Introduction
Rho-associated kinase (ROCK) is a serine/threonine
kinase and one of the major downstream effectors of the small
GTPase RhoA. The RhoA/ROCK pathway is closely correlated with the
pathogenesis of several central nervous system (CNS) disorders and
is involved in a number of aspects of neuronal functions, including
neurite outgrowth and retraction (1). The effects of these axon growth
inhibitors are reversed by blocking the RhoA/ROCK pathway in
vitro (2–4), and inhibition of the RhoA/ROCK
pathway promotes axon regeneration and functional recovery in the
injured CNS in vivo (2,5,6).
Erythropoietin (EPO) has been found to act on the
CNS as a neurotrophic and neuroprotective factor, particularly in
conditions of neural damage, such as hypoxia, ischemia or brain
hemorrhage (7–10). Previous studies in animal models
have indicated that EPO is effective in enhancing neurological
recovery following experimental spinal cord injury (11–13).
EPO has also been shown to protect retinal ganglion cells (RGCs) in
rat models of glaucoma (14,15),
axotomy-induced degeneration (16,17)
and retinal ischemia (18) and to
promote the axonal regeneration of RGCs following optic nerve
transection (19). Previous
studies also showed that there is crosstalk between hypoxia
inducible factor-1 (HIF-1) and the ROCK pathways in neuronal
differentiation of mesenchymal stem cells, neurospheres and in PC12
neurite outgrowth (20), and that
EPO is one of the major target genes of HIF-1. Therefore, crosstalk
may also exist between EPO and the RhoA/ROCK pathway. There are,
however, few data available concerning the effects of EPO on the
RhoA/ROCK pathway. Therefore, the aim of the present study was to
clarify whether EPO regulates the RhoA/ROCK protein expression in
rat retinal explants cultured with glutamate.
Materials and methods
Animals
Animals included in this study were treated in
accordance with the ARVO Statement for the Use of Animals in
Ophthalmic and Vision Research. In addition, the Ethics Committee
of Ruijin Hospital (Shanghai, China) approved the experiments.
Sprague-Dawley (SD) rat pups were used in all experiments, kept
under conditions of constant temperature and humidity and fed by
their mothers. The day of birth was counted as postnatal day (P)0
and P2-3 rats were used in our experiment. A total of 150 rats was
used for this study.
Retinal explant dissection
The SD rats were sacrificed on P2-3 by decapitation.
Tissue soaked in 70% ethanol was used to wipe clean and wrap the
removed heads. The wrapped heads were transported into a culture
room to a laminar flow cabinet from which point onwards all
handling was performed aseptically. The eyes were enucleated and
incubated in serum-free R16 nutrient medium. The anterior segment,
vitreous body and sclera were then removed and the retinal explants
were collected. Approximately 20 eyes were collected for each
experiment.
Retinal explant culture
The complete list of chemicals making up the
originally developed R16 nutrient medium for brain tissue
(Gibco/BRL, Carlsbad, CA, USA) has been published previously
(21,22). The R16 powder is composed of 41
ingredients that can be divided into three groups: group 1
consisted of salts; group 2 included the amino acids with the
exception of the potentially neurotoxic amino acids glutamate and
aspartate; and group 3 included sugars and vitamins. The retinal
explants were cultured in 12-well culture plates with serum-free
R16 nutrient medium. Each well contained 4 retinal explants which
were cultured in a humidified incubator at 37°C in an atmosphere of
5% CO2-95% O2.
Drug treatment
EPO was prepared by dissolving 10 μg EPO (R&D
Systems, Minneapolis, MN, USA) in 200 μl distilled water (8.3
μg=1,000 units EPO). The retinal explants were cultured for 24 h as
described above and then divided into three groups: the control,
glutamate and glutamate + EPO groups. The retinal explants in the
control group were continually cultured with serum-free R16
nutrient medium; the retinal explants in the glutamate group were
continually cultured with serum-free R16 nutrient medium containing
5 mM/l glutamate (Sigma-Aldrich, St. Louis, MO, USA); and the
retinal explants in the glutamate + EPO group were continually
cultured with serum-free R16 nutrient medium containing 5 mM/l
glutamate and 6.0 U/ml EPO. The retinal explants in the three
groups were continually cultured for another 72 h. The doses of
glutamate and EPO used in the present study were selected according
to our previous study in which it was shown that 6.0 U/ml EPO
significantly improved the survival of cultured retinal neurocytes
incubated with 5 mM/l glutamate (23).
Reverse transcription-polymerase chain
reaction (RT-PCR)
The mRNA levels of the genes were measured by
RT-PCR. Total RNA was isolated from the individual samples using
the TRIzol® reagent (Invitrogen, Carlsbad, CA, USA). The
concentration and purity of the preparations were determined by
measuring the absorbance at 260/280 nm using a spectrophotometer
(Beckman Coulter, Miami, FL, USA). Total RNA was
reverse-transcribed into cDNA in a 20-μl reaction volume containing
2 μg RNA, 4 μl 5X M-MLV buffer, 2 μl dNTP, 1 μl random hexamer
primer, 0.5 μl RNase inhibitor and 1 μl M-MLV RTase. The reactions
were performed at 25°C for 10 min, at 42°C for 60 min and at 70°C
for 10 min.
The nucleotide sequences of the primers were based
on previously published sequences (24,25).
The primer sequences used for RT-PCR were: RhoA, 5′-GTGATTGTTGGT
GATGGAGC-3′ and 5′-CTCGTGGCCATCTCAAAAAC-3′; ROCK-1,
5′-TGCGGGAGTTACAAGATCAGCT-3′ and 5′-TTTCCGTCAGTCTCATCAGCAC-3′;
ROCK-2, 5′-TCTG AAAGGAGGGACCGAACC-3′ and 5′-GTTCCTGTTT
GTGTCGAGCCATCA-3′; glyceraldehyde 3-phosphate dehydrogenase
(GAPDH), 5′-ATGGGGAAGGTGAAGGTCGG-3′ and 5′-CAGGAGGCATTGCTGATGAT-3′.
The PCR protocol comprised an initial incubation for 5 min at 94°C;
30 cycles (for RhoA, ROCK-1 and ROCK-2) or 25 cycles (for GAPDH) of
45 sec at 94°C, 45 sec at 55°C and 2 min at 72°C and a final
incubation for 7 min at 72°C. The PCR products were separated by 2%
agarose gel electrophoresis and stained with 0.5 μg/ml ethidium
bromide, and the band signals were exposed to ultraviolet radiation
before they were scanned and quantified with a gel image analyzer
(GelDoc Quantity One; Bio-Rad, Hercules, CA, USA). Band intensities
were quantified and normalized against those of GAPDH. Each set of
experiments was repeated in triplicate for statistical
analysis.
Western blot analysis
Total retinal protein was extracted from pulverized
samples using modified radioimmunoprecipitation (modified RIPA)
buffer with a Halt™ protease and phosphatase inhibitor cocktail
(Thermo Scientific, Rockford, IL, USA). The protein concentrations
were determined by the Bradford protein assay (Bio-Rad). Each
sample contained 4 retinal explants. Equal amounts of protein (20
μg/lane) were separated on polyacrylamide gels and then
electrotransferred onto a nitrocellulose membrane (Amersham,
Buckinghamshire, UK). After blocking for 3 h in Tris-buffered
saline with 0.1% Tween-20 (TBST) and 3% bovine serum albumin (BSA),
the membranes were incubated overnight at 4°C with primary
antibodies (RhoA, dilution 1:50, sc-418; ROCK-1, dilution 1:50,
sc-6056; ROCK-2, dilution 1:50, sc-5561; Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) in TBST containing 3% BSA. The membranes
were then washed and incubated with alkaline phosphatase-conjugated
secondary antibodies in TBST for 2 h and developed using nitro blue
tetrazolium chloride (NBT)/5-bromo-4-chloro-3-indolyl phosphate
(BCIP) substrate (Promega, Madison, WI, USA). The densities of the
bands on the membrane were scanned and analyzed using Image Pro
Plus version 6.0 (Media Cybernetics, Silver Spring, MD, USA). Each
set of experiments was repeated in triplicate for statistical
analysis.
RhoA activity assay
Active RhoA was assayed in tissue lysates using a
Rho activation assay kit (Upstate Biotechnology, Milton Keynes,
UK), following the manufacturer’s instructions as described
previously (26,27). Briefly, the retinal protein (200
μg) was mixed with glutathione-S-transferase (GST)-Rho-binding
domain (RBD) fusion protein (20 μl) in an ice bath and the mixture
was incubated for 45 min at 4°C with intermittent mixing. After the
mixture was centrifuged (13,000 × g) for 10 min at 4°C, the
precipitate was suspended with Mg2+ lysis/wash buffer
(500 μl) and centrifuged for 10 min. This procedure was repeated
three times. The final precipitate was used for active RhoA assay
by western blot analysis. Each set of experiments was repeated in
triplicate for statistical analysis.
Statistical analysis
Data were presented as the mean ± standard
deviation, unless otherwise stated. Statistical analyses were
performed using the SPSS software (IBM SPSS Statistics 19.0, SPSS,
Inc., Chicago, IL, USA). To compare data among three groups,
one-way analysis of variance (ANOVA) was performed followed by post
hoc tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
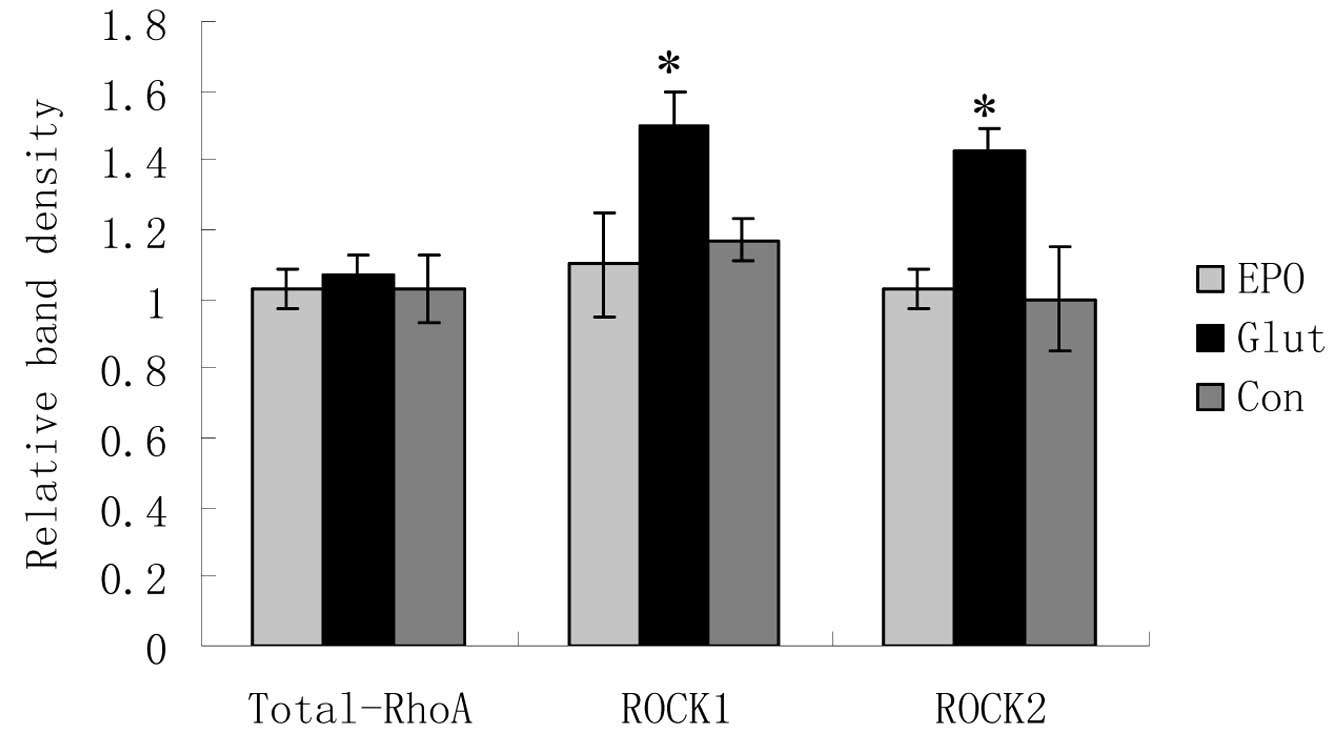
Effect of EPO on RhoA, ROCK1 and ROCK2
mRNA expression in retinal explants cultured with glutamate
RT-PCR analysis revealed that the RhoA mRNA
expression did not differ substantially between the control,
glutamate and glutamate + EPO groups. Compared with the control
group, the glutamate increased ROCK1 and ROCK2 mRNA expression in
cultured retinal explants (P<0.05; Fig. 1). The ROCK1 and ROCK2 mRNA
expression in the glutamate + EPO group was significantly lower
than that in the simple glutamate group (P<0.05; Fig. 1) and similar to that in the control
group.
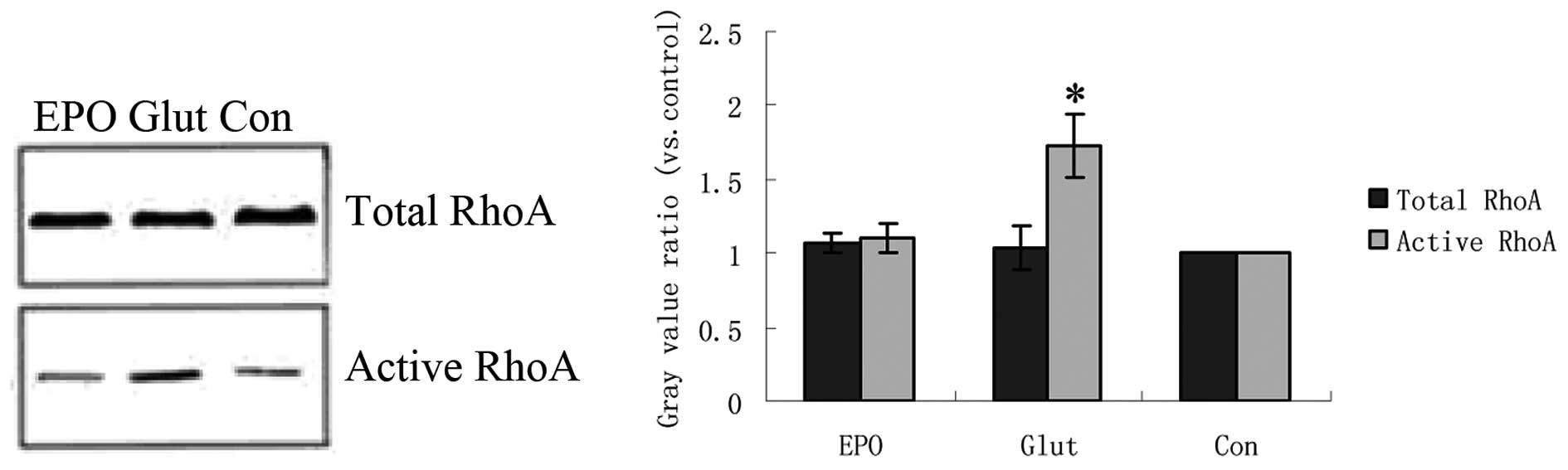
Effect of EPO on RhoA, ROCK1 and ROCK2
protein expression in retinal explants cultured with glutamate
Western blot analysis revealed that the protein
expression of total-RhoA did not differ substantially between the
control, glutamate and glutamate + EPO groups, which was in
accordance with the expression of the RhoA mRNA (Fig. 2). However, compared with the
control group, the glutamate increased active RhoA expression in
cultured retinal explants (P<0.05; Fig. 2). The protein expression of active
RhoA in the glutamate + EPO group was significantly lower than that
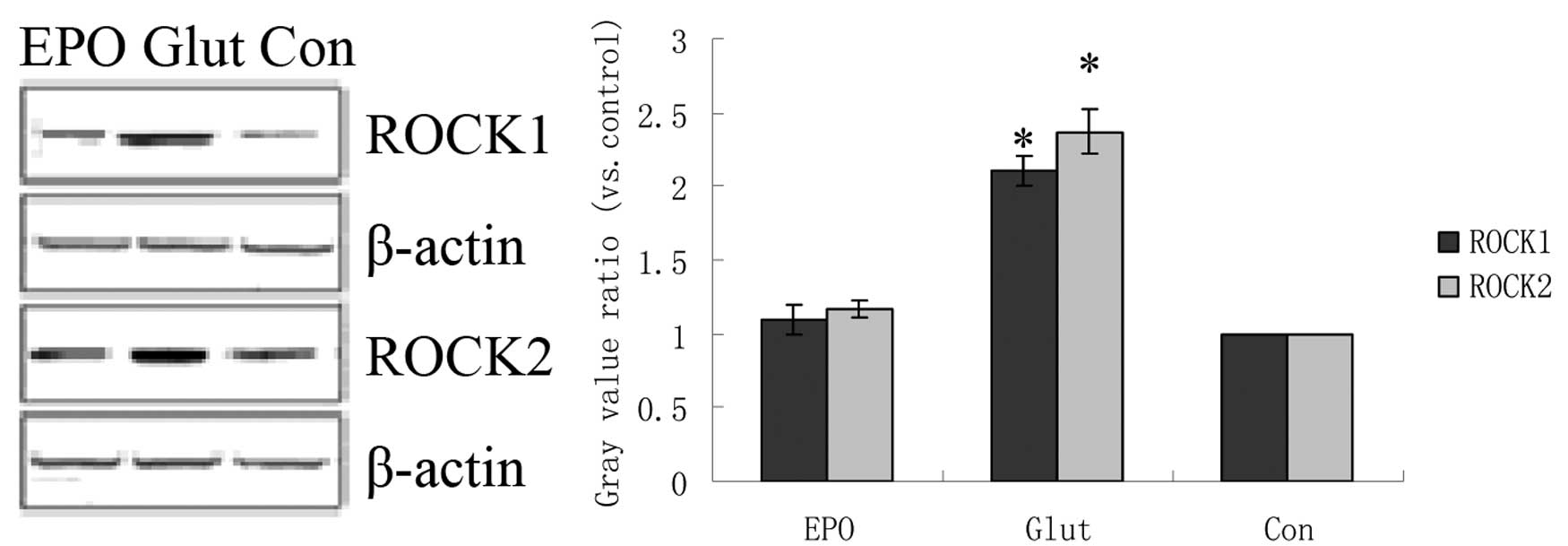
in the simple glutamate group (P<0.05; Fig. 2). Compared with the control group,
the glutamate increased ROCK1 and ROCK2 expression in cultured
retinal explants (P<0.05; Fig.
3). The protein expression of ROCK1 and ROCK2 in the glutamate
+ EPO group was significantly lower than that in the simple
glutamate group (P<0.05; Fig.
3), and similar to that in the control group.
Discussion
A number of studies have confirmed that Rho and its
associated signaling molecules are involved in and mediate the
biological processes of axon regeneration, extension and fiber
projection (28–31). Rho regulates the cell actin
cytoskeleton by its downstream effective factor ROCK, which is
extensively involved in the biological processes of cell migration,
movement, apoptosis, gene transcription and nerve regeneration
(32). An important reason that
axon regeneration is difficult following CNS damage in adult
mammals is the existence of certain growth suppression molecules in
the damaged environment. Three types of molecules derived from
myelin which can suppress axon growth have been identified thus
far: Nogo-A, myelin-associated glycoprotein (MAG) and
oligodendrocyte-myelin glycoprotein (Omgp) (1). Previous studies indicate that Nogo-A,
MAG and Omgp may activate Rho by common or different pathways and
subsequently cause growth cone collapse (33,34).
Our previous studies demonstrated that EPO had a
neuroprotective effect in vivo (15) and a neurite outgrowth promotion
effect on retinal neurons in vitro (23). However, the mechanism of the axonal
regeneration effect of EPO on retinal neurons has not been fully
clarified. A previous study has shown that EPO promotes the
regeneration of adult CNS neurons via activation of the JAK2/STAT3
and PI3K/AKT pathways, and that EPO-facilitated neuritogenesis is
paralleled by the upregulation of Bcl-X(L) (35). However, the induced expression of
Bcl-X(L) alone cannot completely neutralize the inhibition of
axonal growth (36). Thus, the
axonal regeneration mechanism of EPO in damaged RGCs in adult rats
may also involve other signaling pathways or factors. To gain a
deeper understanding of the EPO-dependent axonal regeneration
process, we studied the effects of EPO on RhoA/ROCK expression in
rat retinal explants cultured with 5 mM/l glutamate. Our results
show that glutamate increased the active RhoA, ROCK1 and ROCK2
expression in cultured retinal explants, and that the expression of
active RhoA, ROCK1 and ROCK2 in the glutamate + EPO group was
significantly lower than that in the simple glutamate group, and
similar to that in the control group. This suggests that EPO
downregulated the active RhoA, ROCK1 and ROCK2 expression in
retinal explants cultured with glutamate.
There were a few limitations in our study. Firstly,
the present study did not explore the axonal regeneration of
retinal neurons, making it difficult to determine the relationship
between the RhoA/ROCK expression and axonal regeneration. Secondly,
the study did not determine where the active RhoA, ROCK1 and ROCK2
expression occurred in the cultured retinal explants. Further
investigations are required to demonstrate the locations of active
RhoA, ROCK1 and ROCK2 expression and the correlation between
RhoA/ROCK expression and the axonal regeneration of retinal
neurons.
In conclusion, our results suggest that glutamate
increases the active RhoA, ROCK1 and ROCK2 expression in cultured
retinal explants, and that EPO downregulates the active RhoA, ROCK1
and ROCK2 expression in retinal explants cultured with
glutamate.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China (Nos. 81070728 and 81000373), Shanghai
Natural Science Foundation (Nos. 08ZR1413900 and 11ZR1422000),
Shanghai Municipal Education Committee Project (No. 10YZ38),
Shanghai leading Academic Discipline Project (No. S30205) and
Shanghai ‘Science and Technology Innovation Action Plan’ Basic
Research Key Project (Nos. 11JC1407700 and 11JC1407701).
References
|
1
|
Tan HB, Zhong YS, Cheng Y and Shen X:
Rho/ROCK pathway and neural regeneration: a potential therapeutic
target for central nervous system and optic nerve damage. Int J
Ophthalmol. 4:652–657. 2011.PubMed/NCBI
|
|
2
|
Lingor P, Teusch N, Schwarz K, Mueller R,
Mack H, Bähr M and Mueller BK: Inhibition of Rho kinase (ROCK)
increases neurite outgrowth on chondroitin sulphate proteoglycan in
vitro and axonal regeneration in the adult optic nerve in vivo. J
Neurochem. 103:181–189. 2007.PubMed/NCBI
|
|
3
|
Lingor P, Tönges L, Pieper N, Bermel C,
Barski E, Planchamp V and Bähr M: ROCK inhibition and CNTF interact
on intrinsic signalling pathways and differentially regulate
survival and regeneration in retinal ganglion cells. Brain. 131(Pt
1): 250–263. 2008.PubMed/NCBI
|
|
4
|
Zhang Z, Ottens AK, Larner SF, Kobeissy
FH, Williams ML, Hayes RL and Wang KK: Direct Rho-associated kinase
inhibition [correction of inhibition] induces cofilin
dephosphorylation and neurite outgrowth in PC-12 cells. Cell Mol
Biol Lett. 11:12–29. 2006.
|
|
5
|
Dergham P, Ellezam B, Essagian C,
Avedissian H, Lubell WD and McKerracher L: Rho signaling pathway
targeted to promote spinal cord repair. J Neurosci. 22:6570–6577.
2002.PubMed/NCBI
|
|
6
|
Chan CC, Khodarahmi K, Liu J, Sutherland
D, Oschipok LW, Steeves JD and Tetzlaff W: Dose-dependent
beneficial and detrimental effects of ROCK inhibitor Y27632 on
axonal sprouting and functional recovery after rat spinal cord
injury. Exp Neurol. 196:352–364. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sakanaka M, Wen TC, Matsuda S, Masuda S,
Morishita E, Nagao M and Sasaki R: In vivo evidence that
erythropoietin protects neurons from ischemic damage. Proc Natl
Acad Sci USA. 95:4635–4640. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bernaudin M, Marti HH, Roussel S, Divoux
D, Nouvelot A, Mackenzie ET and Petit E: A potential role for
erythropoietin in focal permanent cerebral ischemia in mice. J
Cereb Blood Flow Metab. 19:643–651. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brines ML, Ghezzi P, Keenan S, Agnello D,
de Lanerolle NC, Cerami C, Itri LM and Cerami A: Erythropoietin
crosses the blood-brain barrier to protect against experimental
brain injury. Proc Natl Acad Sci USA. 97:10526–10531. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Calapai G, Marciano MC, Corica F, Allegra
A, Parisi A, Frisina N, Caputi AP and Buemi M: Erythropoietin
protects against brain ischemic injury by inhibition of nitric
oxide formation. Eur J Pharmacol. 401:349–356. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Celik M, Gökmen N, Erbayraktar S,
Akhisaroglu M, Konakc S, Ulukus C, Genc S, Genc K, Sagiroglu E,
Cerami A and Brines M: Erythropoietin prevents motor neuron
apoptosis and neurologic disability in experimental spinal cord
ischemic injury. Proc Natl Acad Sci USA. 99:2258–2263. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gorio A, Gökmen N, Erbayraktar S, Yilmaz
O, Madaschi L, Cichetti C, Di Giulio AM, Vardar E, Cerami A and
Brines M: Recombinant human erythropoietin counteracts secondary
injury and markedly enhances neurological recovery from
experimental spinal cord trauma. Proc Natl Acad Sci USA.
99:9450–9455. 2002. View Article : Google Scholar
|
|
13
|
Sekiguchi Y, Kikuchi S, Myers RR and
Campna WM: ISSLS prize winner: erythropoietin inhibits spinal
neuronal apoptosis and pain following nerve root crush. Spine.
28:2577–2584. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsai JC, Wu L, Worgul B, Forbes M and Cao
J: Intravitreal administration of erythropoietin and preservation
of retinal ganglion cells in an experimental rat model of glaucoma.
Curr Eye Res. 30:1025–1031. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhong YS, Liu XH, Cheng Y and Min YJ:
Erythropoietin with retrobulbar administration protects retinal
ganglion cells from acute elevated intraocular pressure in rats. J
Ocular Pharmacol Ther. 24:453–459. 2008. View Article : Google Scholar
|
|
16
|
Weishaupt JH, Rohde G, Pölking E, Siren
AL, Ehrenreich H and Bähr M: Effect of erythropoietin
axotomy-induced apoptosis in rat retinal ganglion cells. Invest
Ophthalmol Vis Sci. 45:1514–1522. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kilic Ü, Kilic E, Soliz J, Bassetti Cl,
Gassmann M and Hermann DM: Erythropoietin protects from
axotomy-induced degeneration of retinal ganglion cells by
activating ERK-1/-2. FASEB J. 19:249–251. 2005.PubMed/NCBI
|
|
18
|
Junk AK, Mammis A, Savitz SI, Singh M,
Roth S, Malhotra S, Rosenbaum PS, Cerami A, Brines M and Rosenbaum
DM: Erythropoietin administration protects retinal neurons from
acute ischemia reperfusion injury. Proc Natl Acad Sci USA.
99:10659–10664. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
King CE, Rodger J, Bartlett C, Esmaili T,
Dunlop SA and Beazley LD: Erythropoietin is both neuroprotective
and neuroregenerative following optic nerve transection. Exp
Neurol. 205:48–55. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pacary E, Tixier E, Coulet F, Roussel S,
Petit E and Bernaudin M: Crosstalk between HIF-1 and ROCK pathways
in neuronal differentiation of mesenchymal stem cells, neurospheres
and in PC12 neurite outgrowth. Mol Cell Neurosci. 35:409–423. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Romijn HJ: Development and advantages of
serum-free, chemically defined nutrient media for culturing of
nerve tissue. Biol Cell. 63:263–268. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Caffé AR, Ahuja P, Holmqvist B, Azadi S,
Forsell J, Holmqvist I, Söderpalm AK and van Veen T: Mouse retina
explants after long-term culture in serum free medium. J Chem
Neuroanat. 22:263–273. 2001.PubMed/NCBI
|
|
23
|
Zhong Y, Yao H, Deng L, Cheng Y and Zhou
X: Promotion of neurite outgrowth and protective effect of
erythropoietin on the retinal neurons of rats. Graefes Arch Clin
Exp Ophthalmol. 245:1859–1867. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim BK, Kim HM, Chung KS, Kim DM, Park SK,
Song A, Won KJ, Lee K, Oh YK, Lee K, et al: Upregulation of RhoB
via c-Jun N-terminal kinase signaling induces apoptosis of the
human gastric carcinoma NUGC-3 cells treated with NSC12618.
Carcinogenesis. 32:254–261. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nakamura M, Nagano T, Chikama T and
Nishida T: Role of the small GTP-binding protein Rho in epithelial
cell migration in the rabbit cornea. Invest Ophthalmol Vis Sci.
42:941–947. 2001.PubMed/NCBI
|
|
26
|
Dubreuil CI, Winton MJ and McKerracher L:
Rho activation patterns after spinal cord injury and the role of
activated Rho in apoptosis in the central nervous system. J Cell
Biol. 162:233–243. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ahmed Z, Suggate EL, Brown ER, Dent RG,
Armstrong SJ, Barrett LB, Berry M and Logan A: Schwann cell-derived
factor-induced modulation of the NgR/p75NTR/EGFR axis disinhibits
axon growth through CNS myelin in vivo and in vitro. Brain. 129(Pt
6): 1517–1533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sivasankaran R, Pei J, Wang KC, Zhang YP,
Shields CB, Xu XM and He Z: PKC mediates inhibitory effects of
myelin and chondroitin sulfate proteoglycans on axonal
regeneration. Nat Neurosci. 7:261–268. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou FQ, Walzer M, Wu YH, Zhou J, Dedhar S
and Snider WD: Neurotrophins support regenerative axon assembly
over CSPGs by an ECM-integrin-independent mechanism. J Cell Sci.
119(Pt 13): 2787–2796. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Douglas MR, Morrison KC, Jacques SJ,
Leadbeater WE, Gonzalez AM, Berry M, Logan A and Ahmed Z:
Off-target effects of epidermal growth factor receptor antagonists
mediate retinal ganglion cell disinhibited axon growth. Brain.
132(Pt 11): 3102–3121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duffy P, Schmandke A, Schmandke A,
Sigworth J, Narumiya S, Cafferty WB and Strittmatter SM:
Rho-associated kinase II (ROCKII) limits axonal growth after trauma
within the adult mouse spinal cord. J Neurosci. 29:15266–15276.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bhadriraju K, Yang M, Alom Ruiz S, Pirone
D, Tan J and Chen CS: Activation of ROCK by RhoA is regulated by
cell adhesion, shape, and cytoskeletal tension. Exp Cell Res.
313:3616–3623. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schimchowitsch S and Cassel JC: Polyamine
and aminoguanidine treatments to promote structural and functional
recovery in the adult mammalian brain after injury: a brief
literature review and preliminary data about their combined
administration. J Physiol Paris. 99:221–231. 2006. View Article : Google Scholar
|
|
34
|
Schweigreiter R, Walmsley AR, Niederöst B,
Zimmermann DR, Oertle T, Casademunt E, Frentzel S, Dechant G, Mir A
and Bandtlow CE: Versican V2 and the central inhibitory domain of
Nogo-A inhibit neurite growth via p75NTR/NgR-independent pathways
that converge at RhoA. Mol Cell Neurosci. 27:163–174. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kretz A, Happold CJ, Marticke JK and
Isenmann S: Erythropoietin promotes regeneration of adult CNS
neurons via Jak2/Stat3 and PI3K/AKT pathway activation. Mol Cell
Neurosci. 29:569–579. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dietz GP, Dietz B and Bähr M: Bcl-x(L)
increases axonal numbers but not axonal elongation from rat retinal
explants. Brain Res Bull. 70:117–123. 2006. View Article : Google Scholar : PubMed/NCBI
|