Introduction
Cancer is a serious threat to human life and health.
With the progression and development of cancer research,
accumulating evidence has shown that the majority of cancer
patients do not die from local complications of the primary tumor
growth, but from the development and spread of tumor metastasis
(1). Therefore, the focus of
medical treatment gradually shifts from treating a single tumor and
prolonging the survival rate, to prolonging and improving the
quality of life in cancer patients through multi-channel
development. The development of metastasis consists of a complex
series of linked and sequential steps, which include the detachment
of cancer cells from the primary tumor, the adhesion and invasion
of cancer cells into the blood or lymphatic vessels, extravasation
from the tissue, invasion with the target tissue and finally, the
formation of new tissue (2).
The process of tumor metastasis is accompanied with
changes in gene expression, including mutation, overexpression,
loss and activation or inactivation of a number of genes (3). Matrix metalloproteinases (MMPs) is a
family of proteins that play an important role in cancer
metastasis, and are related to cancer cell invasion, metastasis and
angiogenesis (4). Among these
MMPs, MMP-2 (gelatinase-A) and MMP-9 (gelatinase-B) are
particularly important in cancer metastasis and are associated with
the invasion and metastatic spread of cancer cells by degrading
type IV collagen, a major component of the basement membrane
(5–7). With the overexpression of MMP-2 and
MMP-9, breast carcinoma metastasizes to the bone, leading to poorer
prognosis and an increased risk of relapse (8,9).
MMP-2 and MMP-9 downregulation may be the mechanism behind the
anticarcinogenic effects of various medicines on tumor invasion
(10–12). Thus, MMP-2 and MMP-9 have been
considered as a target in the development of medicine against tumor
invasion and metastasis.
The mitogen-activated protein kinase (MAPK) pathway
is associated with tumor proliferation and survival, motility and
invasion (13,14). Extracellular signal-regulated
kinase 1 and 2 (ERK1/2), p38 MAPK and c-Jun N-terminal kinase (JNK)
are three major MAPKs, which are related to tumor metastasis. They
play a central role in regulating the expression of MMPs. The up-
or downregulation of MAPKs and their phosphorylation are involved
in the regulation of MMP-9 expression in cancer cells (15,16).
The saponin monomer of the Dwarf lilyturf tuber,
ruscogenin
1-O-[β-D-glucopyranosyl-(1→2)]-[β-D-xylopyranosyl-(1→3)]-β-D-fucopyranoside
(DT-13) (Fig. 1), was isolated
from the roots of Liriope muscari (Decn.) Bailey (Fujian,
China) (17), with the structure
of ruscogenin saponin. The Dwarf lilyturf tuber is used widely in
clinics, mainly for the treatment of cardiovascular diseases
(18), with many beneficial
effects, such as the enhancement of the macrophage effect in the
immune system (19). As expected,
several extracts and their principles from the Dwarf lilyturf tuber
have shown promising activities, such as anti-inflammatory and
antitumor activities (20). Among
them, DT-13 has been shown to improve the immunoinammatory liver
injury and inhibit lymphocyte adhesion to the extracellular matrix.
Pharmacological investigations regarding the activities of
ruscogenin saponin have mainly focused on its anti-inflammatory and
anti-oxidant abilities (21,22).
Ruscogenin has been shown to significantly suppress leukocyte
migration in vivo, inhibit the TNF-α-induced overexpression
of ICAM-1 and to suppress NF-κB activation (23).
In a recent study, DT-13 was found to decrease
cancer cell metastasis in vivo and in vitro,
potentially through the downregulation of TF expression under
hypoxia, and to decrease Egr-1 expression (24). However, direct evidence for the
mechanism of DT-13 on tumor metastasis is lacking. Thus, the
antitumor metastatic ability of DT-13 was detected in the present
study, with the help of the MDA-MB-435 highly metastatic human
cancer cells. We found that DT-13 suppressed the adhesion and
invasion of MDA-MB-435 cells by inhibiting the activity of MMP-2
and MMP-9 and p-p38. Taken together, DT-13 may be considered as an
inhibitor of MMP or p38 for cancer therapy.
Materials and methods
Materials
DT-13 was isolated from the root of Liriope
muscari (Denc.) Bailey (Fujian, China), isolated in our own
laboratory. DT-13 was dissolved in dimethyl sulfoxide (DMSO) and
diluted with DMEM medium without serum before each experiment. The
final DMSO in the culture medium never exceeded 0.1‰ (v/v), a
concentration known not to affect cell proliferation, and the
control groups were always treated with 0.1‰ DMSO in the
corresponding experiments.
Cell lines and culture
MDA-MB-435 human cancer cells were obtained from
Keygene Corporation (Nanjing, China), grown in high glucose DMEM
medium supplemented with 20% heat-inactivated fetal bovine serum
(FBS), 100 U/ml penicillin, 100 U/ml streptomycin and 3.7 g/l
sodium bicarbonate. Human umbilical veinendothelial cells (HUVECs)
were isolated from human umbilical veins and harvested by enzymatic
treatment with trypsin. HUVECs were grown in Medium 199,
supplemented with 10% FBS, 30 mg/l endothelial cell growth factor
(Sigma, USA), 0.1% heparin, 100 U/ml penicillin, 100 U/ml
streptomycin and 3.7 g/l sodium bicarbonate. These cells were
maintained in logarithmic phase under a humidified atmosphere of
95% air and 5% CO2 at 37°C.
Cell cytotoxicity assay (MTT assay)
Cells were seeded in 96-well plates at a density of
104 cells/well overnight and then treated with DT-13.
The medium solution was removed after 72 h. Subsequently,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was added to the plate and the cells were cultured for another 3 h
(25). The medium solution was
then removed and DMSO was added to the plate. After agitation for
10 min at room temperature, the cytotoxicity was determined by
measuring the absorbance of the converted dye at a measure
wavelength of 570 nm and reference wavelength of 650 nm in an ELISA
reader (Tecan, Grödig, Austria).
Cell adhesion to HUVECs and
fibronectin
HUVECs were seeded in 96-well plates in complete
HUVECs-medium at a density of 104 cells/well overnight
and washed with PBS. MDA-MB-435 cells were suspended at a final
concentration of 5×105 cells/ml in various
concentrations of DT-13, dissolved in serum-free DMEM medium. After
3 h, the ratio of adhesion was detected by measuring the absorbance
of Rose Bengal at a measure wavelength of 600 nm in an ELISA
reader.
For the adhesion to fibronectin, 96-well plates were
pre-coated with fibronectin (Merck) at 4°C overnight. The
MDA-MB-435 cells, which were suspended in various concentrations of
DT-13, were cultured in a cell incubator for 3 h. The unattached
cells were then removed and washed with PBS twice. The follow steps
were the same as in the cell cytotoxicity assay.
Cell invasion assay
The Transwell membrane (8 μm pore size, 6.5 mm
diameter; Corning Costar Corporation) was pre-coated with Matrigel
(BD Corporation), and then activated by 10 mg/ml BSA at 37°C for 30
min. MDA-MB-435 cells, treated with various concentrations of DT-13
in serum-free DMEM medium, were added to the upper well and 500 μl
10% FBS DMEM were added to the lower well. The chamber was placed
into cell incubator for 20 h, then fixed with 90% ethanol and wiped
on the cells on the upper side using the cotton tips. The chamber
was stained with 0.1% Methyl Violet (Sinopharm Chemical Reagent
Co., Shanghai, China) and scanned with a microscope at ×200
mangification (AxioVision Rel 4.8; Carl Zeiss, Oberkochen,
Germany). Finally, the chamber was extracted with 10% acetic acid
and the absorbance was measured at a measure wavelength of 570 nm
in an ELISA reader.
Gelatin zymography
The activities of MMP-2 and MMP-9 were assayed by
gelatin zymography (7). After
treatment with DT-13 for 20 h, the supernatants were collected.
Samples were mixed with loading buffer and electrophoresed with 10%
SDS-polyacrylamide gel containing 0.1% gelatin. Gels were washed
twice in zymography washing buffer for 45 min at room temperature,
followed by rinsing twice in washing buffer for 20 min. The gels
were then incubated at 37°C for 40 h in zymography reaction buffer,
stained with Coomassie blue R-250 for 3 h and destained with
destaining solution. The field of the non-stained band was
quantified by Quantity One System.
Western blot analysis
MDA-MB-435 cells were incubated with various
concentrations of DT-13 for 20 h. The cells were then collected,
lysed and equal amounts of protein were separated by SDS-PAGE and
transferred onto a PVDF membrane (Millipore, Billerica, MA, USA),
which was blocked for 1.5 h at room temperature, followed by
overnight incubation at 4°C in the relevant primary antibody, and
finally blocked for 1 h with a HRP-conjugated secondary antibody.
The band detection was revealed by enhanced chemiluminescence using
an ECL kit and detected by ChemiDoc-It Imaging System (UVP, Upland,
CA, USA).
Statistical analysis
Data are expressed in terms of the means and
standard deviation (means ± SD). The experimental data were
analyzed using Microsoft Excel software (Microsoft Software Inc.).
All comparisons were made relative to the control groups and
*P<0.05 and **P<0.01 indicated
statistically significant differences.
Results
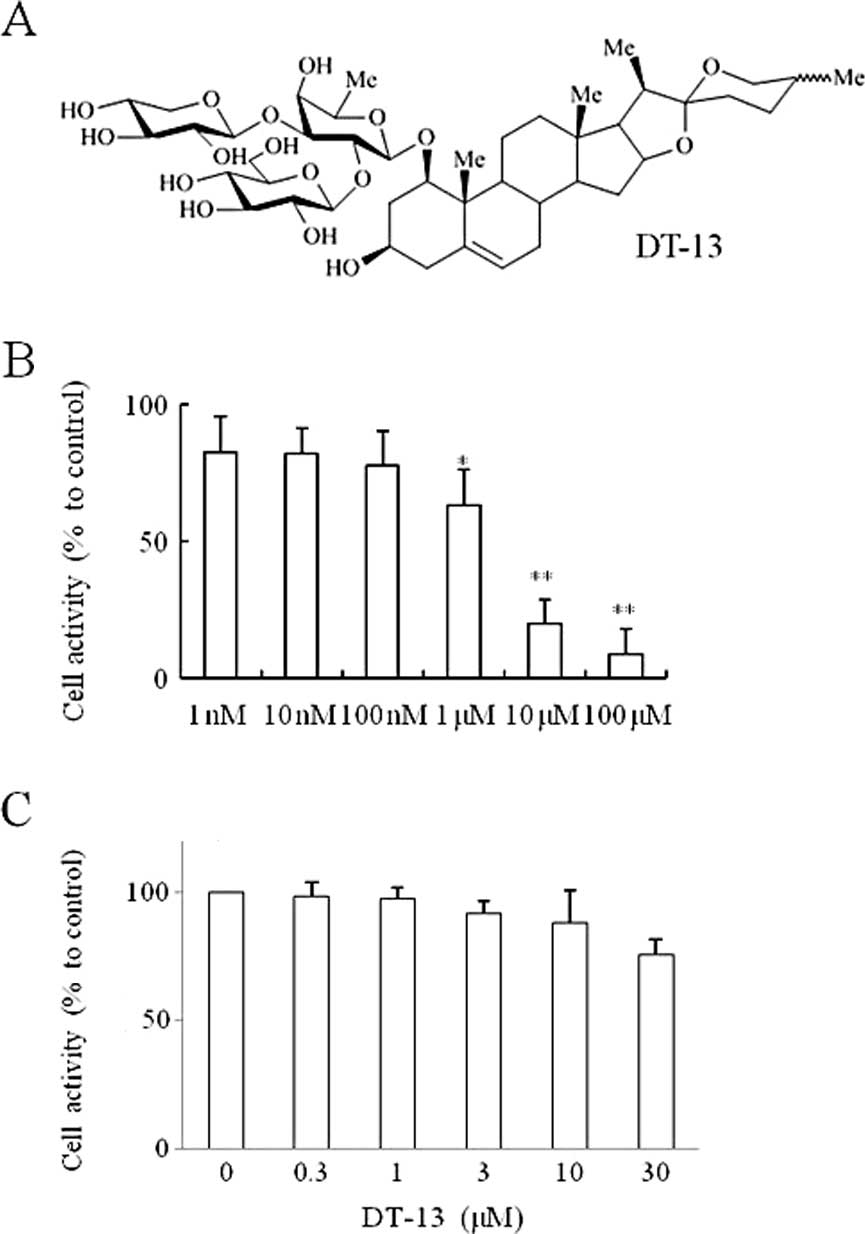
Cell cytotoxicity effect of DT-13 on
MDA-MB-435 cells
The proliferation of MDA-MB-435 cells at 72 h was
inhibited by DT-13 (Fig. 1B). The
results of the MTT assay also showed that 24-h treatments of DT-13
at various concentrations (0–30 μM) exhibited no obvious
cytotoxicity in MDA-MB-435 cells (Fig.
1C). DT-13 at the concentrations of 0.3, 1, 3, 10 and 30 μM,
was used in our experiment. These concentrations were then applied
to all subsequent experiments.
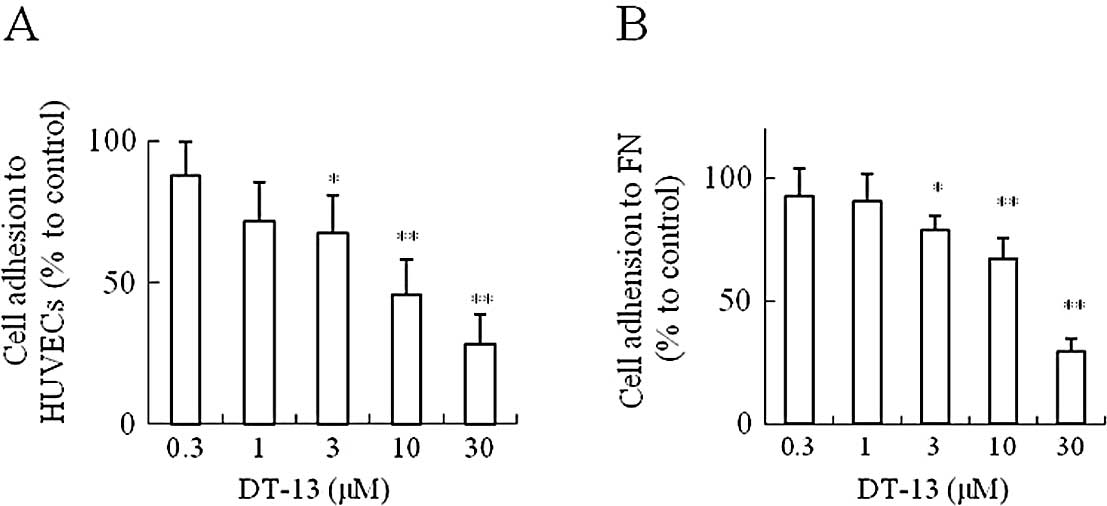
Effects of DT-13 on MDA-MB-435 cell
adhesion
As shown in Fig.
2A, DT-13 inhibited the adhesion of MDA-MB-435 cells to HUVECs.
DT-13 (10 and 30 μM) significantly reduced the number of adhered
MDA-MB-435 cells to HUVECs, and the inhibition rate was
approximately 54 and 72%, respectively. At the same time, the
MDA-MB-435 cell adhesion to fibronectin was detected for a more
accurate result. Fig. 2B shows the
effect of DT-13-inhibited adhesion to fibronectin. At 30 μM, DT-13
dramatically inhibited the adhesion of MDA-MB-435 cells to
fibronectin, and the inhibition rate was approximately 74%.
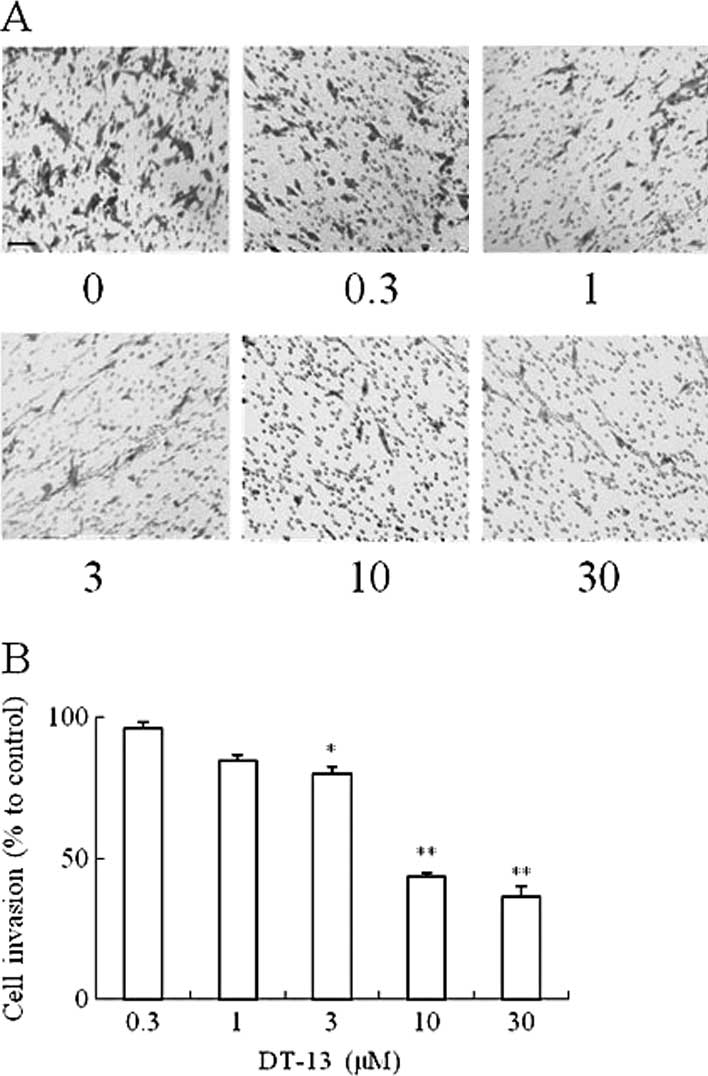
Effects of DT-13 on MDA-MB-435 cell
invasion
After treatment with DT-13 for 20 h, MDA-MB-435
cells invaded the lower side of the filter of the Transwell
chamber. Fig. 3A indicates that
DT-13 dramatically inhibited the cell invasion from the upper to
the lower chamber compared to the control group. The quantification
of cells in the lower chamber in Fig.
3B indicates that DT-13 significantly inhibited the invasion of
MDA-MB-435 cells. The inhibition rate of 10 and 30 μM of DT-13 was
approximately 57 and 64%, respectively.
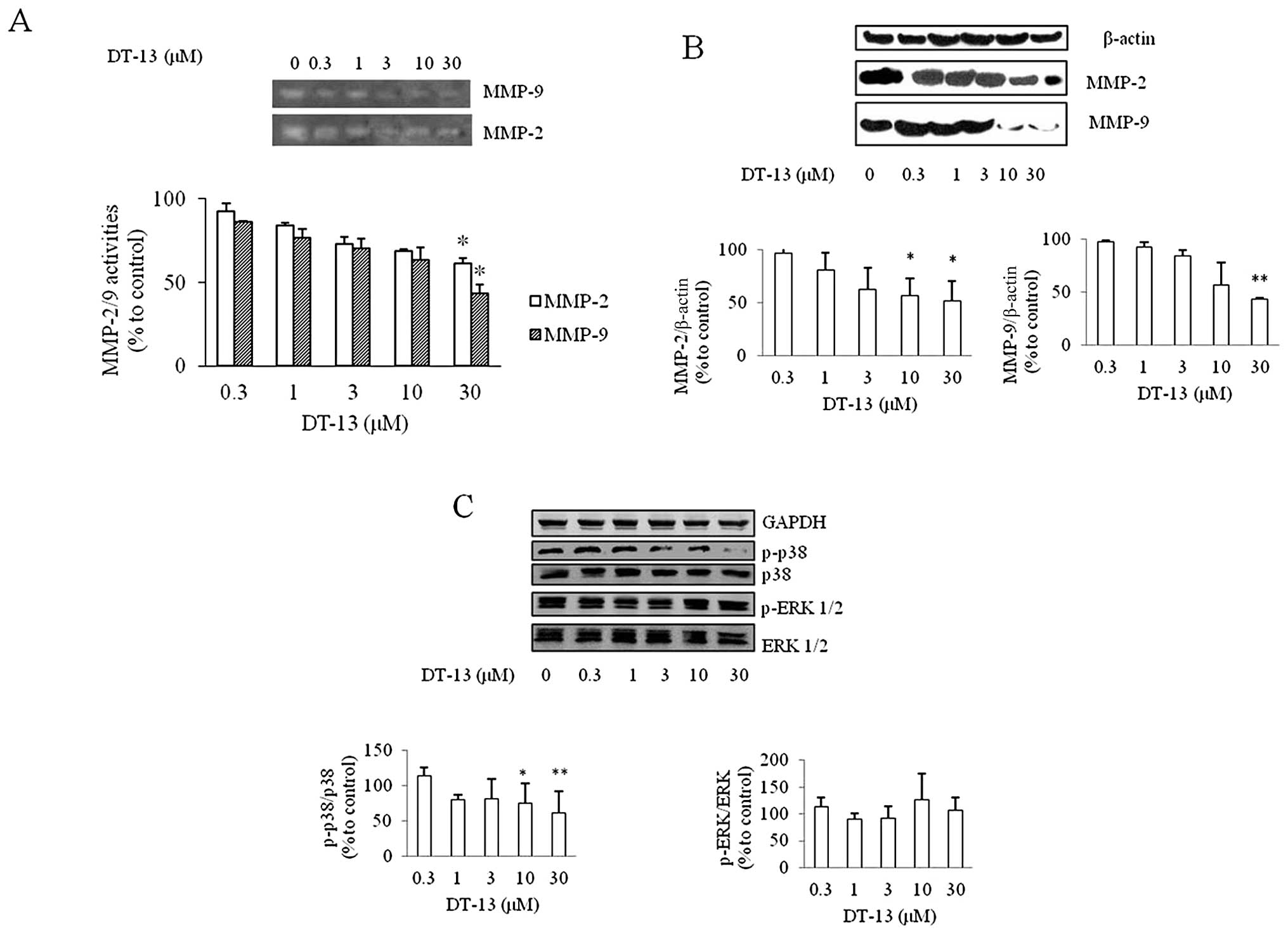
Effects of DT-13 on MMP-2 and MMP-9
enzyme activity
The expression of MMP-2 and MMP-9 has been reported
to play a critical role in degrading the basement membrane in tumor
invasion and migration (26,27).
The activity of MMP-2/9 decreased with the increasing
concentrations of DT-13 (Fig. 4A).
The inhibition rate of MMP-2 was 12, 14, 32, 33 and 42%, and that
of MMP-9 was 13, 28, 35, 44 and 61%, with 0.3, 1, 3, 10 and 30 μM
of DT-13, respectively.
Effects of DT-13 on the expression of
MMP-2/9 and MAPK pathway protein
The effects of DT-13 on the expression of MMP-2/9
were detected by western blot analysis. It was found that DT-13
decreased the expression of MMP-2/9 at the concentration of 30 μM
(Fig. 4B). With the DT-13
concentration increasing, the expression of MMP-2/9 decreased. The
expression and phosphorylation of ERK1/2 and p38 MAPK was examined
to detect whether the MAPK signaling pathway was affected by the
DT-13-treated cells (Fig. 4C). The
expression of p-p38 was inhibited at the concentrations of 10 and
30 μM, while the expression of phosphorylated-ERK (p-ERK) was not
inhibited by DT-13.
Discussion
Tumor growth, invasion and metastasis are multistep
and complex processes that include cell division and proliferation,
adhesion and cell migration through basement membranes (28). This study illustrates that DT-13
regulates several steps in cancer metastasis. The proliferation,
adhesion and invasion of MDA-MB-435 cells were inhibited by DT-13.
Additionally, the secretion and expression of MMP-2/9, which are
important proteins in cancer metastasis, were inhibited in
MDA-MB-435 cells. The MAPK pathway was involved in the
DT-13-treated cells. DT-13 inhibited cancer metastasis, and may be
a possible treatment candidate for inhibiting cancer
metastasis.
MMPs play a significant role in cancer metastasis,
degrading the extracellular matrix. The activities of MMP-2 and
MMP-9 are related to the adhesion, migration, invasion and
angiogenesis of cancer metastasis (27). Due to the significant role of MMPs
in cancer metastasis as well as in additional human pathologies,
interest has focused on identifying natural and synthetic compounds
that inhibit MMP activity. The field of non-stained bands in
gelatin zymography and the area of the bands area in the western
blots demonstrated that the activities of MMP-2 and MMP-9 were
suppressed by DT-13. The inhibition of MMP-9 was slightly greater
than MMP-2, not only in gelatin zymography, but also in western
blot analysis. These results illustrate that MMP-9 plays a more
important role in DT-13 inhibiting tumor metastasis compared to
MMP-2.
To detect whether the MAPK signaling pathway was
involved, we examined the expression and phosphorylation of ERK1/2
and p38 MAPK in DT-13-treated MDA-MB-435 cells. The expression of
p-p38 was inhibited by DT-13, while the expression of p-ERK did not
change. These results indicate that DT-13 inhibits MDA-MB-435 cell
metastasis possibly through the inactivation of p38, but not
ERK1/2. It has been reported that the p38 pathway inhibitor reduces
the MMP-9 expression and secretion, and the in vitro
invasion of cancer cells (29).
According to our result, DT-13 reduced the expression and secretion
of MMP-9 and the expression of p38 MAPK, and inhibited the invasion
of MDA-MB-435 cells. Therefore, it can be concluded that DT-13 has
the ability to act as a p38 inhibitor to tumor metastasis. It is
essential to carry out further investigation as to how DT-13
affects the MAPK pathway and other proteins involved in tumor
metastasis. Further investigation is required to detect the
mechanism of DT-13 on cancer metastasis and the interaction of
these proteins.
In conclusion, the results from this study
demonstrate that DT-13 inhibits MDA-MB-435 cell adhesion and
invasion in vitro, accompanied with the downregulation of
MMP-2/9 and the inactivation of the p38 MAPK signaling pathway. The
results show that DT-13 may have potential as a drug in antitumor
metastasis, since it is able to target diverse mechanisms involved
in the cell cycle, adhesion, invasion and metastasis. Further
investigation is essential concerning the mechanism of DT-13
inhibiting tumor metastasis.
Acknowledgements
This study was supported by grants from the Major
Scientific and Technological Specialized Project for ‘New Drugs
Development’ (no. 2009ZX09103-308), a project funded by the
Priority Academic Program Development of Jiangsu Higher Education
Institutions (PAPD), and the ‘111 Project’ from the Ministry of
Education of China and the State Administration of Foreign Expert
Affairs of China (no. 111-2-07).
Abbreviations:
|
DT-13
|
ruscogenin
1-O-[β-D-glucopyranosyl-(1→2)]-[β-D-xylopyranosyl-(1→3)]-β-D-fucopyranoside
|
|
MMP
|
matrix metal- loproteinase
|
|
MAPK
|
mitogen-activated protein kinase
|
References
|
1
|
Bohle AS and Kalthoff H: Molecular
mechanisms of tumor metastasis and angiogenesis. Langenbecks Arch
Surg. 384:133–140. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hart IR and Saini A: Biology of tumour
metastasis. Lancet. 339:1453–1457. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bogenrieder T and Herlyn M: Axis of evil:
molecular mechanisms of cancer metastasis. Oncogene. 22:6524–6536.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoon SO, Park SJ, Yun CH and Chung AS:
Roles of matrix metalloproteinases in tumor metastasis and
angiogenesis. J Biochem Mol Biol. 36:128–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoneda T: Cellular and molecular
mechanisms of breast and prostate cancer metastasis to bone. Eur J
Cancer. 34:240–245. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
7
|
Song HY, Ju SM, Goh AR, Kwon DJ, Choi SY
and Park J: Suppression of TNF-alpha-induced MMP-9 expression by a
cell-permeable superoxide dismutase in keratinocytes. BMB Rep.
44:462–467
|
|
8
|
Kang JH, Han IH, Sung MK, et al: Soybean
saponin inhibits tumor cell metastasis by modulating expressions of
MMP-2, MMP-9 and TIMP- 2. Cancer Lett. 261:84–92. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gonzalez LO, Pidal I, Junquera S, et al:
Overexpression of matrix metalloproteinases and their inhibitors in
mononuclear inflammatory cells in breast cancer correlates with
metastasis-relapse. Br J Cancer. 97:957–963. 2007.PubMed/NCBI
|
|
10
|
Tan ML, Choong PF and Dass CR: Direct
anti-metastatic efficacy by the DNA enzyme Dz13 and downregulated
MMP-2, MMP-9 and MT1-MMP in tumours. Cancer Cell Int. 10:92010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim S, Choi JH, Lim HI, et al: Silibinin
prevents TPA-induced MMP-9 expression and VEGF secretion by
inactivation of the Raf/MEK/ERK pathway in MCF-7 human breast
cancer cells. Phytomedicine. 16:573–580. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weifeng T, Feng S, Xiangji L, et al:
Artemisinin inhibits in vitro and in vivo invasion and metastasis
of human hepatocellular carcinoma cells. Phytomedicine. 18:158–162.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trusolino L and Comoglio PM:
Scatter-factor and semaphorin receptors: cell signalling for
invasive growth. Nat Rev Cancer. 2:289–300. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fecher LA, Amaravadi RK and Flaherty KT:
The MAPK pathway in melanoma. Curr Opin Oncol. 20:183–189. 2008.
View Article : Google Scholar
|
|
15
|
Kim IY, Yong HY, Kang KW and Moon A:
Overexpression of ErbB2 induces invasion of MCF10A human breast
epithelial cells via MMP-9. Cancer Lett. 275:227–233. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SO, Jeong YJ, Kim M, Kim CH and Lee
IS: Suppression of PMA-induced tumor cell invasion by capillarisin
via the inhibition of NF-kappaB-dependent MMP-9 expression. Biochem
Biophys Res Commun. 366:1019–1024. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu B-Y: Comparative studies on the
constituents of Ophiopogonis tuber and its congeners. VI
Studies on the constituents of the subterranean part of Liriope
spicata var prolifera and L muscari. Chem Pharm Bull.
38:1931–1935. 1990.
|
|
18
|
Fang XC, Yu BY, Xiang BR and An DK:
Application of pyrolysis-high-resolution gas chromatography-pattern
recognition to the identification of the Chinese traditional
medicine mai dong. J Chromatogr. 514:287–292. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kamei J, Nakamura R, Ichiki H and Kubo M:
Antitussive principles of Glycyrrhizae radix, a main
component of the Kampo preparations Bakumondo-to
(Mai-men-dong-tang). Eur J Pharmacol. 469:159–163. 2003.
|
|
20
|
Mimaki Y, Nakamura O, Sashida Y, et al:
Structures of steroidal saponins from the tubers of Brodiaea
californica and their inhibitory activity on tumor
promoter-induced phospholipid metabolism. Chem Pharm Bull (Tokyo).
43:971–976. 1995.PubMed/NCBI
|
|
21
|
Wu F, Cao J, Jiang J, Yu B and Xu Q:
Ruscogenin glycoside (Lm-3) isolated from Liriope muscari
improves liver injury by dysfunctioning liver-infiltrating
lymphocytes. J Pharm Pharmacol. 53:681–688. 2001.PubMed/NCBI
|
|
22
|
Liu J, Chen T, Yu B and Xu Q: Ruscogenin
glycoside (Lm-3) isolated from Liriope muscari inhibits
lymphocyte adhesion to extracellular matrix. J Pharm Pharmacol.
54:959–965. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang YL, Kou JP, Ma L, Song JX and Yu BY:
Possible mechanism of the anti-inflammatory activity of ruscogenin:
role of intercellular adhesion molecule-1 and nuclear
factor-kappaB. J Pharmacol Sci. 108:198–205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun L, Lin S, Zhao R, Yu B, Yuan S and
Zhang L: The saponin monomer of dwarf lilyturf tuber, DT-13,
reduces human breast cancer cell adhesion and migration during
hypoxia via regulation of tissue factor. Biol Pharm Bull.
33:1192–1198. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Szliszka E and Krol W: Soy isoflavones
augment the effect of TRAIL-mediated apoptotic death in prostate
cancer cells. Oncol Rep. 26:533–541. 2011.PubMed/NCBI
|
|
26
|
Vihinen P and Kahari VM: Matrix
metalloproteinases in cancer: prognostic markers and therapeutic
targets. Int J Cancer. 99:157–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Foda HD and Zucker S: Matrix
metalloproteinases in cancer invasion, metastasis and angiogenesis.
Drug Discov Today. 6:478–482. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun Y, Lu N, Ling Y, et al: Oroxylin A
suppresses invasion through down-regulating the expression of
matrix metalloproteinase-2/9 in MDA-MB-435 human breast cancer
cells. Eur J Pharmacol. 603:22–28. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Simon C, Simon M, Vucelic G, et al: The
p38 SAPK pathway regulates the expression of the MMP-9 collagenase
via AP-1-dependent promoter activation. Exp Cell Res. 271:344–355.
2001. View Article : Google Scholar : PubMed/NCBI
|