma (MTC). Medicina (Kaunas).
57(609)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hammarström S: The carcinoembryonic
antigen (CEA) family: Structures, suggested functions and
expression in normal and malignant tissues. Semin Cancer Biol.
9:67–81. 1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sun W, Liu Y, Shou D, Sun Q, Shi J, Chen
L, Liang T and Gong W: AFP (alpha fetoprotein): Who are you in
gastrology? Cancer Lett. 357:43–46. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kamiimabeppu D, Wakatsuki T, Takahari D,
Fukuda N, Shimozaki K, Osumi H, Nakayama I, Ogura M, Ooki A,
Shinozaki E, et al: Treatment efficacy of ramucirumab-containing
chemotherapy in patients with alpha-fetoprotein producing gastric
cancer. Int J Clin Oncol. 28:121–129. 2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kawahara K, Makino H, Kametaka H, Hoshino
I, Fukada T, Seike K, Kawasaki Y and Otsuka M: Outcomes of surgical
resection for gastric cancer liver metastases: A retrospective
analysis. World J Surg Oncol. 18(41)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mihmanli M, Dilege E, Demir U, Coskun H,
Eroglu T and Uysalol MD: The use of tumor markers as predictors of
prognosis in gastric cancer. Hepatogastroenterology. 51:1544–1547.
2004.PubMed/NCBI
|
|
22
|
Nakajima K, Ochiai T, Suzuki T, Shimada H,
Hayashi H, Yasumoto A, Takeda A, Hishikawa E and Isono K: Impact of
preoperative serum carcinoembryonic antigen, CA 19-9 and alpha
fetoprotein levels in gastric cancer patients. Tumour Biol.
19:464–469. 1998.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Moyer S: Pharmacokinetics of naproxen
sodium. Cephalalgia. 6 (Suppl 4):S77–S80. 1986.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kochi M, Fujii M, Kanamori N, Kaiga T,
Kawakami T, Aizaki K, Kasahara M, Mochizuki F, Kasakura Y and
Yamagata M: Evaluation of serum CEA and CA19-9 levels as prognostic
factors in patients with gastric cancer. Gastric Cancer. 3:177–186.
2000.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Suenaga Y, Kanda M, Ito S, Mochizuki Y,
Teramoto H, Ishigure K, Murai T, Asada T, Ishiyama A, Matsushita H,
et al: Prognostic significance of perioperative tumor marker levels
in stage II/III gastric cancer. World J Gastrointest Oncol.
11:17–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wakatsuki K, Matsumoto S, Migita K,
Kunishige T, Nakade H, Miyao S and Sho M: Risk factors and risk
scores for predicting early recurrence after curative gastrectomy
in patients with stage III gastric cancer. J Gastrointest Surg.
24:1758–1769. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yagi S, Kumagai K, Nunobe S, Ishizuka N,
Yamaguchi T, Imai Y, Tsuda M, Haruta S, Fukunaga H, Yamada T and
Goto M: Risk factors for early recurrence after radical gastrectomy
followed by adjuvant chemotherapy for stage II or III gastric
cancer: A multicenter, retrospective study. Jpn J Clin Oncol.
54:403–415. 2024.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Nobre KEL, Pereira MA, Ramos MFKP, Ribeiro
U, Zilberstein B, Cecconello I and Dias AR: Recurrence in PN0
gastric cancer: Risk factors in the occident. Arq Bras Cir Dig.
34(e1562)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Jeong SA, Yook JH, Yoo MW, Kim BS, Lee IS,
Kim S, Gong CS and Ko CS: Analysis of risk factors affecting
long-term survival in elderly patients with advanced gastric
cancer. Aging Clin Exp Res. 35:2211–2218. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ma X, Zhou X, Guo J, Feng X, Zhao M, Zhang
P, Zhang C, Gong S, Wu N, Zhang Y, et al: CA19-9 is a significant
prognostic factor in stage III gastric cancer patients undergoing
radical gastrectomy. BMC Surg. 24(31)2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
You W, Cai Z, Sheng N, Yan L, Wan H, Wang
Y, Ouyang J, Xie L, Wu X and Wang Z: Construction and validation of
convenient clinicopathologic signatures for predicting the
prognosis of stage I-III gastric cancer. Front Oncol.
12(848783)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kodera Y, Yamamura Y, Torii A, Uesaka K,
Hirai T, Yasui K, Morimoto T, Kato T and Kito T: The prognostic
value of preoperative serum levels of CEA and CA19-9 in patients
with gastric cancer. Am J Gastroenterol. 91:49–53. 1996.PubMed/NCBI
|