Introduction
CD4+CD25+Foxp3+regulatory T cells
(Tregs) have a broad immunosuppressive capacity and are central in
regulating self-tolerance and homeostasis in the immune system
(1). Th17 cells are important
contributors to the inflammatory response (2). A number of studies have demonstrated
that Th17 cells and Tregs exhibit unique profiles of cytokines and
regulatory transcription factors (3–5). The
differentiation and development of Th17 cells is dependent on the
transcription factor, ROR-γt (6,7),
while Tregs require TGF-β and the forkhead transcription factor,
Foxp3 (8).
Although a number of T cell lineages exhibit
distinct gene expression and regulation signatures, each subset
retains substantial developmental plasticity (4). However, it is suggested that Th17
cells and Tregs exhibited greater developmental plasticity than Th1
and Th2 cells (9). A number of
studies have shown that Tregs are able to differentiate into
CD4+Foxp3+interleukin-17 (IL-17)+
T cells in the colitic microenvironment, colon carcinoma,
psoriasis, allergic rhinitis and polyposis (10–12).
However, the biological mechanisms of
CD4+Foxp3+IL-17+ T cells remain
poorly understood in allergic asthma. The mechanisms of Treg
suppression on effector T cells (Teffs) remain unclear, but include
cell-cell contact and the release of the soluble mediators, IL-10
and TGF-β. Surface molecules linked to Treg suppression, include
the CCR4 and CCR8 chemokine receptors, CTLA-4, the CD103 integrin,
the CD62L selectin and CD127 (13–17).
CD39 is an ectonucleotidase that catalyzes ATP/ADP to form AMP,
which is cleared by CD73 to form adenosine and CD39. CD73
expression was observed on the surface of CD4+ T cells,
particularly in a subpopulation of Tregs (18,19).
Extracellular ATP has multiple proinflammatory effects, including
promoting the secretion of IL-17 and the maturation of dendritic
cells, and inducing the apoptosis of Tregs (18–20);
thus, its removal may result in anti-inflammatory effects.
Adenosine, which functions via the A2A adenosine
receptor expressed on the surface of T cells, is critical in
inhibiting the functions of activated Teffs (21). A previous study observed that
CD39+ Treg cells from patients with multiple sclerosis
(MS) suppressed pathogenic Th17 cells (20). However, the involvement of
CD39+ Tregs in allergic asthma remains unclear.
In the current study, the unrecognized functions of
CD39 expressed by CD4+ T cells and Tregs in controlling
Th17 cells were investigated. An understanding of these functions
may demonstrate the uncontrolled function of pathogenic T cells in
allergic asthma.
Subjects and methods
Subjects and sample preparation
Patients with allergic asthma from outpatient
clinics at the Department of Pulmonary Medicine, Ruijin Hospital
(Shanghai, China) were consecutively recruited into the study.
Asthma severity was assessed based on the Global Initiative for
Asthma (GINA) (22). All
participants performed a forced expiratory volume in the first
second (FEV1 %pred) test, asthma control questionnaire (ACQ) and
allergen tests. Patients had not been treated with systemic
glucocorticoids for one month prior to the study and had never been
treated with other immunosuppressive agents or undergone
desensitization therapy. Healthy donors, with normal pulmonary
function and negative allergy tests, were selected as normal
controls. Heparinized peripheral venous blood (8 ml) was collected
from each participant. Written informed consent was obtained from
all individuals and the study received ethical approval from the
Research Ethics Board of Ruijin Hospital, Shanghai Jiao Tong
University School of Medicine (Shanghai, China).
Flow cytometry and antibodies
Expression markers on the surface of T cells were
determined by fluorescence-activated cell sorting analysis,
following surface staining or intracellular staining with specific
anti-human antibodies. Antibodies included CD4/CD25-fluorescein
isothiocyanate (FITC)/allophycocyanin (APC), CD39-PE-cy7,
CD73-Percp5.5, Foxp3-PE, IL-17A-FITC and CD4-PE-cy5 (eBiosciences,
San Diego, CA, USA).
For the analysis of Th17 cells and
CD4+Foxp3+IL-17+ T cells, 1 ml
blood was added to 1 ml Dulbecco’s modified Eagle’s medium
(Gibco-BRL, Carlsbad, CA, USA) and the mixture was stimulated with
20 ng/ml phorbol 12-myristate-13-acetate and 1 μg/ml ionomycin in
the presence of 2 mmol/ml monensin (eBiosciences). Following
culture (4 h; 37°C; 5% CO2), red cell lysing solution
was added to lyse red blood cells and cells were washed once in
phosphate-buffered saline. Cells were incubated with CD4-PE-cy5 at
4°C for 30 min, fixed and permeabilized with Perm/Fix solution
(eBiosciences) according to the manufacturer’s instructions, and
were stained with Foxp3-PE and IL-17A-FITC. For the analysis of
CD39+ and CD73+ Tregs, cells were incubated
with CD4/CD25-FITC/APC and CD39-PE-cy7 or CD73-Percp5.5 at 4°C for
30 min, fixed and permeabilized with Perm/Fix solution, and stained
with Foxp3-PE.
Quantitative PCR (qPCR)
CD4+ T cells were obtained from
peripheral blood using a human CD4+ T cell enrichment
cocktail (Stem Cell Technologies Inc., Vancouver, BC, Canada) by
Ficoll-Hypaque density centrifugation. Total RNA in the
CD4+ T cells was isolated with TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA) and was reverse
transcribed into cDNA (Promega Corporation, Madison, WI, USA).
Primers were designed by Invitrogen Life Technologies and
synthesized by BioTNT (Shanghai, China), according to the
manufacturer’s instructions. For amplification, the SYBR-Green I
qPCR kit was used (BioTNT). Each reaction was run in triplicate on
the ABI villa7 real time PCR system (Invitrogen Life Technologies,
Carlsbad, CA, USA) and was normalized to housekeeping gene β-actin
transcripts. Specific primers used are listed in Table I.
 | Table IPrimer sequences of CD39, CD73,
Foxp3, ROR-γt and β-actin. |
Table I
Primer sequences of CD39, CD73,
Foxp3, ROR-γt and β-actin.
| Gene | Primer | Length (bp) |
|---|
| CD39 | 5′-CTG ATT CCT GGG
AGC ACA T-3′ | 143 |
| 5′-GAC ATA GGT GGA
GTG GGA GAG-3′ | |
| CD73 | 5′-TTC TAA ACA GCA
GCA TTC CT-3′ | 214 |
| 5′-AAC ATT TCA TCC
GTG TGT CT-3′ | |
| Foxp3 | 5′-ATG CGA CCC CCT
TTC ACC TAC-3′ | 155 |
| 5′-TGG CGG ATG GCG
TTC TTC-3′ | |
| ROR-γt | 5′-GGC TCC CTG GAT
GAA TAG AAT G-3′ | 190 |
| 5′-AGG CAG AGG CAG
AAA ATG TAA AG-3′ | |
| β-actin | 5′-AAG GTG ACA GCA
GTC GGT T-3′ | 195 |
| 5′-TGT GTG GAC TTG
GGA GAG G-3′ | |
Statistical analysis
GraphPad Prism 5 software (GraphPad Software, Inc.,
La Jolla, CA, USA) was used for statistical analysis. Homogeneity
of variance in the three groups was tested first. If each group
showed homogeneity, analysis was performed using one way analysis
of variance followed by Student-Newman-Keuls test and the data are
presented as the mean ± SD. When heteroscedasticity was present in
each group, data were analyzed using the Mann-Whitney test and are
presented as medians (interquartile range). Pearson’s correlation
was used to analyze the relevance. P<0.05 was considered to
indicate a statistically significant difference.
Results
General characteristics of subjects
According to the GINA guidelines, allergic
asthmatics may be divided into two subgroups, intermittent to mild
and moderate to severe. No significant differences were identified
in terms of age and gender between the patients and normal control
groups. FEV1 (%pred) in patients with moderate to severe asthma was
significantly lower than those with intermittent to mild asthma,
but the scores for ACQ were of the opposite trend (Tables II and III; note that the number of patients
differs between Tables II and
III as the patients were
recalled for the analysis in table
III and not all were available).
 | Table IICharacteristics of subjects for
analyzing the expression of CD39 and CD73 protein in
CD4+ T cells and Treg cells. |
Table II
Characteristics of subjects for
analyzing the expression of CD39 and CD73 protein in
CD4+ T cells and Treg cells.
|
Characteristics | Normal control
(n=20) | Intermittent to
mild asthma (n=23) | Moderate to severe
asthma (n=15) |
|---|
| Allergen test | − | + | + |
| Age (years) | 35.45±11.9 | 39.52±12.28 | 43.60±15.07 |
| Gender
(male/female) | 6/14 | 11/12 | 4/11 |
| FEV1 (% pred) | 94.14±5.77 | 79.16±12.60a | 66.51±7.04ab |
| ACQ | − | 12.11±1.73 | 18.42±1.38b |
 | Table IIICharacteristics of subjects for
analyzing the expression of CD39, CD73, Foxp3 and ROR-γt mRNA in
CD4+ T cells. |
Table III
Characteristics of subjects for
analyzing the expression of CD39, CD73, Foxp3 and ROR-γt mRNA in
CD4+ T cells.
|
Characteristics | Normal control
(n=23) | Intermittent to
mild asthma (n=17) | Moderate to severe
asthma (n=12) |
|---|
| Allergen test | − | + | + |
| Age (years) | 37.26±11.88 | 40.24±11.92 | 44.33±14.29 |
| Gender
(male/female) | 10/13 | 7/10 | 3/9 |
| FEV1 (%pred) | 94.03±5.39 | 81.24±11.35a | 64.34±6.03ab |
| ACQ | − | 11.52±2.29 | 18.07±1.44b |
Tregs differentiate into
CD4+Foxp3+IL-17+ T cells in the
microenvironment of allergic asthma
The relative frequency of
CD4+Foxp3+IL-17+ T cells over
total Tregs was 4.69% (2.90–8.01) in healthy controls. However, the
frequency in patients with intermittent to mild asthma and moderate
to severe asthma was 6.10 (4.63–8.70) and 16.80%(9.13–32.65),
respectively. CD4+Foxp3+IL-17+ T
cells were detected in the peripheral blood of patients with
allergic asthma. Higher levels were observed in patients with
moderate to severe asthma compared with those with intermittent to
mild asthma (P<0.01) and healthy controls (P<0.01); however,
no significant difference was identified between intermittent to
mild asthma and healthy controls (Fig.
1).
Decreased CD39 and CD73 on the surface of
CD4+ T cells in allergic asthma contributes to the
imbalance of Th17 Tregs
Results demonstrated that the proportions of Th17
cells (23) and
CD4+Foxp3+IL-17+ T cells were
increased in asthma patients. Therefore, increased Th17 cells and
CD4+Foxp3+IL-17+ T cells were
hypothesized to be correlated with decreased
CD4+CD39+ T cells and
CD4+CD73+ T cells. Thus, the mRNA and protein
levels of CD39 and CD73 in CD4+ T cells were
investigated. The correlation between the levels CD39 and CD73
expressed by CD4+ T cells and Th17 cells,
CD4+Foxp3+IL-17+ T cells and
disease severity were analyzed.
CD39 mRNA in CD4+ T cells was
significantly lower in patients with moderate to severe asthma
compared with those with intermittent to mild asthma [0.15
(0.10–0.19)×10−3 vs. 0.27 (0.19–0.39)×10−3;
P<0.01] and healthy controls [0.15 (0.10–0.19)×10−3
vs. 0.55 (0.34–0.70)×10−3; P<0.001] and there was
also a significant difference between intermittent to mild asthma
and healthy controls (P<0.01; Fig.
2); however, CD73 mRNA in CD4+ T cells was
significantly lower in patients with intermittent to mild asthma
compared with healthy controls (Fig.
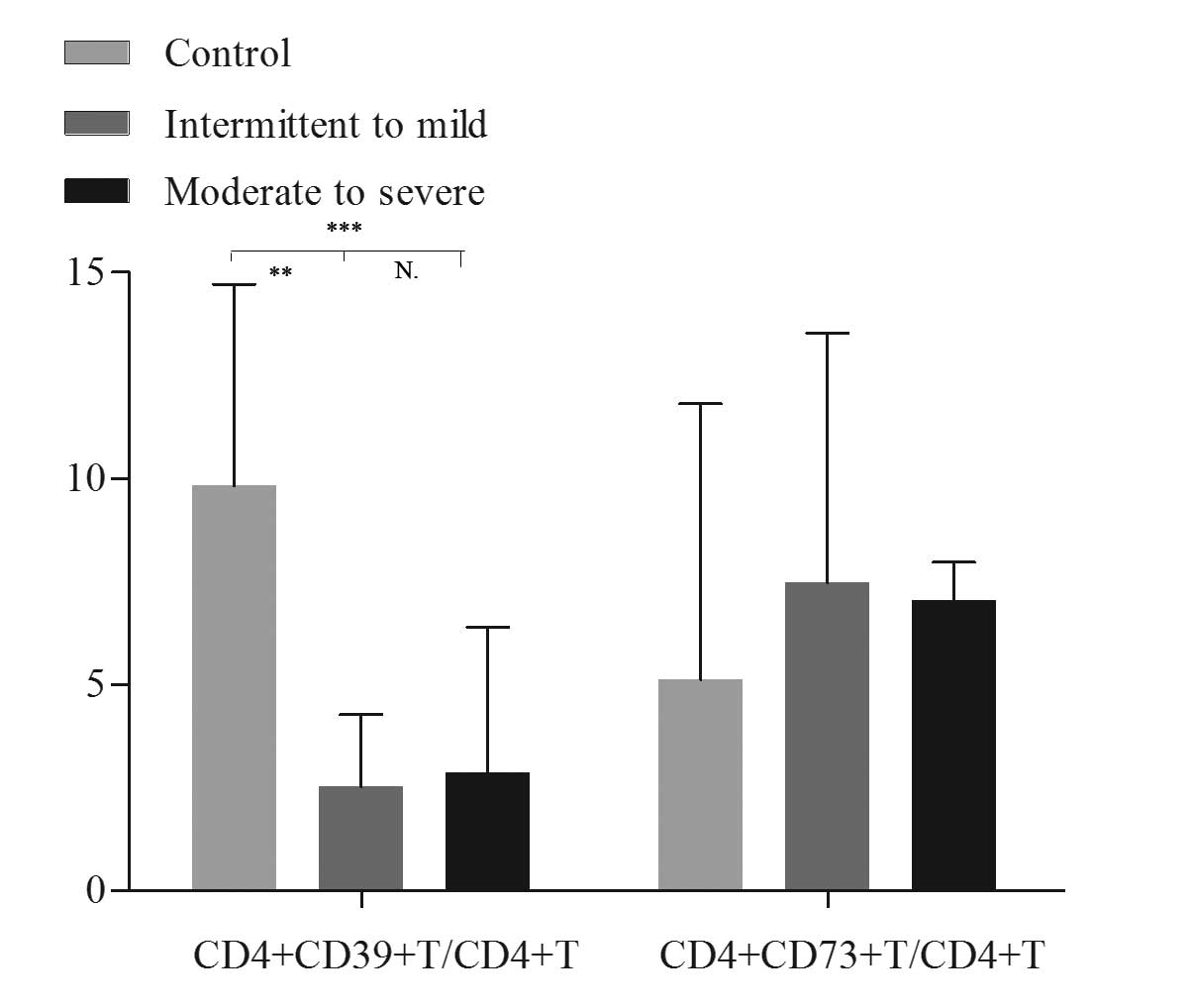
3). The relative frequency of CD4+CD39+ T
cells in the peripheral blood was significantly higher in healthy
controls compared with patients with intermittent to mild asthma
(10.77±3.94 vs. 3.84±2.58%; P<0.001) and with moderate to severe
asthma (10.77±3.94 vs. 4.43±4.13%; P<0.001); however, there was
no significant difference identified between the two subgroups of
asthma (Fig. 4). No significant
differences in the levels of CD4+CD73+ T cell
levels were identified among these three groups (Fig. 4).
Correlation analysis indicated that CD39 mRNA
expression was positively correlated with Foxp3 mRNA expression
(r=0.484; P<0.001) and negatively correlated with ROR-γt
(r=−0.272, P=0.051). In addition, the frequency of Th17 cells was
negatively correlated with the relative frequencies of
CD4+CD39+ T cells and
CD4+CD73+ T cells (r=−0.348, P<0.05 and
r=−0.428, respectively; P<0.05; Table IV). However, the frequency of
CD4+Foxp3+IL-17+ T cells was not
observed to be correlated with the relative frequencies of
CD4+CD39+ T cells and
CD4+CD73+ T cells (Table IV).
 | Table IVResults of correlation analysis. |
Table IV
Results of correlation analysis.
|
CD4+CD39+ T
cells |
CD4+CD73+ T
cells | CD39+
Tregs | CD73+
Tregs |
|---|
|
|
|
|
|
|---|
| Cell type | r-value | P-value | r-value | P-value | r-value | P-value | r-value | P-value |
|---|
|
CD4+Foxp3+IL-17+
T cells | r=−0.20 | P=0.915 | r=−0.031 | P=0.872 | r=−0.038 | P=0.841 | r=−0.052 | P=0.784 |
| Th17 cells | r=−0.348 | P<0.05 | r=−0.428 | P<0.05 | r=−0.377 | P<0.05 | r=−0.428 | P<0.05 |
CD39+and CD73+ Treg
cells are significantly decreased in the peripheral blood of
patients with allergic asthma
Impaired function of Tregs was responsible for
airway inflammation in allergic asthma. Preliminary studies
indicated that the functional deficiency of Tregs results from a
decrease in the number and capacity of cells secreting IL-10 and
TGF-β, as well as a number of other factors. Tregs have been
observed to coexpress CD39 and CD73 enzymes, which catalyze ATP and
ADP into adenosine where adenosine binds to the A2A
receptor expressed by Teffs to suppress inflammatory responses
(18). CD39+ Treg cells
have been reported to be important in constraining pathogenic Th17
cells in MS (21). Further studies
are required to determine the mechanisms of the imbalance of Th17
and Tregs in allergic asthma.
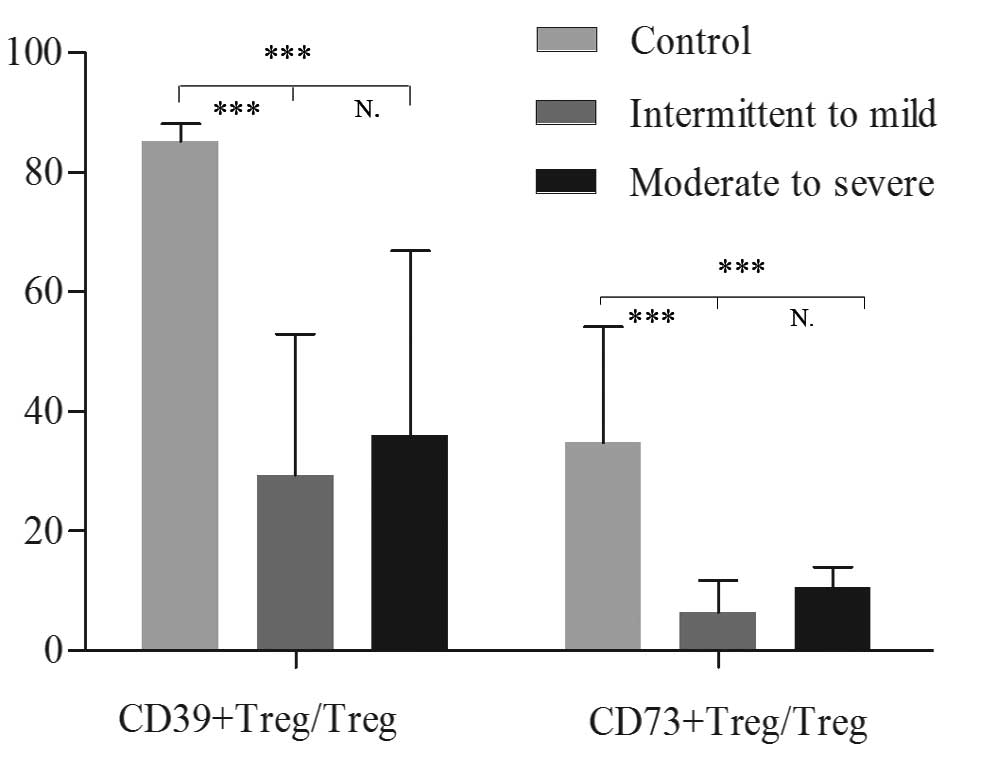
The proportions of CD39+ and
CD73+ Treg cells over total Tregs in the peripheral
blood were significantly higher in healthy controls compared with
patients with intermittent to mild asthma [85.18 (79.04–88.12) vs.
29.41 (19.80–52.98)%; P<0.001 and 34.78 (26.30–54.23) vs. 6.46
(4.98–11.71)%; P<0.001, respectively] and with moderate to
severe asthma [85.18 (79.04–88.12) vs. 36.02 (27.47–66.91)%,
P<0.001 and 34.78 (26.30–54.23) vs. 10.50 (7.69–13.98)%;
P<0.001, respectively]. No significant differences were
identified between the two subgroups of asthma (P=0.226 and
P=0.051, respectively; Figs. 5 and
6). The relative frequency of Th17
cells demonstrated a similar trend in the opposite direction. The
frequency of Th17 cells was observed to be negatively correlated
with the relative frequencies of CD39+ and
CD73+ Treg cells (r=−0.377, P<0.05 and r=−0.428,
P<0.05, respectively; Table
IV). The relative frequency of
CD4+Foxp3+IL-17+ T cells was not
shown to be correlated with the relative frequencies of
CD39+ and CD73+ Tregs (Table IV).
Discussion
Our previous study demonstrated an increased
proportion of Th17 cells and a decreased frequency of Tregs in
asthma (23). Correlation analysis
indicated that the frequency of peripheral blood Th17 cells was
negatively correlated with the percentage of Tregs (23). Thus, the immunosuppressive capacity
of Tregs was decreased in the inhibition of inflammatory responses
mediated by Th17 cells and there was an imbalance of Th17 cells and
Tregs in asthma. However, the underlying mechanisms remain
unclear.
It has been shown that Tregs may be converted to
Th17 cells (10,12). In the current study, Tregs in
patients with asthma were observed to exhibit the capacity to
produce IL-17 (CD4+Foxp3+IL-17+ T
cells). CD4+Foxp3+IL-17+ T cells
were hypothesized to represent Tregs in the middle stages of
transformation into Th17 cells. Furthermore,
CD4+Foxp3+IL-17+ T cell levels
were associated with disease severity. Thus, the imbalance of Th17
cells and Tregs in asthma was hypothesized to be due to the
increased capacity of Tregs to transform into Th17 cells. A balance
of Foxp3 and ROR-γt has been reported in Tregs, and Foxp3 is known
to inhibit the expression of ROR-γt (4). In addition, CD39 promotes the
expression of Foxp3, which amplifies and stabilizes the expression
of CD39 (24). In the present
study, CD39 mRNA was observed to be negatively correlated with
ROR-γt mRNA and positively correlated with Foxp3 mRNA in
CD4+ T cells. This observation indicates that decreased
Foxp3 and CD39 and increased ROR-γt in asthma may be a mechanism of
plasticity of Tregs transforming to Th17 cells in asthma.
The correlation between the imbalance of Th17 and
Tregs in asthma and the expression of CD39 and CD73 by
CD4+ T cells, particularly Tregs, was then investigated.
To the best of our knowledge, this study demonstrated for the first
time that a subset of human Tregs expressed CD39 and CD73 in asthma
and the relative frequencies of CD4+CD39+ T
cells, CD39+ Tregs and CD73+ Tregs in the
peripheral blood were significantly lower in patients with
intermittent to mild asthma compared with healthy controls. These
results suggested that CD39 and CD73 may be involved in the
occurrence and progression of allergic asthma. CD39 and CD73
expression by Tregs was not identified to be significantly
different between asthma subgroups, but there was an increasing
trend in the expression observed in patients with moderate to
severe asthma. Tregs from individuals with asthma were hypothesized
to constitute a deficiency in the mechanism of immunosuppression by
increasing CD39 and CD73 expression, although Tregs do not inhibit
the progression of asthma.
A number of studies have indicated that human
Foxp3+ Tregs, while capable of suppressing proliferation
and IFN-γ production, do not suppress the IL-17 production of Teffs
(25,26). Mechanisms of suppression of
Foxp3+ Tregs include cell-cell contact, the release of
soluble mediators (IL-10 and TGF-β) and the consumption of IL-2. In
the present study, another mechanism, the negative correlation
between the decreased relative frequency of CD39+ Tregs
and increased Th17 cells, was introduced. Thus, the results
indicated that CD39+ Tregs may inhibit the production of
IL-17, which is not consistent with certain studies, which have
indicated that total Foxp3+ Tregs do not suppress IL-17
production by T cells.
The relative frequencies of CD39+ and
CD73+ Tregs were shown to be reduced in intermittent to
mild asthma and there was an increasing trend in moderate to severe
asthma. However, the increased relative frequency of
CD39+ and CD73+ Tregs did not inhibit the
progression of asthma. These observations indicated that
CD39+ and CD73+ Treg populations in moderate
to severe asthma are less effective at suppressing Th17 responses
than the same cells from healthy controls. The current study
indicated that the upregulation of Th17 cells in asthma may be due
to a decrease in the relative frequency and impaired function of
CD39+ and CD73+ Tregs in asthma.
In the present study, the relative frequencies of
CD4+Foxp3+IL-17+ T cells, Th17
cells and CD39 and CD73 expression by CD4+ T cells and
Tregs was determined and their correlation was analyzed. Future
studies are likely to investigate the suppressive function of
CD39+ Tregs and their biological characteristics in
asthma.
In conclusion, the plasticity of Tregs transforming
to IL-17+Foxp3CD4+ T cells, the reduced
frequency CD39+ Tregs and the efficacy of suppression of
IL-17 production by residual CD39+ Tregs, may lead to
the imbalance of Th17 and Tregs in allergic asthma. The
observations indicate that enhancing CD39 activity may be
beneficial in preventing the progression of asthma.
References
|
1
|
Shevach EM:
CD4+CD25+ suppressor T cells: more questions
than answers. Nat Rev Immunol. 2:389–400. 2002.
|
|
2
|
Tesmer LA, Lundy SK, Sarkar S and Fox DA:
Th17 cells in human disease. Immunol Rev. 223:87–113. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weaver CT, Harrington LE, Mangan PR,
Gavrieli M and Murphy KM: Th17: an effector CD4 T cell lineage with
regulatory T cell ties. Immunity. 24:677–688. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou L, Chong MM and Littman DR:
Plasticity of CD4+ T cell lineage differentiation.
Immunity. 30:646–655. 2009.
|
|
5
|
Dong C: TH17 cells in development: an
updated view of their molecular identity and genetic programming.
Nat Rev Immunol. 8:337–348. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Q, Yang W, Gupta S, Biswas P, Smith
P, Bhagat G and Pernis AB: IRF-4-binding protein inhibits
interleukin-17 and interleukin-21 production by controlling the
activity of IRF-4 transcription factor. Immunity. 29:899–911. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ivanov II, McKenzie BS, Zhou L, Tadokoro
CE, Lepelley A, Lafaille JJ, Cua DJ and Littman DR: The orphan
nuclear receptor RORgammat directs the differentiation program of
proinflammatory IL-17+ T helper cells. Cell.
126:1121–1133. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Josefowicz SZ and Rudensky A: Control of
regulatory T cell lineage commitment and maintenance. Immunity.
30:616–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee YK, Mukasa R, Hatton RD and Weaver CT:
Developmental plasticity of Th17 and Treg cells. Curr Opin Immunol.
21:274–280. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kryczek I, Wu K, Zhao E, Wei S, Vatan L,
Szeliga W, Huang E, Greenson J, Chang A, Roliński J, et al:
IL-17+ regulatory T cells in the microenvironments of
chronic inflammation and cancer. J Immunol. 186:4388–4395.
2011.PubMed/NCBI
|
|
11
|
Bovenschen HJ, van de Kerkhof PC, van Erp
PE, Woestenenk R, Joosten I and Koenen HJ: Foxp3+
regulatory T cells of psoriasis patients easily differentiate into
IL-17A-producing cells and are found in lesional skin. J Invest
Dermatol. 131:1853–1860. 2011.
|
|
12
|
Liu T, Song CH, Liu AM, Xie C, Zhao F,
Chen X, Cheng L and Yang PC: Forkhead box P3+ T cells
express interleukin-17 in nasal mucosa of patients with both
allergic rhinitis and polyposis. Clin Exp Immunol. 163:59–64.
2011.
|
|
13
|
Iellem A, Mariani M, Lang R, Recalde H,
Panina-Bordignon P, Sinigaglia F and D’Ambrosio D: Unique
chemotactic response profile and specific expression of chemokine
receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp
Med. 194:847–853. 2001.PubMed/NCBI
|
|
14
|
Paust S, Lu L, McCarty N and Cantor H:
Engagement of B7 on effector T cells by regulatory T cells prevents
autoimmune disease. Proc Natl Acad Sci USA. 101:10398–10403. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lehmann J, Huehn J, de la Rosa M, Maszyna
F, Kretschmer U, Krenn V, Brunner M, Scheffold A and Hamann A:
Expression of the integrin alpha Ebeta 7 identifies unique subsets
of CD25+ as well as CD25- regulatory T cells. Proc Natl
Acad Sci USA. 99:13031–13036. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Salomon B, Lenschow DJ, Rhee L, Ashourian
N, Singh B, Sharpe A and Bluestone JA: B7/CD28 costimulation is
essential for the homeostasis of the
CD4+CD25+ immunoregulatory T cells that
control autoimmune diabetes. Immunity. 12:431–440. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee
MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St
Groth B, et al: CD127 expression inversely correlates with FoxP3
and suppressive function of human CD4+ Treg cells. J Exp
Med. 203:1701–1711. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Deaglio S, Dwyer KM, Gao W, Friedman D,
Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, et al:
Adenosine generation catalyzed by CD39 and CD73 expressed on
regulatory T cells mediates immune suppression. J Exp Med.
204:1257–1265. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Borsellino G, Kleinewietfeld M, Di Mitri
D, Sternjak A, Diamantini A, Giometto R, Höpner S, Centonze D,
Bernardi G, Dell’Acqua ML, et al: Expression of ectonucleotidase
CD39 by Foxp3+ Treg cells: hydrolysis of extracellular
ATP and immune suppression. Blood. 110:1225–1232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fletcher JM, Lonergan R, Costelloe L,
Kinsella K, Moran B, O’Farrelly C, Tubridy N and Mills KH:
CD39+Foxp3+ regulatory T cells suppress
pathogenic Th17 cells and are impaired in multiple sclerosis. J
Immunol. 183:7602–7610. 2009.PubMed/NCBI
|
|
21
|
Huang S, Apasov S, Koshiba M and Sitkovsky
M: Role of A2a extracellular adenosine receptor-mediated signaling
in adenosine mediated inhibition of T-cell activation and
expansion. Blood. 90:1600–1610. 1997.PubMed/NCBI
|
|
22
|
Boulet LP, FitzGerald JM, Levy ML, Cruz
AA, Pedersen S, Haahtela T and Bateman ED: A guide to the
translation of the Global Initiative for Asthma (GINA) strategy
into improved care. Eur Respir J. 39:1220–1229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi YH, Shi GC, Wan HY, Jiang LH, Ai XY,
Zhu HX, Tang W, Ma JY, Jin XY and Zhang BY: Co-existence of Th1/Th2
and Th17/Treg imbalances in patients with allergic asthma and its
significance. Chin Med J (Engl). 124:1951–1956. 2011.PubMed/NCBI
|
|
24
|
Gavin MA, Rasmussen JP, Fontenot JD, Vasta
V, Manganiello VC, Beavo JA and Rudensky AY: Foxp3-dependent
programme of regulatory T cell differentiation. Nature.
445:771–775. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Flores-Borja F, Jury EC, Mauri C and
Ehrenstein MR: Defects in CTLA-4 are associated with abnormal
regulatory T cell function in rheumatoid arthritis. Proc Natl Acad
Sci USA. 105:19396–19401. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Annunziato F, Cosmi L, Santarlasci V,
Maggi L, Liotta F, Mazzinghi B, Parente E, Filì L, Ferri S, Frosali
F, et al: Phenotypic and functional features of human Th17 cells. J
Exp Med. 204:1849–1861. 2007. View Article : Google Scholar : PubMed/NCBI
|