Introduction
Glioblastoma multiforme (GBM) is the most common and
lethal glial tumor of the adult brain, accounting for approximately
50% of all gliomas. GBM is characterized by an aggressive growth
pattern, a marked degree of invasiveness and extremely poor
prognosis. Even after multimodal treatment approaches, the median
survival of patients with GBM following the primary diagnosis
remains poor. An improved understanding of the genetic background
and molecular pathogenic processes involved in the tumorigenesis of
GBM is therefore critical for the development of rational, targeted
therapies (1–5).
Special AT-rich sequence-binding protein 1 (SATB1)
is a cell type-specific nuclear matrix attachment region-binding
protein that links specific DNA elements to its cage-like network
(6) and is predominantly expressed
in thymocytes (7). SATB1
facilitates the formation of an open chromatin structure and is
involved in the regulation of hundreds of genes. SATB1 has recently
received considerable attention from the cancer research field due
to high expression in various malignant tumor tissues (8–10),
indicative of a tumor growth promoter. To explore the roles of
SATB1 in the clinical progression of GBM, the present study
examined the correlation between expression of SATB1 and Bcl-2 in
GBM and tumor apoptosis, in order to shed new light on GBM
therapy.
Materials and methods
Patients and tissue samples
Seventy cases of surgically resected GBMs were
collected from the 2007–2010 pathology files of the Third People's
Hospital Affiliated to Shanghai Jiao Tong University School of
Medicine and Zhongnan Hospital of Wuhan University. Approval was
obtained from the Ethics committee of the Third People's Hospital
Affiliated to Shanghai Jiao Tong University School of Medicine and
Zhongnan Hospital of Wuhan University. Specimens were handled and
made anonymous according to ethical and legal standards. Written
informed consent was obtained from all patients. There were 40
males and 30 females with a mean age of 46 years (range, 29–68).
None of the patients had received chemical therapy or radiotherapy
prior to surgery. Control brain tissues were obtained from 10
individuals, who had died in traffic accidents exhibiting no prior
pathologically detectable condition. Based on the results of
hematoxylin-eosin staining, histopathological diagnosis was
performed by various neuropathologists.
In situ hybridization
Frozen sections were immersed in a solution of 30%
hydrogen dioxide and methanol for 30 min following brief warming at
room temperature, then incubated at 37°C with pepsin diluted by 3%
citric acid. Sections were postfixed for 10 min in 1%
paraformaldehyde and were incubated at 38–42°C with DIG-labeled
antisense cRNA probes overnight in a humidified chamber. Following
multiple washes in 4× SSC at room temperature, the slides were
incubated at 37°C in a blocking reagent for 30 min, a biotinylated
anti-digoxin antibody for 60 min, SABC for 20 min and the
biotinylated peroxydase for 20 min, at 37°C, followed by staining
with DAB (Sigma, St. Louis, MO, USA). Sections were then covered
with glycerol-gelatin and coverslips for microscopic examination
(11,12). The SATB1 probe (sequence:
5′-TCTTTAATTTCTAATATATTTAGAA-3′) was synthesized and labeled with
biotin at the 5′ end by Sangon Bioengineering Technology and
Services Co., Ltd. (Shanghai, China). The probe was replaced with
the dilution solution in control samples.
Immunohistochemical analysis
Antigen retrieval was performed in boiling citrate
buffer for 15 min. Peroxide blocking was performed with 0.3%
peroxide in absolute methanol. The slides were then incubated with
anti-Bcl-2 polyclonal antibody (diluted 1:300; Sigma) or mouse
anti-PCNA monoclonal antibody (diluted 1:100; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C overnight and
washed twice with phosphate-buffered saline prior to incubation
with a secondary antibody (Santa Cruz Biotechnology, Inc.) at room
temperature for 30 min. After washing, the sections were incubated
with immunoglobulins conjugated with horseradish peroxidase. The
reaction was then developed with 3,3′-diaminobenzidine substrate
(2,13). Tissue sections were counterstained
with hematoxylin or methyl green.
Quantification of SATB1 mRNA, Bcl-2 and
PCNA protein
The nucleoli of SATB1-positive cells, cytoplasm of
Bcl-2-positive cells (14) and
nuclei of PCNA-positive cells were stained brown-yellow. Images of
the sections were obtained (magnification, ×100) using the
HPIAS-1000 High Resolution Color Pathological Image Analysis System
(Tongji Medical College Qianping Imaging Engineering Co., Ltd.,
Shanghai, China) (15). Specimens
with a positive cell ratio <30% were defined as negative.
Measurement of apoptosis by flow
cytometry
Tissues collected in RPMI-1640 medium supplemented
with 10% fetal bovine serum were processed routinely to generate
single-cell suspensions (16,17).
Suspensions were then fixed in 70% cold ethanol, treated with 10
g/l RNase and suspended and stained with 10 g/l propidium iodine
(PI). After washing with PBS, the cells were stained directly with
PI at a final concentration of 10 μg/ml and 2% Annexin-V Flous
(Roche, Basel, Swizerland) in incubation buffer for 10 min. The
cells were collected by FACSCalibur (BD Pharmingen, San Diego, CA,
USA) following instrument set-up with controls (non-treated,
stained cells) and two washes in PBS. In this experiment, cells
with early apoptotic signals (stained with Annexin V) and cells
with late death signals (stained with PI) were quantified and
apoptotic cells were analyzed using the CellQuest software
(18,19). Each assay was performed in
triplicate.
Statistical analysis
Quantitative values were expressed as the mean ± SD.
Statistical analysis was performed using the Pearson method and a
Student's or Chi-squared test using SPSS 12.0 (for Windows; SPSS,
Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
SATB1 expression by in situ
hybridization
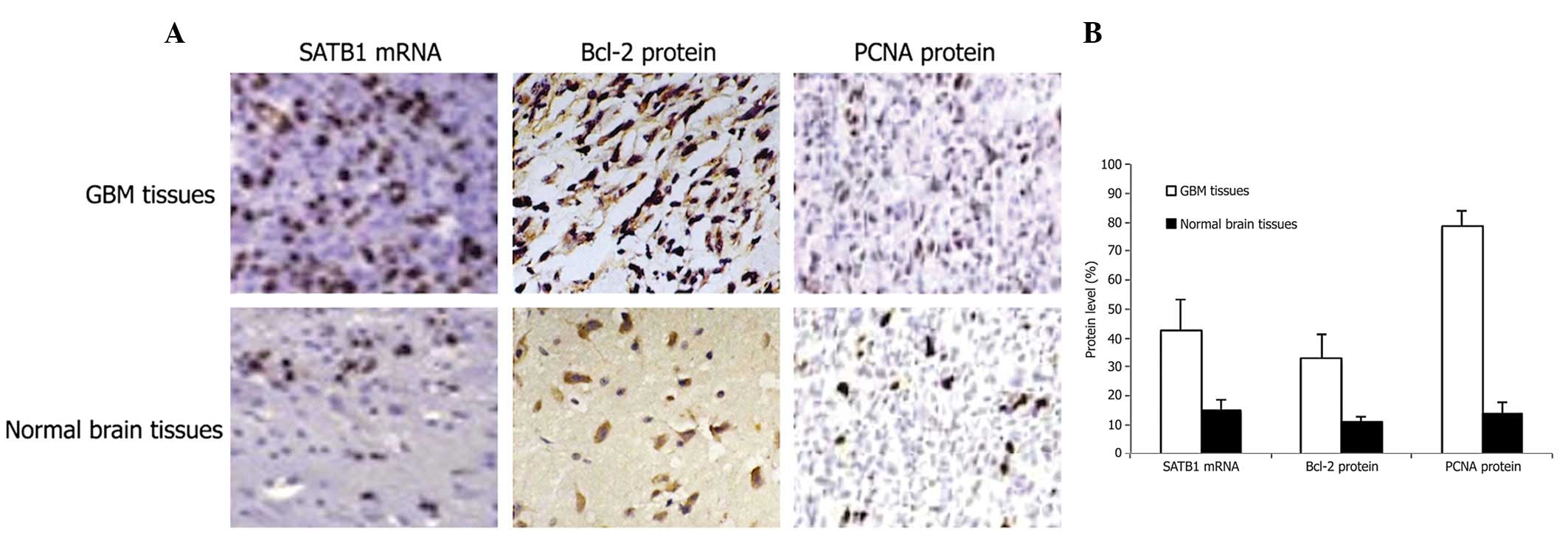
The majority of brown-positive staining for in
situ hybridization of SATB1 mRNA was homogeneously distributed
within the nucleolus (Fig. 1A).
SATB1 mRNA expression levels were found to be significantly higher
in GBM than in the normal brain tissues (Fig. 1B; P<0.01).
Immunohistochemical analysis of Bcl-2 and
PCNA protein expression
Immunohistochemical staining revealed Bcl-2 protein
expression in both the cytoplasm and cell membrane (Fig. 1A). PCNA protein expression was
primarily detected in the nucleolus (Fig. 1A). Expression of Bcl-2 and PCNA
protein was noted at significantly higher levels in GBM tissues
than in normal brain tissues (Fig.
1B, P<0.01).
Measurement of apoptosis by flow
cytometry
To quantify apoptotic cell death in tissues,
~1×106 cells were double stained with Annexin V-FITC and
PI at various times post-transfection. Apoptotic cell death was
detected in GBM and normal brain tissues (Fig. 2A). FACS analysis identified a
significantly higher number of apoptotic cells in GBM tissues than
normal brain tissues (Fig. 2B;
P<0.01).
Correlation between SATB1 and Bcl-2
expression and clinical characteristics of GBM
SATB1 mRNA and Bcl-2 protein expression levels were
associated with the survival rate of patients (P<0.01), but were
not associated with patient gender, age and tumor size and site
(Table I).
 | Table ICorrelation of SATB1 and Bcl-2
expression with clinical variables of GBM. |
Table I
Correlation of SATB1 and Bcl-2
expression with clinical variables of GBM.
| Variables | n | SATB1 (%) | P-value | Bcl-2 (%) | P-value |
|---|
| Gender |
| Male | 40 | 43.63±7.23 | 0.332 | 33.24±5.75 | 0.558 |
| Female | 30 | 41.83±8.14 | 32.35±6.88 | | |
| Age (years) |
| ≥46 | 34 | 41.92±6.96 | 0.302 | 32.23±6.21 | 0.388 |
| <46 | 36 | 43.74±7.65 | 33.45±5.54 | | |
| Tumor size
(cm3) |
| ≥4 | 41 | 44.12±7.45 | 0.109 | 34.02±7.12 | 0.103 |
| <4 | 29 | 41.07±8.12 | 31.21±6.85 | | |
| Tumor site |
| Supratentorial | 45 | 42.79±7.52 | 0.919 | 32.80±5.23 | 0.906 |
| Infratentorial | 25 | 42.98±7.44 | 32.96±5.78 | | |
| Survival rate
(years) |
| ≥1 | 29 | 37.18±7.68 | 0.000 | 28.34±6.23 | 0.000 |
| <1 | 41 | 46.87±8.92 | 36.05±7.48 | | |
Correlation between SATB1 and Bcl-2
expression, PCNA and apoptosis
Statistical analysis revealed a positive correlation
between SATB1 mRNA and Bcl-2 protein levels (P<0.05) and between
SATB1 mRNA and PCNA protein levels (P<0.01). A negative
correlation was identified between SATB1 and apoptosis (P<0.01)
and between Bcl-2 and apoptosis (P<0.01). However, a positive
correlation was observed between Bcl-2 and PCNA (P<0.01),
whereas a negative correlation was found between PCNA and apoptosis
(P<0.01; Table II).
 | Table IICorrelation between SATB1, Bcl-2, PCNA
expression and apoptosis in GBM. |
Table II
Correlation between SATB1, Bcl-2, PCNA
expression and apoptosis in GBM.
| Variables | SATB1 | Bcl-2 | PCNA | Apoptosis |
|---|
| SATB1 | - | 0.542a | 0.615b | −0.534a |
| Bcl-2 | 0.542a | - | −0.536a | −0.586b |
| PCNA | 0.615b | −0.536a | - | −0.532a |
| Apoptosis | −0.534a | −0.586b | −0.532a | - |
Co-expression of SATB1 and Bcl-2
SATB1-positive cases (+) were divided into two
groups consisting of a survival rate <1 and ≥1 year. Differences
between the groups were evaluated according to the Bcl-2 protein
expression and identified as χ2=20.95 (P<0.001;
Table III).
 | Table IIICorrelation between SATB1, Bcl-2 and
survival time. |
Table III
Correlation between SATB1, Bcl-2 and
survival time.
| Survival time |
|---|
|
|
|---|
| Variables | ≥1 year | <1 year |
|---|
|
S+B+ | 6 | 29 |
|
S+B− | 16 | 4 |
Discussion
SATB1 is a tissue-specific nuclear matrix-attachment
DNA-binding protein, which is located on chromosome 3p23. SATB1 has
previously attracted considerable attention in the cancer research
field due to its high expression in tumor tissues of a variety of
malignancies (8–10), indicative of a crucial role in the
promotion of tumor growth and prediction of tumor prognosis. It was
previously demonstrated that overexpression of SATB1 correlates
with the metastatic potential of human gastric cancer and may be
suitable for use as a novel independent prognostic marker for the
prediction of gastric cancer outcome (20). Bcl-2 is expressed in various
tissues under normal conditions, with the physiological function of
the modulation of apoptotis and cell number balance (21). Two important factors of cell number
control are rate of apoptosis and proliferation (22,23).
Overexpression of SATB1 and/or Bcl-2 disturbs this balance and
contributes to the proliferation and anti-apoptotic functions of
the abnormal cell.
Results of the present study have demonstrated that
expression of SATB1 mRNA and Bcl-2 protein is significantly higher
in GBM tissues than in the normal brain tissues. With regard to
clinical features, expression of SATB1 and Bcl-2 was correlated
with patient survival, but was not associated with patient gender,
age and tumor size and site. Overexpression of SATB1 mRNA and Bcl-2
protein was higher in the survival <1 year group than the ≥1
year and a significant positive correlation between SATB1 and Bcl-2
was observed. We analyzed the correlation between SATB1, Bcl-2,
PCNA and apoptosis. A positive correlation between SATB1 mRNA and
PCNA was observed. A negative correlation between SATB1 mRNA and
apoptosis and between Bcl-2 and apoptosis was observed and a
positive correlation was found between Bcl-2 and PCNA. These data
suggest that SATB1 functions in the promotion of cell proliferation
and inhibition of apoptosis. Function of Bcl-2 is restricted to
inhibiting apoptosis. Consistent with this hypothesis, cases
positive for SATB1 and Bcl-2 were associated with poor prognosis,
thus, assessment of SATB1 and Bcl-2 co-expression may provide
useful information for the diagnosis, therapy and prognosis of
GBM.
Acknowledgements
This study was supported by grants from the
Innovation Program of Shanghai Municipal Education Commission
(12YZ046) and the New One Hundred Person Project of Shanghai Jiao
Tong University of School of Medicine (10XBR01).
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chu SH, Ma YB, Feng DF, Zhang H, Zhu ZA,
Li ZQ and Jiang PC: Correlation of low SLC22A18 expression with
poor prognosis in patients with glioma. J Clin Neurosci. 19:95–98.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chu SH, Feng DF, Ma YB, Zhang H, Zhu ZA,
Li ZQ and Jiang PC: Promoter methylation and downregulation of
SLC22A18 are associated with the development and progression of
human glioma. J Transl Med. 9:1562011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chu SH, Ma YB, Feng DF, Zhang H, Qiu JH
and Zhu ZA: Effect of 5-Aza-2′-deoxycytidine on SLC22A18 in glioma
U251 cells. Mol Med Rep. 5:138–141. 2012.
|
|
5
|
Yang FY, Teng MC, Lu M, Liang HF, Lee YR,
Yen CC, Liang ML and Wong TT: Treating glioblastoma multiforme with
selective high-dose liposomal doxorubicin chemotherapy induced by
repeated focused ultrasound. Int J Nanomedicine. 7:965–974. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kouzarides T: Histone acetylases and
deacetylases in cell proliferation. Curr Opin Genet Dev. 9:40–48.
1999. View Article : Google Scholar
|
|
7
|
Dickinson LA, Joh T, Kohwi Y and
Kohwi-Shigematsu T: A tissue-specific MAR/SAR DNA-binding protein
with unusual binding site recognition. Cell. 70:631–645. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han HJ, Russo J, Kohwi Y and
Kohwi-Shigematsu T: SATB1 reprogrammes gene expression to promote
breast tumour growth and metastasis. Nature. 452:187–193. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meng WJ, Yan H, Zhou B, Zhang W, Kong XH,
Wang R, Zhan L, Li Y, Zhou ZG and Sun XF: Correlation of SATB1
overexpression with the progression of human rectal cancer. Int J
Colorectal Dis. 27:143–150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen H, Takahara M, Oba J, Xie L, Chiba T,
Takeuchi S, Tu Y, Nakahara T, Uchi H, Moroi Y and Furue M:
Clinicopathologic and prognostic significance of SATB1 in cutaneous
malignant melanoma. J Dermatol Sci. 64:39–44. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu M, Xiao GG, Rong P, Zhang Z, Dong J,
Zhao H, Li H, Li Y, Pan J, Liu H, Wang W, Zha Q and Ju D:
Therapeutic effects of Radix Dipsaci, Pyrola Herb and
Cynomorium Songaricum on bone metabolism of ovariectomized
rats. BMC Complement Altern Med. 12:672012.
|
|
12
|
Chu SH, Yuan XH, Jiang PC, Li ZQ, Zhang J,
Wen ZH, Zhao SY, Chen XJ and Cao CJ: The expression of hepatocyte
growth factor and its receptor in brain astrocytomas. Zhonghua Yi
Xue Za Zhi. 85:835–838. 2005.(In Chinese).
|
|
13
|
Chu SH, Feng DF, Ma YB, Zhang H, Zhu ZA,
Li ZQ and Zhang ZH: Expression of HGF and VEGF in the cerebral
tissue of adult rats with chronic hydrocephalus after subarachnoid
hemorrhage. Mol Med Rep. 4:785–791. 2011.PubMed/NCBI
|
|
14
|
Chu SH, Ma YB, Feng DF, Zhang H, Qiu JH
and Zhu ZA: Elevated expression of solute carrier family 22 member
18 increases the sensitivity of U251 glioma cells to BCNU. Oncol
Lett. 2:1139–1142. 2011.PubMed/NCBI
|
|
15
|
Zhang H, Liu L, Huang G, Zhou L, Wu W,
Zhang T and Huang H: Protective effect of electroacupuncture at the
Neiguan point in a rabbit model of myocardial ischemia-reperfusion
injury. Can J Cardiol. 25:359–363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Demurtas A, Aliberti S, Bonello L, Di
Celle PF, Cavaliere C, Barreca A, Novero D and Stacchini A:
Usefulness of multiparametric flow cytometry in detecting composite
lymphoma: study of 17 cases in a 12-year period. Am J Clin Pathol.
135:541–555. 2011.PubMed/NCBI
|
|
17
|
Wang E, Hutchinson CB, Huang Q, Lu CM,
Crow J, Wang FF, Sebastian S, Rehder C, Lagoo A, Horwitz M,
Rizzieri D, Yu J, Goodman B, Datto M and Buckley P: Donor
cell-derived leukemias/myelodysplastic neoplasms in allogeneic
hematopoietic stem cell transplant recipients: a clinicopathologic
study of 10 cases and a comprehensive review of the literature. Am
J Clin Pathol. 135:525–540. 2011. View Article : Google Scholar
|
|
18
|
Chu SH, Feng DF, Zhang H, Chen ET, Duan
ZX, Li XY, Li J, Ma YB, Zhu ZA and Qiu JH: c-Met-targeted RNA
interference inhibits growth and metastasis of glioma U251 cells in
vitro. J Neurooncol. 93:183–189. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chu S, Yuan X, Li Z, Jiang P and Zhang J:
C-Met antisense oligodeoxynucleotide inhibits growth of glioma
cells. Surg Neurol. 65:533–538. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu X, Cheng C, Zhu S, Yang Y, Zheng L,
Wang G, Shu X, Wu K, Liu K and Tong Q: SATB1 is an independent
prognostic marker for gastric cancer in a Chinese population. Oncol
Rep. 24:981–987. 2010.PubMed/NCBI
|
|
21
|
Wensveen FM, Alves NL, Derks IA, Reedquist
KA and Eldering E: Apoptosis induced by overall metabolic stress
converges on the Bcl-2 family proteins Noxa and Mcl-1. Apoptosis.
16:708–721. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang MS, Hu AH, Qiu H, Xiong HH and Chen
Y: The correlation between IGF-II and Bcl-2 expression in
colorectal adenocarcinoma. Med Oncol. 29:928–932. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
An YL, Nie F, Wang ZY and Zhang DS:
Preparation and characterization of realgar nanoparticles and their
inhibitory effect on rat glioma cells. Int J Nanomedicine.
6:3187–3194. 2011. View Article : Google Scholar : PubMed/NCBI
|