1. Introduction
Programmed cell death protein 1 (PD-1) and its
ligand PD-L1 are crucial immune checkpoint molecules which regulate
the immune response. PD-1 is a receptor protein present on the
surface of T cells and other immune cells. It plays a role in
suppressing T-cell activity for appropriate immune response control
(1). PD-1 was discovered in 1992
as a gene associated with programmed cell death (2). However, subsequent research revealed
that PD-1 primarily functions as an immune checkpoint (3). PD-L1 protein is present mainly on the
surface of tumor cells and some normal cells (3). PD-L1 is expressed in numerous
tissues, but is relatively more abundant on tumor cells (4). When PD-L1 binds to PD-1, it
suppresses T-cell attack and weakens the immune response (3), allowing tumor cells to evade the
immune system. The PD-1/PD-L1 pathway is an important target in
cancer immunotherapy (4). Drugs
that block PD-1 and PD-L1 (immune checkpoint inhibitors; ICIs) have
enabled great progress in cancer treatment (5). Their effectiveness has been confirmed
in numerous types of cancer, including melanoma, non-small cell
lung cancer and renal cell carcinoma (6). Recently, systematic reviews have
addressed the relationship between PD-1/PD-L1 immune checkpoint
mechanisms and bone and soft tissue tumors; however, limited
comprehensive reviews have summarized the long-term outcomes of
ICIs, including their side effects. This gap highlights the need
for more robust evidence regarding the efficacy and safety of these
therapies in the context of sarcomas (7-9).
The present review examines PD-1/PD-L1 expression in bone and soft
tissue tumors, focusing on their clinical significance, based on
studies conducted between 2009-2024.
2. Biological role and mechanism of
PD-1/PD-L1
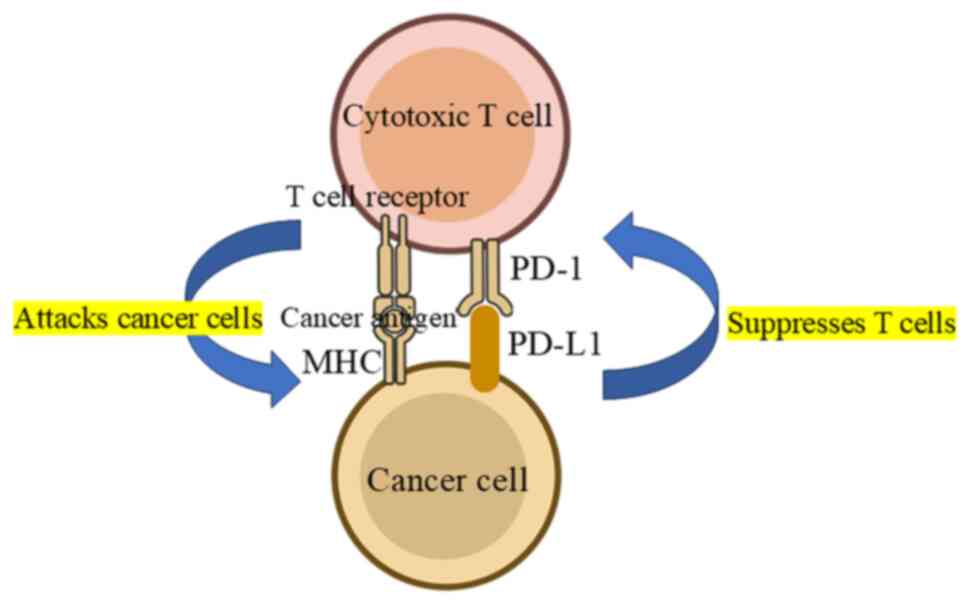
PD-1 is an inhibitory receptor expressed on
activated T cells, B cells, and myeloid cells. Its ligand, PD-L1,
is expressed on cells, including tumor cells within the tumor
microenvironment (Figs. 1 and
2). The interaction between PD-1
and PD-L1 inhibits T-cell function, allowing tumors to evade immune
surveillance. This mechanism is central to the development of ICIs
which block PD-1/PD-L1 interaction, restoring T-cell activity and
enhancing antitumor immunity (10,11).
3. PD-1/PD-L1 expression in bone and soft
tissue tumors
Osteosarcoma
Osteosarcoma is the most common primary malignant
bone tumor and predominantly affects adolescents and young adults.
PD-L1 expression in osteosarcoma is associated with poor prognosis,
indicating its potential as a prognostic marker (11). Previously, the authors evaluated
preoperative needle biopsy specimens from 16 patients with
osteosarcoma, performing immunostaining for CD4, CD8, PD-1 and
PD-L1(11). The findings revealed
that 75% of specimens were positive for both CD4 and CD8, while
PD-1 and PD-L1 positivity rates were 18.7 and 62.5%, respectively.
Notably, the tumors were larger in PD-L1-negative cases than those
in PD-L1-positive cases. Furthermore, high PD-L1 expression
correlates with increased tumor invasiveness and metastatic
potential, suggesting that PD-1/PD-L1 blockade may be a suitable
therapeutic strategy (11). Other
studies have supported that PD-L1 expression in osteosarcoma cells
may contribute to immune evasion and tumor progression (Fig. 3). Paydas et al (12) found that PD-L1 expression is
significantly higher in metastatic osteosarcoma than that in
non-metastatic cases and correlates with shorter overall and
disease-free survival.
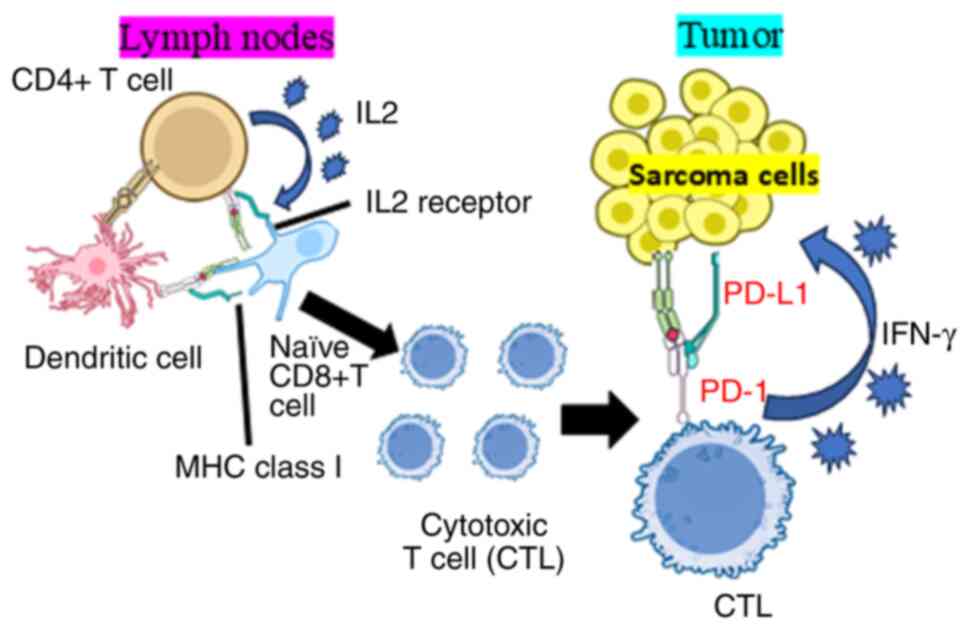 | Figure 3Potential PD-1/PD-L1 immune
checkpoint mechanism in the bone and soft tissue tumor
microenvironment. The PD-1/PD-L1 immune checkpoint mechanism
involves both CD4+ and CD8+ T cells in
regulating immune responses against tumors. CD8+ T
cells, which are crucial for directly eliminating cancer cells,
express PD-1 which binds to PD-L1 on tumor cells, inhibiting their
function. Similarly, activated CD4+ T cells can also
express PD-1, further contributing to immune suppression. Tumors
often exploit this pathway by upregulating PD-L1, allowing them to
evade immune detection. Immune checkpoint inhibitors targeting PD-1
or PD-L1 can block this interaction, restoring the activity of both
T cell populations and enhancing antitumor immunity. PD-1,
programmed cell death 1; PD-L1, programmed cell death ligand-1;
MHC, major histocompatibility complex; IFN, interferon. |
Soft tissue sarcomas (STSs)
STSs are a heterogeneous group of malignancies
arising from mesenchymal tissues. PD-L1 expression varies among STS
subtypes. The expression rate of PD-1 is in the range of 12.2-28.3%
and that of PD-L1 is 10.7-31.7% (13-16).
In addition, dedifferentiated liposarcoma exhibits higher
expression levels of PD-1, PD-L1 and PD-L2 than those in other
sarcoma subtypes. These elevated expression levels suggest a
potential immunosuppressive environment which may contribute to the
tumor's ability to evade immune detection and response.
Investigating these expression patterns is crucial for developing
targeted therapies that leverage ICIs in treating dedifferentiated
liposarcoma (15). It was
previously demonstrated that PD-L1 is more frequently expressed in
high-grade sarcomas (17), which
are associated with poor clinical outcomes, suggesting that
PD-1/PD-L1 inhibitors may benefit patients harboring tumors with
high PD-L1 expression. Studies by Kim et al (18) and Anastasiou et al (9) revealed that PD-L1 expression is
prevalent in certain STS subtypes, such as undifferentiated
pleomorphic sarcoma and leiomyosarcoma, suggesting a potential role
of ICIs in these subtypes, particularly when conventional therapies
fail. They found that PD-L1 is expressed in significant number of
cases and is associated with relatively poor overall survival (OS)
rates (11 and 19 months for patients positive and negative for
PD-L1, respectively). In addition, Anastasiou et al
(9) reviewed the role of ICIs in
sarcomas, highlighting their potential and limitations. They
reported that monotherapies with ICIs exhibit inadequate responses
in sarcomas, while combinations with targeted therapies, such as
TKIs and anti-CTLA-4, have demonstrated promising results,
particularly in alveolar soft part sarcomas. The aforementioned
study emphasized that although certain sarcoma subtypes, such as
undifferentiated pleomorphic sarcoma, exhibit the prevalence of
tertiary lymphoid structures and may have improved response to
ICIs, further research is necessary to identify predictive
biomarkers for treatment efficacy.
In high-grade STSs, PD-L1 expression is associated
with increased tumor aggressiveness and poor prognosis. Despite
multidisciplinary treatments, including extensive resection,
high-grade STSs have a high recurrence rate and poor prognosis
(19). It was found that PD-1 and
PD-L1 expression levels in these sarcomas can serve as biomarkers
for identifying patients who may benefit from immune checkpoint
blockade therapy (20,21). These findings are supported by
those of D'Angelo et al (22), who demonstrated the clinical
activity of pembrolizumab, an anti-PD-1 antibody, in patients with
PD-L1-expressing advanced sarcomas.
Desmoid tumors
Desmoid tumors, also known as aggressive
fibromatosis, are benign but locally invasive soft tissue tumors.
The PD-1/PD-L1 immune checkpoint mechanism is reportedly in active
in desmoid tumors (23). This
research included biopsy and resection samples from patients
diagnosed with desmoid tumors, focusing on the immunohistochemical
assessment of PD-L1 expression. The findings revealed that while
PD-L1 was expressed in the tumor microenvironment, all patients
were negative for PD-1. However, low PD-L1 expression was found in
desmoid tumors, which may reflect their benign nature (24); some desmoid tumors exhibit
PD-1/PD-L1 expression, indicating a possible, albeit limited, role
for immunotherapy in selected patients.
Angiosarcoma
Angiosarcoma is a rare and aggressive malignancy of
endothelial cell origin (25). The
importance of the PD-1/PD-L1 pathway in angiosarcoma (26). In particular, high PD-L1 expression
may be a target for immunotherapy (26). The aforementioned study discussed
the role of the PD-1/PD-L1 pathway in cancer, further emphasizing
its significance in immune regulation and tumor progression. PD-L1
serves as a critical immune checkpoint which inhibits T cell
activation when it binds to PD-1, thus promoting immune evasion by
tumors. The authors have explored the following dual functions of
PD-L1: its role in suppressing antitumor immunity and its
pro-oncogenic signaling which enhances cancer cell survival and
resistance to apoptosis. The research also highlights the
therapeutic potential of targeting this pathway with ICIs, which
have demonstrated promising potential in treating various cancers.
High PD-L1 expression has been confirmed in some patients with
angiosarcoma (27), suggesting
that immunotherapy (for example, PD-1/PD-L1 inhibitors) may be
effective. A previous study reported that ~40% of tumor samples
from patients with angiosarcoma are PD-L1-positive. In this patient
population, the use of PD-1 inhibitors resulted in tumor shrinkage
and disease stabilization (28).
Significant tumor shrinkage was observed in some patients with
advanced angiosarcoma in a clinical trial using PD-1/PD-L1
inhibitors (29). It was found
that PD-1, PD-L1 and other immunogenic markers such as NY-ESO-1 and
MAGE-A4 are expressed in cutaneous angiosarcoma, suggesting that
multitargeted immunotherapy may be a promising approach for this
aggressive cancer (20,21).
4. Clinical trials of ICIs
Several clinical trials have investigated the
efficacy of PD-1/PD-L1 inhibitors in bone and soft tissue tumors.
The PD-1 inhibitors pembrolizumab and nivolumab have been evaluated
in various sarcoma subtypes. The SARC028 trial demonstrated
significant activity of pembrolizumab in undifferentiated
pleomorphic sarcoma and liposarcoma, leading to ongoing
investigations into combination therapies and in other sarcoma
subtypes (30). This trial
included two cohorts and enrolled 86 patients, with 84 receiving
pembrolizumab. The overall objective response rate was 18%, with
notable responses observed in undifferentiated pleomorphic sarcoma
(40% response) and dedifferentiated liposarcoma. Despite the
promising results in specific subtypes, the primary endpoint of
overall response was not met for either cohort. The study also
reported various grade 3 or higher adverse events, highlighting the
need for further investigating the efficacy of pembrolizumab in
these sarcomas. Nivolumab in combination with the a CTLA-4
inhibitor ipilimumab showed promising results in heavily pretreated
patients with sarcoma, high-lighting the potential for synergistic
effects in enhancing antitumor immunity (22). A total of 85 eligible patients were
randomized to receive either nivolumab monotherapy or a combination
of nivolumab and ipilimumab. The results showed an objective
response rate of 5% in the nivolumab group and 16% in the
combination group. Serious but manageable treatment-related adverse
events occurred in both the groups. The findings suggest that while
nivolumab alone may not warrant further investigation in unselected
sarcoma populations, the combination with ipilimumab demonstrated
promising efficacy, particularly in certain sarcoma subtypes,
indicating the need for further randomized studies to validate
these results.
5. PD-1/PD-L1 expression and prognosis
PD-1/PD-L1 expression is associated with prognosis
in various bone and soft tissue tumors. High PD-L1 expression
generally correlates with poor outcomes, including low OS and high
recurrence rates. For example, patients with osteosarcoma and high
PD-L1 expression tend to have worse survival rates than those with
low PD-L1 expression. Similarly, in STSs, high PD-L1 expression is
often associated with more aggressive disease and lower survival
rates. Additionally, higher PD-1 expression levels in synovial
sarcoma are associated with shorter progression-free survival (PFS)
rates. This correlation suggests that increased PD-1 expression may
contribute to a more immunosuppressive tumor microenvironment,
negatively impacting the efficacy of the immune response and
leading to poorer clinical outcomes (31). These findings underscore the
potential of PD-1/PD-L1 as both a prognostic marker and a
therapeutic target in such malignancies (11,12).
However, it remains controversial whether high or
low expression of PD-1/PD-L1 immune molecules correlates with
prognosis. One reason for this is the exhaustion of T cells in the
tumor microenvironment (32).
T cells that are definitively induced may no longer
be able to function in the tumor microenvironment, even when they
have colonized it. Miyake et al (15) reported that the expression rates of
PD-1, PD-L1 and PD-L2 are not associated with prognosis; however,
high Ki67 expression was identified as a significant factor for
poor prognosis. This finding highlights the importance of Ki67 as a
marker for tumor proliferation and its potential role in predicting
clinical outcomes in patients (15).
6. Mechanisms of resistance to PD-1/PD-L1
blockade
Despite the promising results of PD-1/PD-L1
inhibitors, resistance to these therapies remains a significant
challenge. Mechanisms of resistance include adaptive immune
resistance, in which tumors upregulate PD-L1 in response to immune
pressure, and intrinsic resistance, involving genetic and
epigenetic changes within the tumor cells. A study by Martin et
al (33) highlighted the role
of the tumor microenvironment in mediating therapy resistance.
Factors such as tumor-associated macrophages, regulatory T cells,
and myeloid-derived suppressor cells can inhibit the antitumor
immune response, even in the presence of PD-1/PD-L1 blockade
(33). Combining PD-1/PD-L1
inhibitors with other therapeutic modalities, such as chemotherapy,
radiotherapy, or other immunotherapies, may help overcome
resistance. Studies are in progress to identify the most effective
combination strategies to enhance the efficacy of ICIs in bone and
soft tissue tumors (22).
7. Clinical and preclinical studies
(2009-2014)
Between 2009 and 2014, foundational studies laid the
groundwork for elucidating the role of PD-1/PD-L1 in various
cancers, including bone and soft tissue tumors. The role of
PD-1/PD-L1 in immune evasion was first characterized in melanoma
and lung cancer, providing a rationale for exploring these pathways
in other cancers types.
Preclinical studies
Preclinical studies have demonstrated that PD-L1 is
upregulated in response to immune attack, suggesting that targeting
this pathway may enhance antitumor immunity. In this regard, Dong
et al (10) established
that PD-L1 on tumor cells inhibits T-cell function, which is
pivotal in understanding the tumor immune escape mechanism. In
sarcomas, initial studies focused on assessing PD-L1 expression and
its impact on the tumor microenvironment. These studies provided
evidence that, similar to other solid tumors, sarcomas can evade
the immune system via PD-1/PD-L1 interaction. Chen and Han
(34) found that PD-L1 expression
in sarcoma cell lines was associated with resistance to T
cell-mediated cytotoxicity, supporting the potential for PD-1/PD-L1
blockade in these tumors.
Clinical studies
Clinical studies conducted between 2009-2014 focused
primarily on the safety and preliminary efficacy of PD-1/PD-L1
inhibitors. Early-phase trials in melanoma and lung cancer
demonstrated durable responses and manageable toxicity, paving the
way for trials in other cancers, including sarcomas. Topalian et
al (35) conducted a land-mark
phase I trial of nivolumab in patients with various cancers,
including melanoma, demonstrating promising antitumor activity and
durable responses. Brahmer et al (36) evaluated PD-1 blockade with
pembrolizumab in solid tumors, including a small cohort of patients
with sarcoma. Although the response rate was lower in sarcomas than
that in melanoma and lung cancer, the aforementioned study
highlighted the potential for PD-1/PD-L1 blockade in sarcomas and
provided a platform for more focused trials.
Clinical studies (2014-2024)
Italiano et al (37) performed a combined analysis of data
from multiple phase II trials to assess the efficacy of
PD-1/PD-L1-targeted therapy in advanced soft tissue sarcoma. This
analysis included 384 patients, 153 of whom received PD-1/PD-L1
inhibitor monotherapy. The overall response rate for monotherapy
was 15.1%, and the non-progression rate was 58.5%. Based on
histology, focal soft-tissue sarcomas and anaplastic pleomorphic
sarcomas had the highest response rates, while leiomyosarcomas had
the lowest response rates. PD-L1 expression rates were generally
low and not consistently associated with objective response. The
investigators concluded that PD-1/PD-L1 inhibitors demonstrate only
limited efficacy in the unselected soft-tissue sarcoma patient
population and recommended stratification for sarcoma heterogeneity
and longitudinal blood and tissue sampling in future studies.
Somaiah et al (38) evaluated the efficacy and safety of
the dervalumab and tremelimumab combination in a single-center
phase II trial in patients with advanced or metastatic soft tissue
and osteosarcoma. Among the 62 enrolled patients, 57 were treated,
with 37.2 months of follow-up and a 12-week point-of-care. The PFS
rate was 49%, and the median OS (mOS) was 17.4 months. The most
common adverse events were lipase elevation, colitis and pneumonia,
with one patient experiencing a grade 5 adverse event. The
investigators concluded that the combination was active against
advanced or metastatic sarcoma and warrants further evaluation in
specific subsets.
Gordon et al (39) conducted a phase I/II trial
evaluating the safety and efficacy of the combination of
ipilimumab, nivolumab and trabectedin (SAINT regimen) as primary
therapy for advanced soft tissue sarcoma. In Phase I, the maximum
tolerated dose of trabectedin was 1.2 mg/m² in nine previously
treated patients. In Phase II, 79 untreated patients were
evaluated; the results showed six complete responses, 14 partial
responses and 49 stable responses, with a best response rate of
25.3% and a disease control rate of 87.3%. The median PFS (mPFS)
was 6.7 months and the mOS was 24.6 months. In terms of safety, the
most common Grade 3/4 treatment-related adverse events reported
were alanine aminotransferase elevation (25%), fatigue (8.7%),
aspartate aminotransferase elevation (8.7%), neutropenia (5.4%) and
anemia (4.6%). The researchers concluded that the SAINT regimen is
a safe and effective first-line treatment for advanced soft-tissue
sarcoma. The aforementioned study represents a new therapeutic
approach combining ICIs and conventional chemotherapy and provides
important implications for treatment strategies against soft-tissue
sarcoma.
The SU2C-SARC032 trial is a Phase II randomized
study focusing on 127 patients with high-risk extremity soft tissue
sarcoma, primarily including undifferentiated pleomorphic sarcoma
and dedifferentiated/mixed-type liposarcoma across 20 medical
institutions (40). The trial
assessed the efficacy of pembrolizumab administered preoperatively
for three cycles and postoperatively for up to 14 cycles, with the
primary endpoint being the two-year disease-free survival rate.
Results indicated that pembrolizumab administration reduced the
risk of cancer recurrence by 43% after two years, highlighting its
effectiveness in treating soft tissue sarcoma.
Liao et al (41) conducted a two-center study
evaluating the efficacy and safety of PD-1 inhibitor-based
immunotherapy in patients with sarcoma, including both monotherapy
and combination therapy. Among 37 patients with advanced or
unresectable tumors, the mPFS was 5 months and mOS was 13 months.
mPFS and mOS were not significantly different between PD-1
inhibitors in combination with targeted therapy and in combination
with chemotherapy. There were no significant differences in mPFS
and mOS between PD-1 inhibitors plus targeted therapy and
chemotherapy. The six patients who received adjuvant therapy
demonstrated a longer mPFS of 15 months. The investigators
concluded that this immunotherapy is effective against sarcoma and
has a manageable safety profile.
Babatunde et al (42) performed a retrospective
single-center study which evaluated the efficacy of ICI-based
therapy in 60 patients with leiomyosarcoma. The findings revealed
an mPFS of 8.43 weeks and an overall response rate of 6.67% (four
patients). Of note, two of the four patients demonstrating partial
responses had BRCA1 or BRCA2 mutations. Thus, while the
aforementioned study suggested that the overall effect of ICI
treatment for leiomyosarcoma is limited, patients with LMS and
specific molecular profiles (such as BRCA1/2 mutations) may benefit
from ICI.
8. Immune-related adverse events
(iRAEs)
PD-1/PD-L1 inhibitors are widely used as part of
cancer immunotherapy, but they can cause side effects called iRAEs
(43). While PD-1/PD-L1 inhibitors
enhance the antitumor activity of T cells and prevent the immune
escape of tumor cells, they can over-activate autoimmune responses,
causing iRAEs (44).
Main iRAEs
iRAEs can affect various organs of the body, and the
following symptoms have been reported (45): Skin disorders, including rash,
itching and psoriasis-like dermatitis. Gastrointestinal disorders
such as diarrhea, colitis and hepatitis have also been identified.
Endocrine disorders comprise thyroid dysfunction, diabetes and
adrenal insufficiency. Reported lung disorders include pneumonia
and interstitial pneumonia, and neuropathies include peripheral
neuropathy and central nervous system disorders (for example,
encephalitis and meningitis).
Frequency and severity of iRAEs
The frequency of iRAEs varies depending on the type
of treatment and the patient's condition, but it is generally
considered that 10-30% of patients develop some form of iRAE
(46). In total, ~3.5% of iRAE
cases are severe and require hospitalization for treatment
(46). In most cases, symptoms are
mild to moderate and can be managed with steroid treatment;
however, in severe cases, treatment may need to be interrupted or
immunosuppressants may be used (47).
Management of iRAEs
Early detection and prompt treatment are important
for managing iRAEs (48). Regular
monitoring and patient education are recommended, and patients are
required to immediately visit a medical institution if symptoms
appear. For treating mild cases, local treatment and low-dose
steroids are used, and for moderate to severe cases, systemic
steroids and immunosuppressants (such as methotrexate and
azathioprine) are used (46,47,49).
9. Combination therapy of PD-1/PD-L1 ICIs
and other treatments
PD-1/PD-L1 ICIs may be not sufficiently effective,
and several combination therapies have recently been evaluated
(50).
PD-1/PD-L1 inhibitors combined with
chemotherapy
Gandhi et al (51) reported that the combination of
chemotherapy and a PD-1 inhibitor (pembrolizumab) is more effective
than conventional treatment in patients with metastatic non-small
cell lung cancer. Lynch et al (52) treated 30 patients with advanced
sarcoma using a combination of doxorubicin and pembrolizumab, and
36.7% (11/30) of the patients responded to the treatment. However,
PFS and OS were relatively short, at 5.7 and 17 months,
respectively.
PD-1/PD-L1 inhibitors in combination
with radiotherapy
Dovedi et al (53) demonstrated that a combination of
radiotherapy and PD-1 inhibitors improved the tumor immune
environment and enhances the therapeutic effect. Patel et al
(54) reported that preoperative
radiation increases the tumor-associated macrophage expression of
PD-L1 in STSs. Katsuki et al (55) reported that radiotherapy is
necessary both pre- and post-operatively when anti-PD-L1 therapy is
used.
Combining PD-1/PD-L1 inhibitors with
other immunotherapies
The combination of PD-1/PD-L1 inhibitors with CTLA-4
inhibitors (for example, ipilimumab) improves the therapeutic
effect on refractory cancers such as advanced melanoma (56). A recent systematic review and
meta-analysis evaluated the efficacy and safety of a combination of
PD-1/PD-L1 inhibitors and CTLA-4 inhibitors as a novel therapy for
solid tumors (57). This
combination showed no significant effect on the overall response
rate, major pathological response, pathological complete response,
surgical resection, radical resection, OS, PFS, recurrence-free
survival, grade 3-4 adverse events, all-cause mortality, and
treatment completion. New ICIs which suppress both PD-1 and CTLA-4
signals have been developed (58).
Combination of PD-1/PD-L1 Inhibitors
and targeted therapy
The combination of a PD-L1 inhibitor (atezolizumab)
and an angiogenesis inhibitor (bevacizumab) improves survival in
patients with unresectable hepatocellular carcinoma (59). Roussot et al (60) assessed the safety of a combination
of a PD-1/PD-L1 inhibitor and anti-angiogenesis therapy in the
first-line treatment of unresectable hepatocellular carcinoma by
monitoring patients' conditions during and after treatment and
documenting the frequency and severity of adverse reactions; they,
found the regimen to be generally safe and efficacious. They used
the Common Terminology Criteria for Adverse Events to assess the
adverse reactions. iRAEs such as skin rash, diarrhea, liver
dysfunction and endocrine disorders, and radiation-related adverse
reactions such as dermatitis at the irradiated site, fatigue, and
effects on organs at the irradiated site (for example, pneumonia
due to irradiation of the lungs) were reported as the most common
adverse reactions. Mild to moderate side effects were observed in
numerous patients, but these were within the normal range and did
not require intervention. Serious side effects were reported in
some patients but were infrequent and manageable with prompt
therapeutic intervention (60). A
phase III randomized study demonstrated the efficacy of
atezolizumab and bevacizumab plus chemotherapy in patients with
non-small cell lung cancer (61).
A phase I study reported the efficacy of a combination of
atezolizumab and bevacizumab in patients with advanced malignancies
(62). These combination therapies
are promising strategies to maximize the efficacy of PD-1/PD-L1
inhibitors in cancer treatment.
10. Future directions
Future research should focus on several key areas.
Reliable response biomarkers, such as tumor mutation burden and
microsatellite instability, should be identified to predict which
patients will benefit most from PD-1/PD-L1 inhibitors. Combinations
of PD-1/PD-L1 inhibitors with other therapies can be explored to
overcome resistance and enhance efficacy (22). To improve therapeutic strategies,
the contributions of the tumor microenvironment and genetic
alterations to resistance and the underlying mechanisms should be
elucidated (33). Finally,
additional immune checkpoints and pathways that may synergize with
PD-1/PD-L1 blockade should be identified (20,21).
11. Conclusions
The PD-1/PD-L1 immune checkpoint plays a critical
role in the immune evasion of bone and soft tissue tumors. While
significant progress has been made in elucidating and targeting
this pathway, challenges remain in identifying target patient
populations and in overcoming resistance mechanisms. Ongoing
research and clinical trials continue to explore the potential of
PD-1/PD-L1 inhibitors, alone and in combination with other
therapies, to improve outcomes for patients with these challenging
malignancies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KH, SN and KG conceptualized the study, contributed
to the study methodology, wrote the original draft, and reviewed
and edited the manuscript. All authors read and approved the final
version of the manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lin X, Kang K, Chen P, Zheng Z, Li G,
Xiong W, Yi M and Xiang B: Regulatory mechanisms of PD-1/PD-L1 in
cancers. Mol Cancer. 23(108)2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Pipitone RM, Lupo G, Zito R, Javed A,
Petta S, Pennisi G and Grimaudo S: The PD-1/PD-L1 axis in the
biology of MASLD. Int J Mol Sci. 25(3671)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu J, Chen Z, Li Y, Zhao W, Wu J and
Zhang Z: PD-1/PD-L1 checkpoint inhibitors in tumor immunotherapy.
Front Pharmacol. 12(731798)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yi M, Li T, Niu M, Mei Q, Zhao B, Chu Q,
Dai Z and Wu K: Exploiting innate immunity for cancer
immunotherapy. Mol Cancer. 22(187)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Tang Q, Chen Y, Li X, Long S, Shi Y, Yu Y,
Wu W, Han L and Wang S: The role of PD-1/PD-L1 and application of
immune-checkpoint inhibitors in human cancers. Front Immunol.
13(964442)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gao W, Liu S, Wu Y, Wei W, Yang Q, Li W,
Chen H, Luo A, Wang Y and Liu Z: Enhancer demethylation-regulated
gene score identified molecular subtypes, inspiring immunotherapy
or CDK4/6 inhibitor therapy in oesophageal squamous cell carcinoma.
EBioMedicine. 105(105177)2024.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wood GE, Meyer C, Petitprez F and D'Angelo
SP: Immunotherapy in sarcoma: Current data and promising
strategies. Am Soc Clin Oncol Educ Book. 44(e432234)2024.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Martin-Broto J, Hindi N and Moura DS:
Combination treatment with PD1/PDL-1 inhibitors for sarcomas: State
of the art, next questions. Curr Opin Oncol. 36:269–275.
2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Anastasiou M, Kyriazoglou A, Kotsantis I,
Economopoulou P, Kyrkasiadou M, Giannopoulou A, Kosmidou A, Smerdi
D, Moutafi M, Gavrielatou N and Psyrri A: Immune checkpoint
inhibitors in sarcomas: A systematic review. Immunooncol Technol.
20(100407)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dong H, Strome SE, Salomao DR, Tamura H,
Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, et al:
Tumor-associated B7-H1 promotes T-cell apoptosis: A potential
mechanism of immune evasion. Nat Med. 8:793–800. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Hashimoto K, Nishimura S and Akagi M:
Characterization of PD-1/PD-L1 immune checkpoint expression in
osteosarcoma. Diagnostics (Basel). 10(528)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Paydas S, Bagir EK, Deveci MA and Gonluses
G: Clinical and prognostic significance of PD-1 and PD-L1
expression in sarcomas. Med Oncol. 33(93)2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Torabi A, Amaya CN, Wians FH Jr and Bryan
BA: PD-1 and PD-L1 expression in bone and soft tissue sarcomas.
Pathology. 49:506–513. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Orth MF, Buecklein VL, Kampmann E,
Subklewe M, Noessner E, Cidre-Aranaz F, Romero-Pérez L, Wehweck FS,
Lindner L, Issels R, et al: A comparative view on the expression
patterns of PD-L1 and PD-1 in soft tissue sarcomas. Cancer Immunol
Immunother. 69:1353–1362. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Miyake M, Oda Y, Nishimura N, Morizawa Y,
Ohnishi S, Hatakeyama K, Fujii T, Hori S, Gotoh D, Nakai Y, et al:
Integrative assessment of clinicopathological parameters and the
expression of PD L1, PD L2 and PD 1 in tumor cells of
retroperitoneal sarcoma. Oncol Lett. 20(190)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Boxberg M, Steiger K, Lenze U, Rechl H,
von Eisenhart-Rothe R, Wörtler K, Weichert W, Langer R and Specht
K: PD-L1 and PD-1 and characterization of tumor-infiltrating
lymphocytes in high grade sarcomas of soft tissue–prognostic
implications and rationale for immunotherapy. Oncoimmunology.
7(e1389366)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hashimoto K, Nishimura S, Ito T and Akagi
M: Characterization of PD-1/PD-L1 immune checkpoint expression in
soft tissue sarcomas. Eur J Histochem. 65(3203)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kim C, Kim EK, Jung H, Chon HJ, Han JW,
Shin KH, Hu H, Kim KS, Choi YD, Kim S, et al: Prognostic
implications of PD-L1 expression in patients with soft tissue
sarcoma. BMC Cancer. 18:1–9. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guo J, Li YM, Guo H, Hao DP, Xu JX, Huang
CC, Han HW, Hou F, Yang SF, Cui JL and Wang HX: Parallel CNN-deep
learning clinical-imaging signature for assessing pathologic grade
and prognosis of soft tissue sarcoma patients. J Magn Reson
Imaging. 61:807–819. 2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hashimoto K, Nishimura S, Shinyashiki Y,
Ito T, Tanaka H, Ohtani K, Kakinoki R and Akagi M: PD-1, PD-L1,
NY-ESO-1, and MAGE-A4 expression in cutaneous angiosarcoma: A case
report. Medicine (Baltimore). 101(e29621)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hashimoto K, Nishimura S, Ito T, Kakinoki
R and Akagi M: Immunohistochemical expression and
clinicopathological assessment of PD-1, PD-L1, NY-ESO-1, and
MAGE-A4 expression in highly aggressive soft tissue sarcomas. Eur J
Histochem. 66(3393)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
D'Angelo SP, Mahoney MR, Van Tine BA,
Atkins J, Milhem MM, Jahagirdar BN, Antonescu CR, Horvath E, Tap
WD, Schwartz GK and Streicher H: Nivolumab with or without
ipilimumab treatment for metastatic sarcoma (Alliance A091401): Two
open-label, non-comparative, randomized, phase 2 trials. Lancet
Oncol. 19:416–426. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yildirim AN, Akyurek M, Okay E,
Zenginkinet T, Iyetin Y and Ozkan K: Programmed cell death ligand-1
(PD-L1) expression in desmoid tumors: A retrospective study. Eur
Rev Med Pharmacol Sci. 27:5223–5229. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hashimoto K, Nishimura S, Shinyashiki Y,
Ito T, Kakinoki R and Akagi M: Clinicopathological assessment of
PD-1-comp/PD-L1 immune checkpoint expression in desmoid tumors. Eur
J Histochem. 67(3688)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Young RJ, Brown NJ, Reed MW, Hughes D and
Woll PJ: Angiosarcoma. Lancet Oncol. 11:983–991. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ai L, Xu A and Xu J: Roles of PD-1/PD-L1
pathway: Signaling, cancer, and beyond. Adv Exp Med Biol.
1248:33–59. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lee JB, Ahn BC, Kim SH, Lee YH, Han JW,
Jeon MK, Kim SH and Kim HS: Prognostic implications of PD-L1
expression in patients with angiosarcoma. Future Sci OA.
7(FSO691)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shimizu A, Kaira K, Okubo Y, Utsumi D,
Yasuda M, Asao T, Nishiyama M, Takahashi K and Ishikawa O: Positive
PD-L1 expression predicts worse outcome in cutaneous angiosarcoma.
J Glob Oncol. 3:360–369. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wen W, Zhang Y, Zhang H and Chen Y:
Clinical outcomes of PD-1/PD-L1 inhibitors in patients with
advanced hepatocellular carcinoma: A systematic review and
meta-analysis. J Cancer Res Clin Oncol. 149:969–978.
2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tawbi HA, Burgess M, Bolejack V, Van Tine
BA, Schuetze SM, Hu J, D'Angelo S, Attia S, Riedel RF, Priebat DA,
et al: Pembrolizumab in advanced soft-tissue sarcoma and bone
sarcoma (SARC028): A multicentre, two-cohort, single-arm,
open-label, phase 2 trial. Lancet Oncol. 18:1493–1501.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nowicki TS, Akiyama R, Huang RR, Shintaku
IP, Wang X, Tumeh PC, Singh A, Chmielowski B, Denny C, Federman N
and Ribas A: Infiltration of CD8 T cells and expression of PD-1 and
PD-L1 in synovial sarcoma. Cancer Immunol Res. 5:118–126.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Huang Q, Wu X, Wang Z, Chen X, Wang L, Lu
Y, Xiong D, Liu Q, Tian Y, Lin H, et al: The primordial
differentiation of tumor-specific memory CD8+ T cells as bona fide
responders to PD-1/PD-L1 blockade in draining lymph nodes. Cell.
185:4049–4066.e25. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Martin AM, Nirschl TR, Nirschl CJ,
Francica BJ, Kochel CM, van Bokhoven A, Meeker AK, Lucia MS, Anders
RA, Demarzo AM and Drake CG: Paucity of PD-L1 expression in
prostate cancer: Innate and adaptive immune resistance. Prostate
Cancer Prostatic Dis. 18:325–332. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen L and Han X: Anti-PD-1/PD-L1 therapy
of human cancer: Past, present, and future. J Clin Invest.
125:3384–3391. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ,
Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Italiano A, Bellera C and D'Angelo S:
PD1/PD-L1 targeting in advanced soft-tissue sarcomas: A pooled
analysis of phase II trials. J Hematol Oncol. 13(55)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Somaiah N, Conley AP, Parra ER, Lin H,
Amini B, Soto LS, Salazar R, Barreto C, Chen H, Gite S, et al:
Durvalumab plus tremelimumab in advanced or metastatic soft tissue
and bone sarcomas: A single-centre phase 2 trial. Lancet Oncol.
23:1156–1166. 2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gordon EM, Chawla SP, Tellez WA, Younesi
E, Thomas S, Chua-Alcala VS, Chomoyan H, Valencia C, Brigham DA,
Moradkhani A, et al: SAINT: A phase I/Expanded phase II study using
safe amounts of ipilimumab, nivolumab and trabectedin as first-line
treatment of advanced soft tissue sarcoma. Cancers (Basel).
15(906)2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Saif A, Verbus EA, Sarvestani AL, Teke ME,
Lambdin J, Hernandez JM and Kirsch DG: A randomized trial of
pembrolizumab & radiotherapy versus radiotherapy in high-risk
soft tissue sarcoma of the extremity (SU2C-SARC032). Ann Surg
Oncol. 30:683–685. 2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Liao Z, Teng J, Li T, Liu H, Li T, Zhang
C, Xing R, Teng S, Yang Y, Zhao J, et al: Evaluation of the
efficacy and safety of immunotherapy in sarcoma: A two-center
study. Front Immunol. 15(1292325)2024.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Babatunde O, Rosenbaum E, Kelly CM,
Desir-Camille R, Keohan ML, Banks L, Avutu V, Chan J, Reed D,
Gounder M, et al: 1752P A retrospective single-center study of
outcomes to immune checkpoint blockade-based therapy in
leiomyosarcoma. Ann Oncol. 35(S1045)2024.
|
|
43
|
Sonpavde GP, Grivas P, Lin Y, Hennessy D
and Hunt JD: Immune-related adverse events with PD-1 versus PD-L1
inhibitors: A meta-analysis of 8730 patients from clinical trials.
Future Oncol. 17:2545–2558. 2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Baxi S, Yang A, Gennarelli RL, Khan N,
Wang Z, Boyce L and Korenstein D: Immune-related adverse events for
anti-PD-1 and anti-PD-L1 drugs: Systematic review and
meta-analysis. BMJ. 360(k793)2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liu X, Shi Y, Zhang D, Zhou Q, Liu J, Chen
M, Xu Y, Zhao J, Zhong W and Wang M: Risk factors for
immune-related adverse events: What have we learned and what lies
ahead? Biomark Res. 9(79)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kalinich M, Murphy W, Wongvibulsin S,
Pahalyants V, Yu KH, Lu C, Wang F, Zubiri L, Naranbhai V, Gusev A,
et al: Prediction of severe immune-related adverse events requiring
hospital admission in patients on immune checkpoint inhibitors:
Study of a population level insurance claims database from the USA.
J Immunother Cancer. 9(e001935)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Schneider BJ, Naidoo J, Santomasso BD,
Lacchetti C, Adkins S, Anadkat M, Atkins MB, Brassil KJ, Caterino
JM, Chau J, et al: Management of immune-related adverse events in
patients treated with immune checkpoint inhibitor therapy: ASCO
guideline update. J Clin Oncol. 39:4073–4126. 2021.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728.
2018.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yi M, Zheng X, Niu M, Zhu S, Ge H and Wu
K: Combination strategies with PD-1/PD-L1 blockade: Current
advances and future directions. Mol Cancer. 21(28)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092.
2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lynch MM, Alexiev BA, Schroeder BA and
Pollack SM: Combinations of chemotherapy and PD-1/PD-L1 inhibitors
in sarcoma. Curr Treat Options Oncol. 23:1861–1876. 2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Dovedi SJ, Adlard AL, Lipowska-Bhalla G,
McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M,
Steward R, et al: Acquired resistance to fractionated radiotherapy
can be overcome by concurrent PD-L1 blockade. Cancer Res.
74:5458–5468. 2014.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Patel KR, Martinez A, Stahl JM, Logan SJ,
Perricone AJ, Ferris MJ, Buchwald ZS, Chowdhary M, Delman KA,
Monson DK, et al: Increase in PD-L1 expression after pre-operative
radiotherapy for soft tissue sarcoma. Oncoimmunology.
7(e1442168)2018.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Katsuki S, Takahashi Y, Tamari K, Minami
K, Takenaka W, Ibuki Y, Yamamoto J, Tatekawa S, Hayashi K, Seo Y,
et al: Radiation therapy enhances systemic antitumor efficacy in
PD-L1 therapy regardless of sequence of radiation in murine
osteosarcoma. PLoS One. 17(e0271205)2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Wolchok JD, Kluger H, Callahan MK, Postow
MA, Rizvi NJ, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K,
et al: Nivolumab plus ipilimumab in advanced melanoma. N Engl J
Med. 369:122–133. 2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Huang S, Zheng G and Yang K: Neoadjuvant
PD-1/PD-L1 combined with CTLA-4 inhibitors for solid malignancies:
A systematic review and meta-analysis. World J Surg Oncol.
21(349)2023.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Willsmore ZN, Coumbe BGT, Crescioli S,
Reci S, Gupta A, Harris RJ, Chenoweth A, Chauhan J, Bax HJ, McCraw
A, et al: Combined anti-PD-1 and anti-CTLA-4 checkpoint blockade:
treatment of melanoma and immune mechanisms of action. Eur J
Immunol. 51:544–556. 2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Roussot N, Fumet JD, Limagne E, Thibaudin
M, Hervieu A, Hennequin A, Zanetta S, Dalens L, Fourrier T, Galland
L, et al: A phase I study of the combination of atezolizumab,
tiragolumab, and stereotactic body radiation therapy in patients
with metastatic multiorgan cancer. BMC Cancer.
23(1080)2023.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Huang D, Ke L, Cui H and Li S: Efficacy
and safety of PD-1/PD-L1 inhibitors combined with anti-angiogenic
therapy for the unresectable hepatocellular carcinoma and the
benefit for hepatitis B virus etiology subgroup: A systematic
review and meta-analysis of randomized controlled trials. BMC
Cancer. 23(474)2023.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Park S, Kim TM, Han JY, Lee GW, Shim BY,
Lee YG, Kim SW, Kim IH, Lee S, Kim YJ, et al: Phase III, randomized
study of atezolizumab plus bevacizumab and chemotherapy in patients
with EGFR- or ALK-mutated non-small-cell lung cancer. J Clin Oncol.
42:1241–1251. 2024.PubMed/NCBI View Article : Google Scholar
|