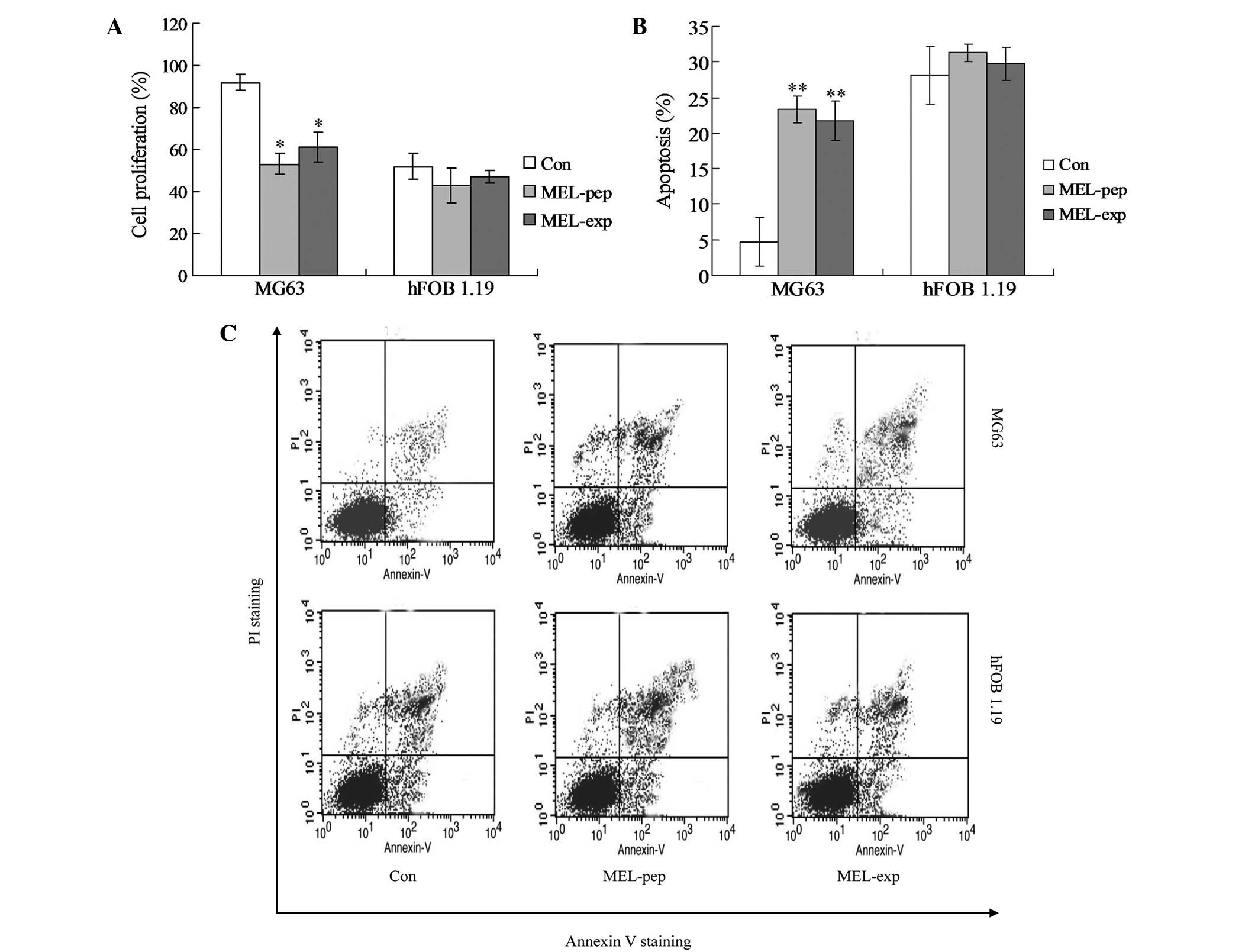
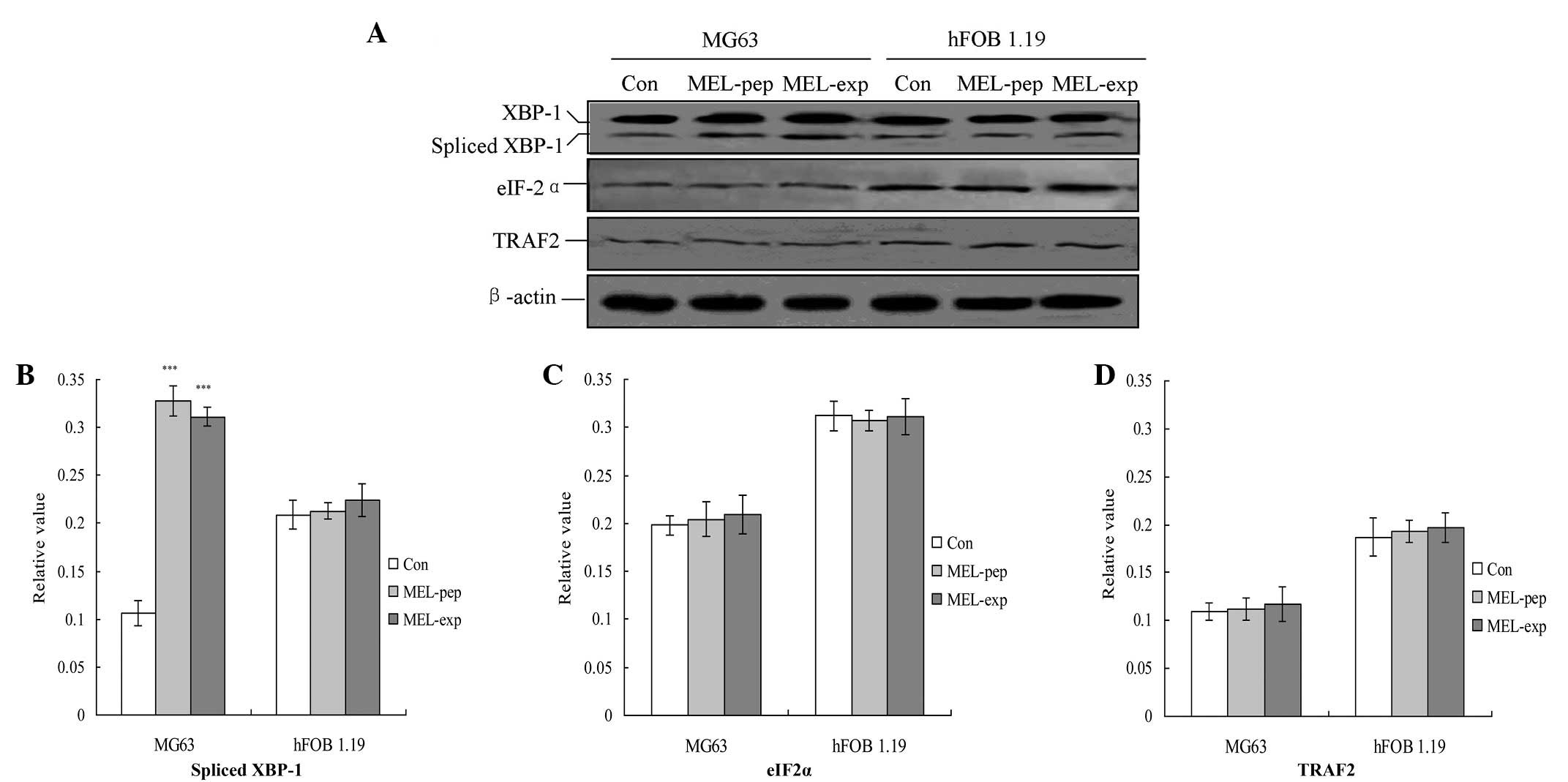
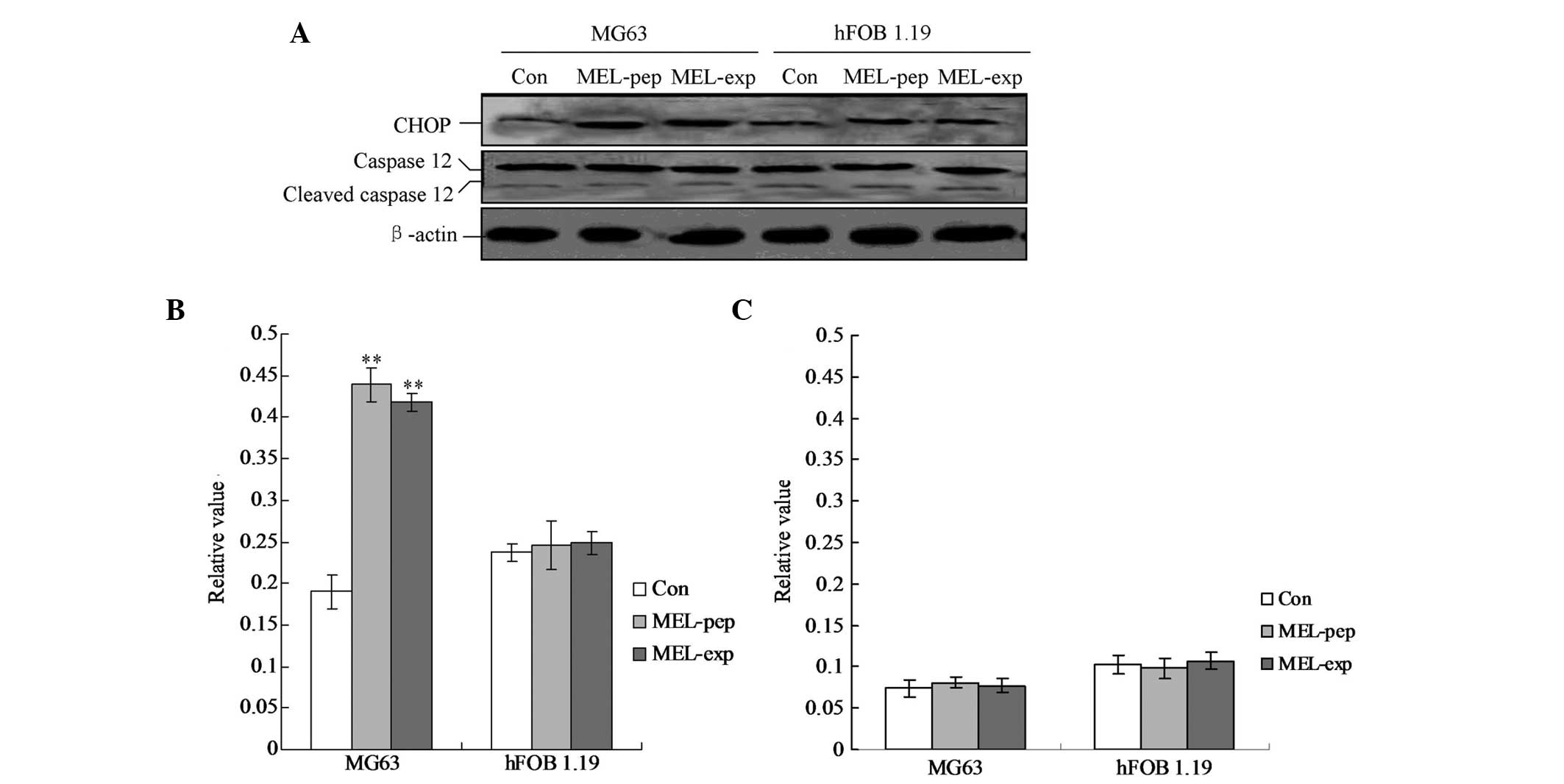
|
1
|
Alvarez-Fischer D, Noelker C, Vulinović F,
Grünewald A, Chevarin C, Klein C, Oertel WH, Hirsch EC, Michel PP
and Hartmann A: Bee Venom and its component apamin as
neuroprotective agents in a Parkinson disease mouse model. PLoS
One. 8:e617002013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park HJ, Lee SH, Son DJ, Oh KW, Kim KH,
Song HS, Kim GJ, Oh GT, Yoon DY and Hong JT: Antiarthritic effect
of bee venom: inhibition of inflammation mediator generation by
suppression of NF-kappaB through interaction with the p50 subunit.
Arthritis Rheum. 50:3504–3515. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hong SJ, Rim GS, Yang HI, Yin CS, Koh HG,
Jang MH, Kim CJ, Choe BK and Chung JH: Bee venom induces apoptosis
through caspase-3 activation in synovial fibroblasts of patients
with rheumatoid arthritis. Toxicon. 46:39–45. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jang MH, Shin MC, Lim S, Han SM, Park HJ,
Shin I, Lee JS, Kim KA, Kim EH and Kim CJ: Bee venom induces
apoptosis and inhibits expression of cyclooxygenase-2 mRNA in human
lung cancer line NCI-H1299. J Pharmacol Sci. 91:95–104. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moon DO, Park SY, Heo MS, Kim KC, Park C,
Ko WS, Choi YH and Kim GY: Key regulators in bee venom-induced
apoptosis are Bcl-2 and caspase-3 in human leukemic U937 cells
through downregulation of ERK and Akt. Int Immunopharmacol.
6:1796–1807. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lariviere WR and Melzack R: The bee venom
test: a new tonic-pain test. Pain. 66:271–277. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Son DJ, Ha SJ, Song HS, Lim Y, Yun YP,
Moon DC, Park YH, Park BS, Song MJ and Hong JT: Melittin inhibits
vascular smooth muscle cell proliferation through induction of
apoptosis via suppression of NF-kappaB and Akt activation and
enhancement of apoptotic protein expression. J Pharmacol Exp Ther.
317:627–634. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shaposhnikova VV, Egorova MV, Kudryavtsev
AA, Levitman MK and Korystov YuN: The effect of melittin on
proliferation and death of thymocytes. FEBS Lett. 410:285–288.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim SK, Park KY, Yoon WC, Park SH, Park
KK, Yoo DH and Choe JY: Melittin enhances apoptosis through
suppression of IL-6/sIL-6R complex-induced NF-κB and STAT3
activation and Bcl-2 expression for human fibroblast-like
synoviocytes in rheumatoid arthritis. Joint Bone Spine. 78:471–477.
2011.PubMed/NCBI
|
|
10
|
Rasheva VI and Domingos PM: Cellular
responses to endoplasmic reticulum stress and apoptosis. Apoptosis.
14:996–1007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ron D and Walter P: Signal integration in
the endoplasmic reticulum unfolded protein response. Nat Rev Mol
Cell Biol. 8:519–529. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, Dong CF, Shi Q, Shi S, Wang GR,
Lei YJ, Xu K, An R, Chen JM, Jiang HY, Tian C, Gao C, Zhao YJ, Han
T and Dong XP: Cytosolic prion protein induces apoptosis in human
neuronal cell SH-SY5Y via mitochondrial disruption pathway. BMB
Rep. 42:444–449. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park C and Lee DG: Melittin induces
apoptotic features in Candida albicans. Biochem Biophys Res
Comm. 394:170–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Shi Q, Xu K, Gao C, Chen C, Li XL,
Wang GR, Tian C, Han J and Dong XP: Familial CJD associated PrP
mutants within transmembrane region induced Ctm-PrP retention in ER
and triggered apoptosis by ER stress in SH-SY5Y cells. PLoS One.
6:e146022011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim KM, Kim HC, Jeon KN, Kim HG, Kang JH,
Hahm JR and Lee GW: Rituximab-CHOP induced interstitial pneumonitis
in patients with disseminated extranodal marginal zone B cell
lymphoma. Yonsei Med J. 49:155–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang SC, Lu MC, Chen HL, Tseng HI, Ke YY,
Wu YC and Yang PY: Cytotoxicity of calotropin is through caspase
activation and downregulation of anti-apoptotic proteins in K562
cells. Cell Biol Int. 33:1230–1236. 2009. View Article : Google Scholar : PubMed/NCBI
|