Introduction
Maintenance of articular cartilage integrity and its
ability to react to mechanical loads and injury requires a properly
orchestrated response of chondrocytes to cellular signals generated
by growth factors, the extracellular matrix and cytokines (1). Under physiological conditions,
programmed cell death in cartilage is uncommon, owing to
maintenance of metabolic homeostasis and chondrocyte adhesion to
extracellular matrix proteins (2).
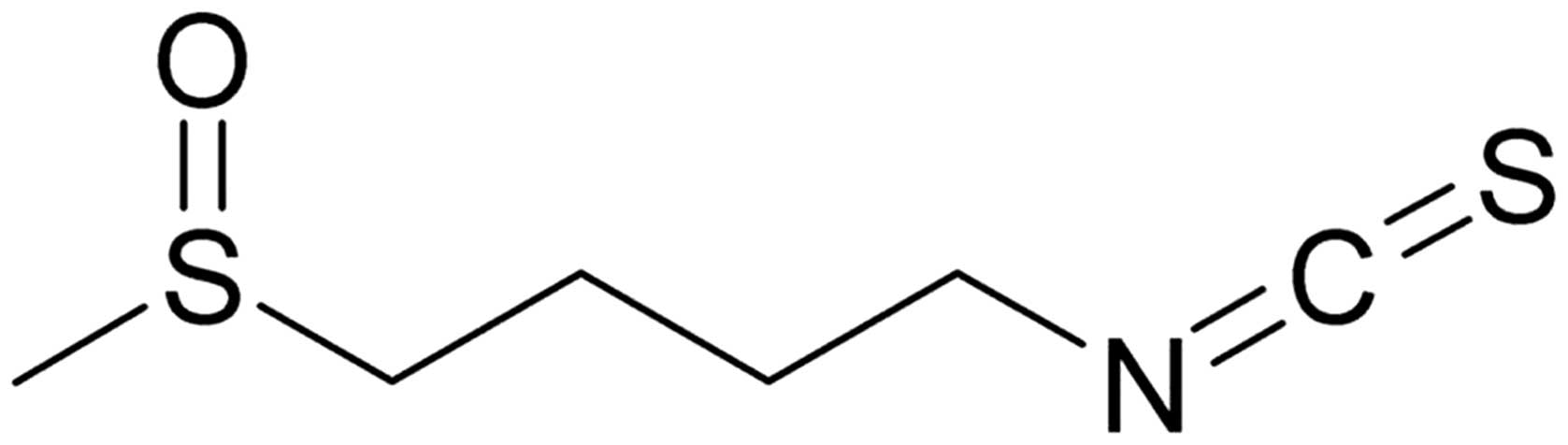
Sulforaphane [SFN;
1-isothiocyanato-4-(methyl-sulfinyl) butane; Fig. 1] is an isothiocyanate identified in
broccoli and other cruciferous vegetables. SFN forms following
hydrolysis of the glucosinolate compound glucoraphanin by
thioglucoside glucohydrolase (3,4). The
compound has been revealed to be a naturally occurring cancer
chemopreventive agent in animal models (5). Previous studies using animals have
demonstrated the protective effect of SFN with regard to chemically
induced carcinogenesis in various organs, including lung,
esophagus, liver, mammary gland, colon (6,7) and
stomach (8). The chemopreventive
activity of SFN has been associated with various effects. However,
the mechanism by which SFN exerts its effects on chondrocytes is
not fully understood.
SFN has been demonstrated to inhibit neoplastic cell
proliferation, block cell cycle progression at G2/M,
cause apoptosis and modulate signal transduction pathways,
suggesting that it may be an effective inhibitor of neoplastic cell
proliferation and cancer promotion/progression (4,9).
Generally, mammalian cells respond to DNA-damaging
agents by activating cell cycle checkpoints, resulting in a delay
of cell cycle progression until errors have been corrected
(10). Cell cycle arrest at the
G2/M phase prevents cells from completing the cell cycle
and proliferating (11). The phase
is regulated by the sequential activation and deactivation of cdc
family protein and cyclin complexes, including the cdc2/cyclin B
complex.
Cyclin-dependent kinases (CDKs), cyclins and CDK
inhibitors (CDKIs) are key molecules that play significant roles in
cell cycle progression (12).
p21WAF1/CIP1 is a CDKI protein essential for cellular
growth, differentiation and apoptosis (13). Therefore, induction of cell cycle
arrest and apoptosis by chemotherapeutic agents is an effective
approach to the inhibition of uncontrolled cell proliferation and
survival in chondrocytes. We confirm that SFN induces cell cycle
arrest at the G2/M phase via the p21WAF1/CIP1
and p53 pathways in rabbit articular chondrocytes. The present
study sought to define the signal transduction pathways involved in
the disruption of cartilage homeostasis, with the aim of
identifying new therapeutic strategies for the prevention of
cartilage destruction.
Materials and methods
Cell culture
Rabbit articular chondrocytes were isolated from
cartilage slices of 2-week-old New Zealand white rabbits by
enzymatic digestion. Cartilage slices were dissociated
enzymatically for 6 h in 0.2% collagenase type II (381 U/mg solid;
Sigma, St. Louis, MO, USA) in DMEM (Gibco-BRL, Gaithersburg, MD,
USA). Individual cells were suspended in DMEM supplemented with 10%
(v/v) fetal bovine serum (Gibco-BRL), 50 g/ml streptomycin (USB,
Staufen, Germany)and 50 U/ml penicillin (Sigma). Following this,
cells were plated on culture dishes at a density of
5×104 cells/cm2. Seeding medium was changed
every 2 days, and cells reached confluence in ~5 days. The 3.5-day
cell cultures were treated with SFN. SFN was purchased from LKT
Laboratories, Inc. (St. Paul, MN, USA). The study was approved by
the ethics committee of Kongju National University, Gongju,
Republic of Korea.
Western blot analysis
Whole cell lysates were prepared by extracting
proteins using a buffer containing 50 mM Tris-HCl (pH 7.4), 150 mM
NaCl, 1% Nonidet P-40 and 0.1% sodium dodecylsulfate (SDS)
supplemented with protease inhibitors (10 g/ml leupeptin, 10 g/ml
pepstatin A, 10 g/ml aprotinin and 1 mM AEBSF) and phosphatase
inhibitors (1 mM NaF and 1 mM Na3VO4).
Lysates were size-fractionated by SDS-polyacrylamide gel
electrophoresis and transferred to a nitrocellulose membrane. The
nitrocellulose sheet was blocked with 5% non-fat dry milk in
Tris-buffered saline. Expression levels of cdc2, cyclin B, cdc25c,
p53, p21 and β-actin were detected using antibodies purchased from
Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Blots were
developed using a peroxidase-conjugated secondary antibody and
imaged using an ImageQuant LAS 4000 system (GE Healthcare
Bio-Sciences Corp., Piscataway, NJ, USA). Quantification of protein
expression was performed using densitometric analysis (Image
J).
Cell cycle distribution by FACS
analysis
Cell cycle distribution was assessed by staining DNA
content with propidium iodide. Briefly, chondrocytes were plated at
a density of 2×104 cells/cm2 and incubated
for 24 h. Fresh media containing SFN were applied to culture dishes
and further incubated for 24 h. Following incubation, cells were
harvested and fixed with 70% ethanol in PBS overnight. Fixed cells
were incubated with RNase A (50 μg/ml) for 25 min, prior to
staining nucleic acid with propidium iodide (50 μg/ml) for 5 min.
The DNA content of 5×104 cells/group was analyzed by
flow cytometry (Partec GmbH, Münster, Germany) and the results
presented as histograms of DNA content. Quantification of the
distinct cell cycle phases was calculated using FloMax analysis
software (Partec GmbH).
Trypan blue dye exclusion assay
The effect of SFN on the survival/proliferation of
rabbit articular chondrocytes was determined by trypan blue dye
exclusion assay. Cells were collected by trypsinization, separated
into single cell suspensions in culture medium and inoculated into
culture dishes at densities of 5×104
cells/cm2. Following culture for 24 h, cells were
exposed to increasing concentrations of SFN or DMSO for 48 h,
followed by trypsinization of cells adherent to the culture dishes
and cell count anaysis by hemocytometer. Each experiment was
performed in triplicate. Results were reported as an average of at
least three separate experiments.
Data analyses and statistics
Results are expressed as the mean ± SE, calculated
from the specified number of determinations. A Student’s t-test was
used to compare individual treatments with their respective control
value. P<0.05 was considered to indicate a statistically
significant difference.
Results
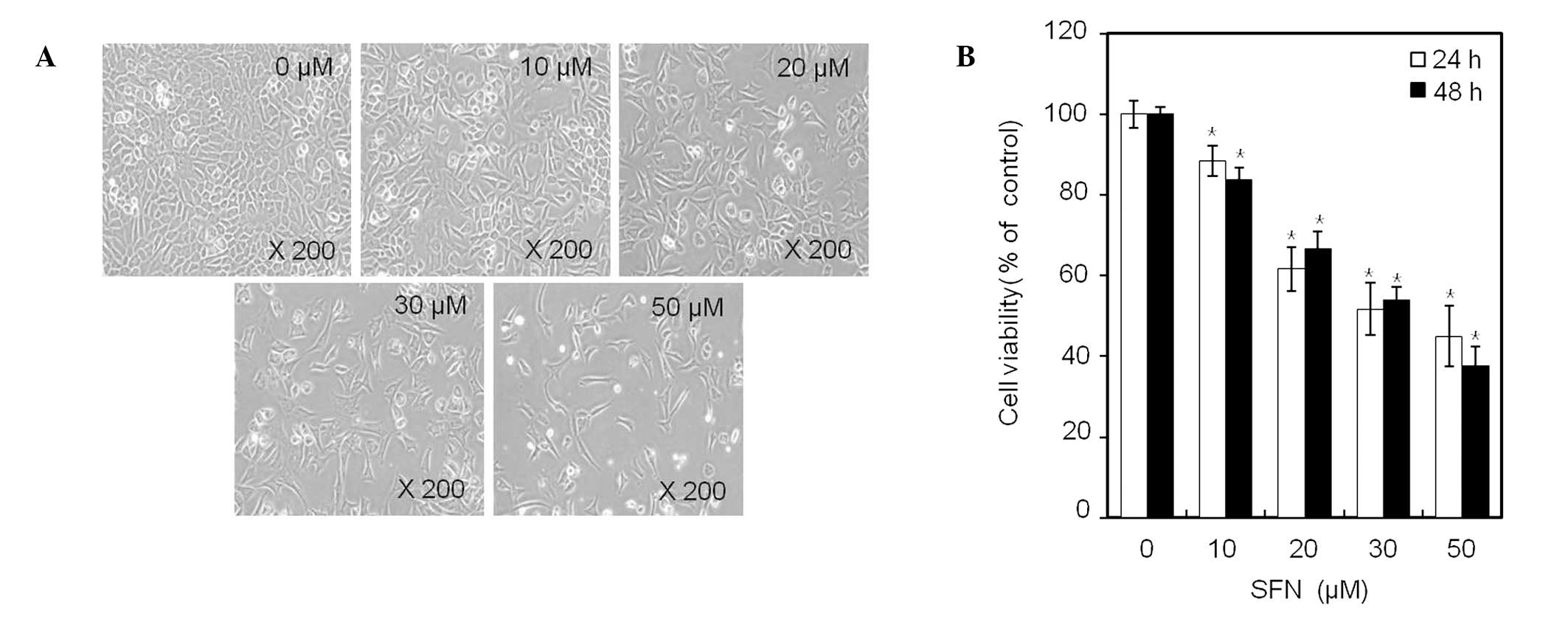
SFN suppresses proliferation of rabbit
articular chondrocytes
Phase contrast microscopy (x400; Fig. 2A) demonstrated that treatment of
chondrocytes for 24 h with an increasing concentration of SFN, up
to 50 μM, inhibited cell proliferation in a dose-dependent manner.
Within 48 h, SFN treatment decreased the number of cells attached
to the plating surface and the total number of cells present in
culture. Treatment with 50 μM SFN for 24 or 48 h inhibited cell
proliferation of chondrocytes by 45 and 38%, respectively (Fig. 2B). These results demonstrate that
SFN has a growth inhibitory effect on cell proliferation in rabbit
articular chondrocytes (Fig.
2).
SFN causes cell cycle arrest at
G2/M phase
Since SFN inhibits cell proliferation of
chondrocytes, we investigated the effects of SFN on cell cycle
distribution by flow cytometric analysis. As demonstrated in
Fig. 3A, FACS analysis revealed
that exposure to SFN for 24 h increased the population of
G2/M phase cells in a dose-dependent manner.
Chondrocytes at the G2/M phase increased from 12.03%
(medium alone) to 36.82%, by treatment with 50 μM SFN (Fig. 3A and B). Exposure of chondrocytes
to a growth suppressive concentration of 30 μM SFN for 48 h
resulted in accumulation of cells in the G2/M phase, and
was accompanied by a decrease in cells in the
G0/G1 phase (Fig. 3C and D). Compared with the control,
the percentage of cells in G2/M phase was increased by
~4-fold, upon treatment with 30 μM SFN for 48 h (Fig. 3C and D). These results suggest that
SFN is effective in suppressing chondrocyte proliferation through
induction of cell cycle arrest at the G2/M phase
(Fig. 3). Inhibition of growth was
not accompanied by an increase of apoptosis at the
G0/G1 phase, as determined by FACS analysis
(Fig. 3).
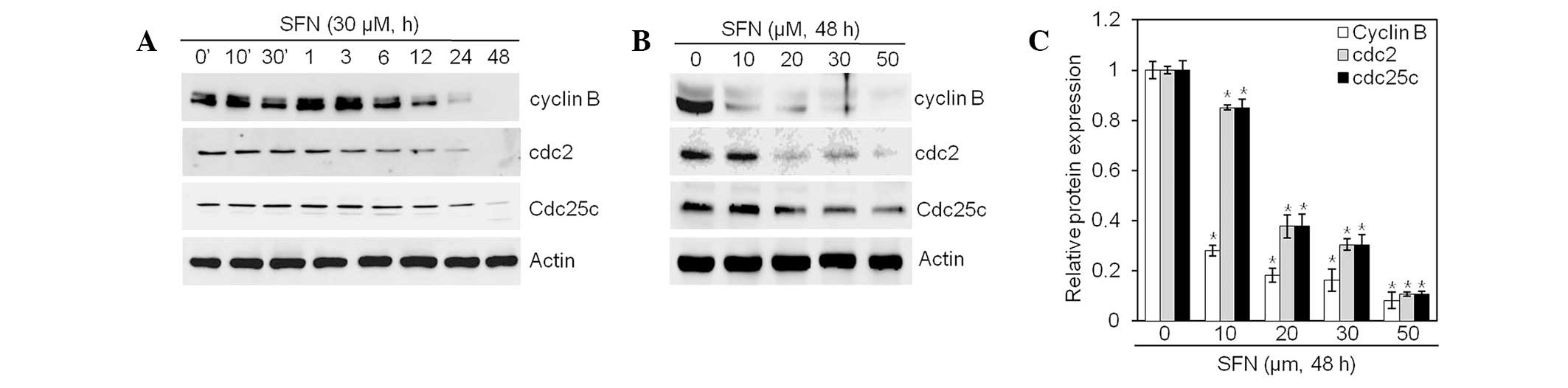
SFN inhibits expression of cyclin B1/cdc2
and cdc25c
To determine the molecular mechanisms by which SFN
induces G2/M phase arrest, we examined cell
cycle-regulatory proteins involved in the G2/M phase.
Western blot analysis was performed, using antibodies against cell
cycle-related proteins (cyclin B1, cdc2 and cdc25c; Fig. 4). SFN treatment resulted in
substantial reductions in levels of cyclin B1, cdc2 and cdc25c
proteins (Fig. 4A and B). Levels
of cyclin 1 and cdc2 decreased faster than levels of cdc25c
(Fig. 4A). Quantification of
protein content identified a significant reduction of cyclin B1,
cdc2 and cdc25c in a dose- and time-dependent manner (Fig. 4C). Collectively, these data
indicate that treatment of articular chondrocyte cells with SFN
induces expression changes in cell cycle regulators, resulting in
subsequent G2/M phase arrest.
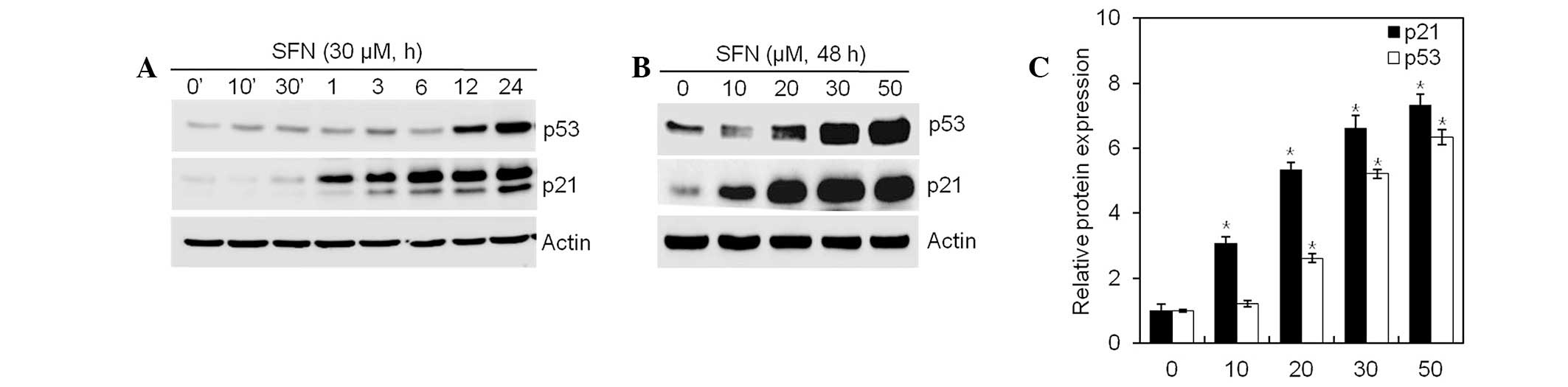
SFN increases p21WAF1/CIP1
expression through a p53-dependent pathway
SFN inhibited cell proliferation and induced
G2/M phase cell cycle arrest, observable by flow
cytometry analysis. Following DNA damage, p53 activates
p21WAF1/CIP1 and causes G2/M phase cell cycle
arrest. Expression of p21WAF1/CIP1 and p53 in articular
chondrocytes exposed to SFN, was examined by western blot analysis.
SFN increased expression of p21WAF1/CIP1 and p53 in a
dose- and time-independent manner (Fig. 5). These data indicate that
SFN-mediated G2/M phase arrest is regulated by the
p53/p21WAF1/CIP1-dependent pathway.
Discussion
Cruciferous or brassica vegetables come from the
brassica genus and include broccoli, brussel sprout and
cauliflower. These vegetables are a good source of glucosinolates,
and their hydrolysis products include indoles and isothiocyanates.
SFN is an isothiocyanate associated with chemopreventive activity,
linked to inhibition of cell growth and disruption of microtubule
polymerization (4). In the present
study, SFN inhibited the growth of articular chondrocytes, as
determined by trypan blue exclusion assay (Fig. 2B).
Various checkpoint mechanisms function to regulate
the cell cycle, ensuring appropriate cellular response to external
stresses, including abnormal mitogenic signaling (14).
In an effort to elucidate the mechanism by which SFN
treament results in the inhibition of cell growth, its effects on
cell cycle progression were assessed. The present study
demonstrated that SFN induces a 27% decrease of
G0/G1 phase and a 24% increase of
G2/M phase, following 24 h treatment with 50 μM SFN
(Fig. 3A and B). Several studies
on SFN have reported that SFN induces cell cycle arrest at the
G2/M phase in cancer cells (15–17).
To the best of our knowledge, activation of cdc2, triggered by a
positive feedback loop at the end of the G2 phase, is
the key event that initiates mitotic entry (18,19).
The present study revealed that SFN treatment, resulting in
G2/M cell cycle arrest in articular chondrocytes, was
correlated with decreased levels of cyclin B1/cdc2 and cdc25c
(Fig. 4).
Cell cycle progression is mediated by various CDKs
whose activities are regulated by CDKIs, including
p21WAF1/CIP1. The tumor suppressor gene p53 also
regulates inhibition of cell growth (20), and expression of p53 has been
revealed to cause a limited arrest in the G1 phase
(21). However, other studies have
demonstrated that p53 leads to G2/M phase progression in
the rat cell line REF52, and a human ovarian cancer cell line
(22,23). p53 directly stimulates expression
of p21WAF1/CIP1 to promote cell cycle inhibition.
p21WAF1/CIP1 competently blocks instigation of early
mitotic progression by inhibiting activation of cyclin B and cdc2
(24). The present study suggests
that activation of p21 and p53 with SFN may regulate
G2/M phase arrest, by inhibiting formation of
cdc2/cyclin B complexes (Figs. 4
and 5). Cdc25c is a major
phosphatase which activates cdc2 by dephosphorylatyion, enabling
formation of the cdc2/cyclin B complex. These formations result in
mitotic transition of cells (25).
In conclusion, our results demonstrate that SFN
inhibits cell growth and induces cell cycle arrest in rabbit
articular chondrocytes.
Acknowledgements
This study was supported by the National Research
Foundation of Korea funded by the Korea Government (MEST;
2011-00027473 and 2012-0004359).
References
|
1
|
Loeser RF, Chubinskaya S, Pacione C and Im
HJ: Basic fibroblast growth factor inhibits the anabolic activity
of insulin-like growth factor 1 and osteogenic protein 1 in adult
human articular chondrocytes. Arthritis Rheum. 52:3910–3917. 2005.
View Article : Google Scholar
|
|
2
|
Pulai JI, Del Carlo M Jr and Loeser RF:
The alpha5beta1 integrin provides matrix survival signals for
normal and osteoarthritic human articular chondrocytes in vitro.
Arthritis Rheum. 46:1528–1535. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gamet-Payrastre L, Lumeau S, Gasc N,
Cassar G, Rollin P and Tulliez J: Selective cytostatic and
cytotoxic effects of glucosinolates hydrolysis products on human
colon cancer cells in vitro. Anticancer Drugs. 9:141–148. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jackson SJ and Singletary KW:
Sulforaphane: a naturally occurring mammary carcinoma mitotic
inhibitor, which disrupts tubulin polymerization. Carcinogenesis.
25:219–227. 2004. View Article : Google Scholar
|
|
5
|
Parnaud G, Li P, Cassar G, et al:
Mechanism of sulforaphane-induced cell cycle arrest and apoptosis
in human colon cancer cells. Nutr Cancer. 48:198–206. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chung FL, Conaway CC, Rao CV and Reddy BS:
Chemoprevention of colonic aberrant crypt foci in Fischer rats by
sulforaphane and phenethyl isothiocyanate. Carcinogenesis.
21:2287–2291. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kassie F, Uhl M, Rabot S, et al:
Chemoprevention of 2-amino-3-methylimidazo[4,5-f]quinoline
(IQ)-induced colonic and hepatic preneoplastic lesions in the F344
rat by cruciferous vegetables administered simultaneously with the
carcinogen. Carcinogenesis. 24:255–261. 2003.
|
|
8
|
Fahey JW, Haristoy X, Dolan PM, et al:
Sulforaphane inhibits extracellular, intracellular, and
antibiotic-resistant strains of Helicobacter pylori and
prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci
USA. 99:7610–7615. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gamet-Payrastre L, Li P, Lumeau S, et al:
Sulforaphane, a naturally occurring isothiocyanate, induces cell
cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer
Res. 60:1426–1433. 2000.PubMed/NCBI
|
|
10
|
Zhang C, Zhu H, Yang X, et al: P53 and p38
MAPK pathways are involved in MONCPT-induced cell cycle G2/M arrest
in human non-small cell lung cancer A549. J Cancer Res Clin Oncol.
136:437–445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen AC and Donovan SM: Genistein at a
concentration present in soy infant formula inhibits Caco-2BBe cell
proliferation by causing G2/M cell cycle arrest. J Nutr.
134:1303–1308. 2004.PubMed/NCBI
|
|
12
|
Schwartz GK and Shah MA: Targeting the
cell cycle: a new approach to cancer therapy. J Clin Oncol.
23:9408–9421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiong Y, Hannon GJ, Zhang H, Casso D,
Kobayashi R and Beach D: p21 is a universal inhibitor of cyclin
kinases. Nature. 366:701–704. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim JH, Han Kwon K, Jung JY, et al:
Sulforaphane increases cyclin-dependent kinase inhibitor, p21
protein in human oral carcinoma cells and nude mouse animal model
to induce G(2)/M cell cycle arrest. J Clin Biochem Nutr. 46:60–67.
2010.PubMed/NCBI
|
|
15
|
Chiao JW, Chung FL, Kancherla R, Ahmed T,
Mittelman A and Conaway CC: Sulforaphane and its metabolite mediate
growth arrest and apoptosis in human prostate cancer cells. Int J
Oncol. 20:631–636. 2002.PubMed/NCBI
|
|
16
|
Fimognari C, Nüsse M, Cesari R, Iori R,
Cantelli-Forti G and Hrelia P: Growth inhibition, cell-cycle arrest
and apoptosis in human T-cell leukemia by the isothiocyanate
sulforaphane. Carcinogenesis. 23:581–586. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Herman-Antosiewicz A, Xiao H, Lew KL and
Singh SV: Induction of p21 protein protects against
sulforaphane-induced mitotic arrest in LNCaP human prostate cancer
cell line. Mol Cancer Ther. 6:1673–1681. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Blethrow JD, Glavy JS, Morgan DO and
Shokat KM: Covalent capture of kinase-specific phosphopeptides
reveals Cdk1-cyclin B substrates. Proc Natl Acad Sci USA.
105:1442–1447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chu WF, Wu DM, Liu W, et al: Sulforaphane
induces G2-M arrest and apoptosis in high metastasis cell line of
salivary gland adenoid cystic carcinoma. Oral Oncol. 45:998–1004.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ho JW, Song JZ and Leung YK: Activation of
p53 by specific agents in potential cancer therapy. Curr Med Chem
Anticancer Agents. 5:131–135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Michalovitz D, Halevy O and Oren M:
Conditional inhibition of transformation and of cell proliferation
by a temperature-sensitive mutant of p53. Cell. 62:671–680. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stewart N, Hicks GG, Paraskevas F and
Mowat M: Evidence for a second cell cycle block at G2/M by p53.
Oncogene. 10:109–115. 1995.PubMed/NCBI
|
|
23
|
Vikhanskaya F, Erba E, D’Incalci M and
Broggini M: Introduction of wild-type p53 in a human ovarian cancer
cell line not expressing endogenous p53. Nucleic Acids Res.
22:1012–1017. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Charrier-Savournin FB, Château MT, Gire V,
Sedivy J, Piette J and Dulic V: p21-Mediated nuclear retention of
cyclin B1-Cdk1 in response to genotoxic stress. Mol Biol Cell.
15:3965–3976. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Su C, Deaton RA, Iglewsky MA, Valencia TG
and Grant SR: PKN activation via transforming growth factor-beta 1
(TGF-beta 1) receptor signaling delays G2/M phase transition in
vascular smooth muscle cells. Cell Cycle. 6:739–749. 2007.
View Article : Google Scholar : PubMed/NCBI
|