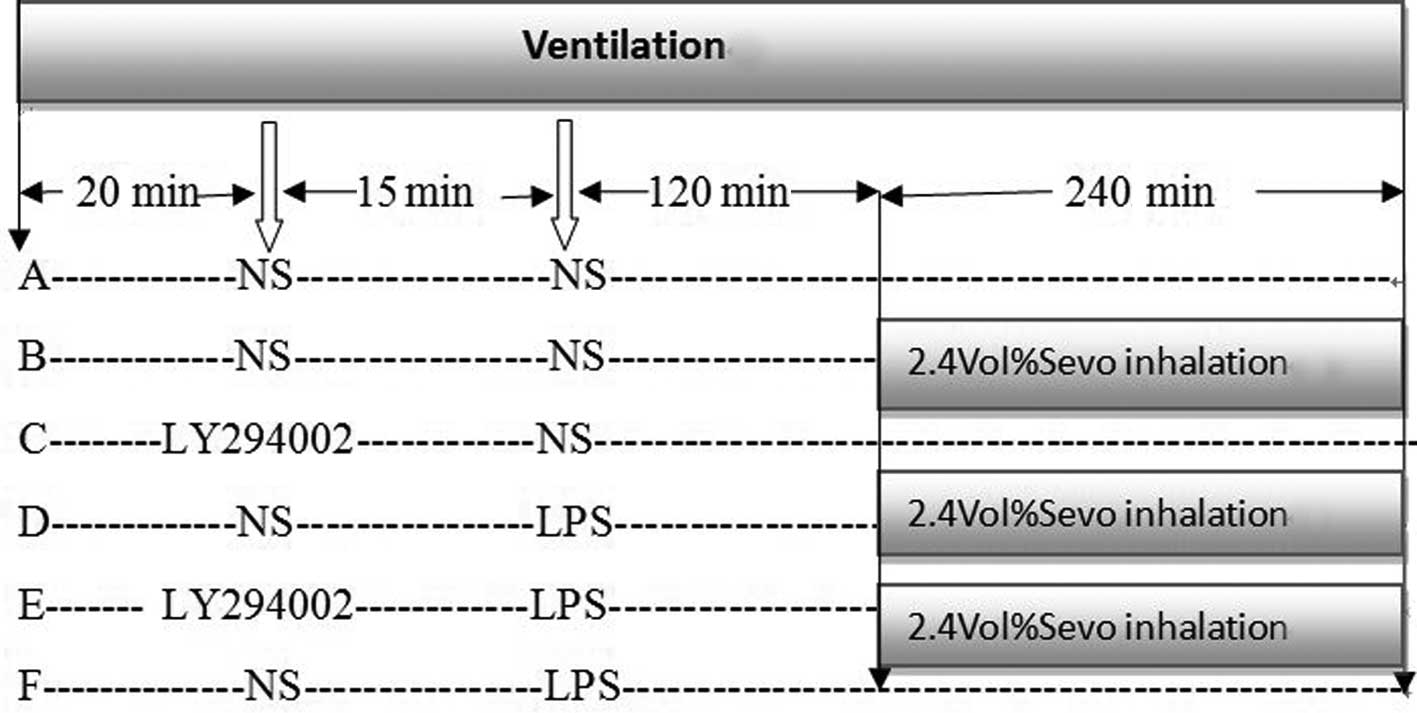
Introduction
Epithelial and endothelial cell perturbation and
inflammatory cell influx are indicative of acute lung injury (ALI),
which is a diffuse heterogeneous lung injury that leads to
hypoxemia, non-cardiogenic pulmonary edema, low lung compliance and
widespread capillary leakage. As an inducible enzyme for the
rate-limiting step in the conversion of heme into biliverdin,
carbon monoxide (CO) and free iron, heme oxygenase-1 (HO-1) is also
widely accepted as a cellular defense mechanism against harmful or
noxious stimuli (1,2). A previous study found that HO-1
expression prevents rat livers from ischemia/reperfusion (I/R)
injury when using anesthetic sevoflurane (Sevo) clinically
(3). Inhalation of Sevo prior to
ischemia increases the activity of HO-1 in the lung and protects
from lung I/R injury in rats (4).
I/R injury is an important model of oxidant-mediated ALI (5). Therefore, Sevo may have an important
role in ALI protection by upregulation of HO-1 expression. However,
the regulatory mechanism of HO-1 expression is complex and is
involved in numerous intracellular signal transduction pathways,
including the phosphatidylinositol 3-kinase/protein kinase B
(PI3K/Akt) pathway (6).
PI3K is a lipid kinase and generates
phosphatidylinositol (3,4,5)-trisphosphate (PIP3). PIP3 is a second
messenger essential for the translocation of Akt to the plasma
membrane where it is phosphorylated and has a pivotal role in
fundamental cellular functions, including cell proliferation and
survival by phosphorylating a variety of substrates (7). A previous report demonstrated that
HO-1-induced protection against hypoxia/reoxygenation is dependent
on biliverdin reductase and its interaction with the PI3K/Akt
pathway (8). In addition, the
PI3K/Akt signaling pathway also has been found to be involved in
HO-1 upregulated expression induced by epigallocatechin,
4-methylcatechol and schisandrin (9–11).
Therefore, in the present study, the role of the PI3K/Akt
regulatory pathway in the upregulated expression of HO-1 induced by
Sevo used for the treatment of lipopolysaccharide (LPS)-induced ALI
was explored.
Materials and methods
Animal preparation
The animal protocol was approved by the
Institutional Animal Care and Use Committee at Central South
University (Changsha, China). Animal models were established
according to the methods of Sun et al (12), Crawford et al (13) and
Coimbra et al (14). Rats
were obtained from Beijing Vital River Laboratory Animal Technology
Co., Ltd. (Beijing, China). They were housed with free access to
food and water under an automatic 12-h light/12-h dark cycle at a
temperature of 24°C and a humidity of 50–60%. Following 12 h
fasting, forty-eight male Sprague-Dawley (SD) rats, 220 to 280 g,
were anesthetized by intraperitoneal injection of 20% urethane (1
g/kg) and placed in a supine position to implement tracheotomy. The
animals were intubated with a homemade tube and mechanically
ventilated for 390 min (with 8 ml/kg tidal volume and 65–70
times/min respiratory frequency). Right femoral artery intubation
was used to measure arterial pressure and blood collection, while
the left femoral vein was used for normal saline (NS) and drug
administration.
Following ventilation for 20 min with stable
conditions, rats were randomized to concurrently receive NS, 2.4%
Sevo (Baxter Healthcare Corporation, Deerfield, IL, USA), delivered
through a rodent ventilator (ALC-V8, Shanghai Alcott Biotech Co.,
Shanghai, China) (13) or LY294002
(a PI3K inhibitor; 0.3 mg/kg, intravenously; Cell Signaling
Technology, Inc., Danvers, MA, USA) (15) to yield the following experimental
groups: Control, 2.4% Sevo only, LY294002 only, LPS (5 mg/kg,
intravenously; Sigma-Aldrich, St. Louis, MO, USA) + 2.4% Sevo,
LY294002 + LPS+ 2.4% Sevo and LPS only (Fig. 1). Sevo, delivered by gaseous
admixture (oxygen) at a concentration of 2.4% via a calibrated
vaporizer, was administered via an endotracheal tube. The inspired
oxygen and Sevo concentrations were also monitored continuously
through the anesthetic agent monitor (Datex Instrumentarium,
Helsinki, Finland). The rectal temperature of all the rats were
maintained between 37–38°C by incandescent bulb heating. Following
6 h drug treatment or saline, all the rats were sacrificed by
exsanguination under anesthesia. The tissue samples of the left
lung were immediately immersed in liquid nitrogen and stored at
−80°C for subsequent activity analysis.
Wet/dry (W/D) lung weight ratios
A total of 100 mg was excised from the right middle
lobe, rinsed briefly in PBS, blotted and weighed to obtain the
‘wet’ weight. Lungs were then dried in an oven at 80°C for 48 h to
obtain the ‘dry’ weight.
MPO, MDA and SOD activity
As an indicator of migration of polymorphonuclear
neutrophils in lung tissue, the activity of myeloperoxidase (MPO)
was measured with a EnzChek® Myeloperoxidase Activity
Assay kit (Thermo Fisher Scientific, Waltham, MA, USA) according to
the manufacturer’s instructions. The malondialdehyde (MDA) assay
was performed to evaluate the severity of lipid peroxidation injury
by the thiobarbituric acid (TBA) colorimetric method using an MDA
Assay kit (Jiancheng Corp., Nanjing, China). This assay is based on
the reaction of MDA with TBA, forming stable thiobarbituric
acid-reactive substances, which has a maximum absorption at 532 nm.
Superoxide dismutase (SOD) activity was determined using an SOD
assay kit (Jiancheng Corp.).
Histological analysis
The pathological changes of lung tissue were
examined by hematoxylin and eosin staining under an optical
microscope (CHBS microscope; Olympus, Tokyo, Japan). The right
upper lobe was dissected, fixed with 4% paraformaldehyde,
dehydrated, embedded in paraffin, sectioned and stained. The degree
of lung injury was assessed using the scoring system described by
Al-Amran et al (16). Each
parameter was graded on a scale of 0–3, as follows: i) 0, Alveolar
septae. 0, Septae thin and delicate; 1, congested alveolar septae
in <1/3 of the field; 2, congested alveolar septae in 1/3–2/3 of
the field; 3, congested alveolar septae in >2/3 of the field.
ii) Intra-alveolar cell infiltrates. 0, <5 intra-alveolar cells
per field; 1, 5–10 intra-alveolar cells per field; 2, 10–20
intra-alveolar cells per field; 3, >20 intra-alveolar cells per
field. iii) Alveolar hemorrhage. 0, No hemorrhage; 1, >5
erythrocytes per alveolus in 1–5 alveoli; 2, >5 erythrocytes in
5–10 alveoli, 3, >5 erythrocytes in >10 alveoli. The total
lung injury score was calculated by adding the individual scores
for each category. The histological sections were evaluated by a
pathologist without prior knowledge of the treatment administered
to the animals.
Quantitative polymerase chain reaction
(qPCR) and western blot analysis
The cDNA was generated from 2 μg total RNA isolated
with TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA,
USA) using the RT-PCR kit (MBI, Fermentas, Vilnius, Lithuania) with
Oligo (dT) primers and GAPDH as the control. The PCR primers used
were: HO-1 forward, 5′-ATGGAGCGCCCACAGCTCGA-3′ and reverse,
5′-CTCCAGAGTGTTCATGCGAG-3′; and GAPDH forward,
5′-CAGCAATTTTCAGTGTCAGAAGCT-3′ and reverse,
5′-TCATCCTGTCCTTGAGGCAGTAT-3′. Western blot analysis was performed
as described previously (17).
Immunoblotting was performed with the following antibodies: Rabbit
anti-mouse HO-1 (Merck, Darmstadt, Germany), rabbit anti-mouse
PI3K-P110α, rabbit anti-mouse phospho-PI3K (pPI3K), rabbit
anti-mouse Akt, rabbit anti-mouse phospho-Akt (pAkt) (all Cell
Signaling Technology, Inc.), and IRDye 800 goat anti-rabbit (LI-COR
Biosciences, Lincoln, NE, USA). Blots were scanned using the
Odyssey infrared imaging system (LI-COR Biosciences). GAPDH and
β-actin were used as a control gene and proteins for normalization
using the AlphaImager 200 Digital Imaging System (Alpha Innotech,
San Leandro, CA, USA).
Statistical analysis
All data were analyzed using SPSS 13.0 (SPSS Inc.,
Chicago, IL, USA) and the data are expressed as the mean ± standard
deviation. A one-way analysis of variance was used for comparisons
between groups. Post-hoc comparisons were performed using a least
significant difference test or Dunnett’s T3 test. P<0.05 was
considered to indicate a statistically significant difference.
Results
LPS injection induces lung tissue damage
with increased W/D ratio, MDA and MPO activity and decreased SOD
activity
As shown in Table
I, no significant differences were observed in the W/D ratio or
the SOD, MDA and MPO activity of lung tissue among groups A, B and
C (P>0.05), while LPS injection (groups D, E and F) caused a
significant increase in the W/D ratio, MDA and MPO activity as
compared with saline-injected rats (group A) (P<0.05), but with
a significant decrease in SOD activity. These results demonstrated
that oxidative damage appeared in lung tissue following LPS
injection. No significant differences were observed in lung W/D
ratios among groups D, E and F (P>0.05).
 | Table IComparison of the W/D ratio, SOD, MDA
and MPO activity in each group. |
Table I
Comparison of the W/D ratio, SOD, MDA
and MPO activity in each group.
| Group (n=8) | W/D ratio | SOD activity
(U/gprot) | MDA activity
(nmol/gprot) | MPO activity
(U/gprot) |
|---|
| A | 4.11±0.35 | 19.55±1.62 | 1.50±0.27 | 5.12±0.41 |
| B | 4.24±0.44 | 20.07±1.96 | 1.67±0.45 | 5.28±0.56 |
| C | 4.04±0.28 | 19.79±2.04 | 1.75±0.43 | 5.23±0.53 |
| D | 4.84±0.39a | 15.32±1.03a | 2.25±0.30a | 6.64±0.74a |
| E | 4.96±0.20a | 12.66±1.97a,b | 2.65±0.53a,c | 7.81±0.74a,b |
| F | 4.85±0.40a | 10.28±0.99a,b | 3.67±0.49a,b | 8.18±0.81a,b |
Sevo decreases lung tissue damage induced
by LPS
As shown in Table
I, compared with group F (only LPS injection), significant
differences were observed in group D (Sevo post-conditioning) with
increased SOD activity and decreased MDA and MPO activity. These
results reflected that Sevo treatment significantly reversed the
oxidant-antioxidant status and had a protective role. Compared with
group D, LY294002 pretreatment led to a decrease in SOD activity
and a slight increase in MDA and MPO activity; however, no
significant difference was observed between groups E and D.
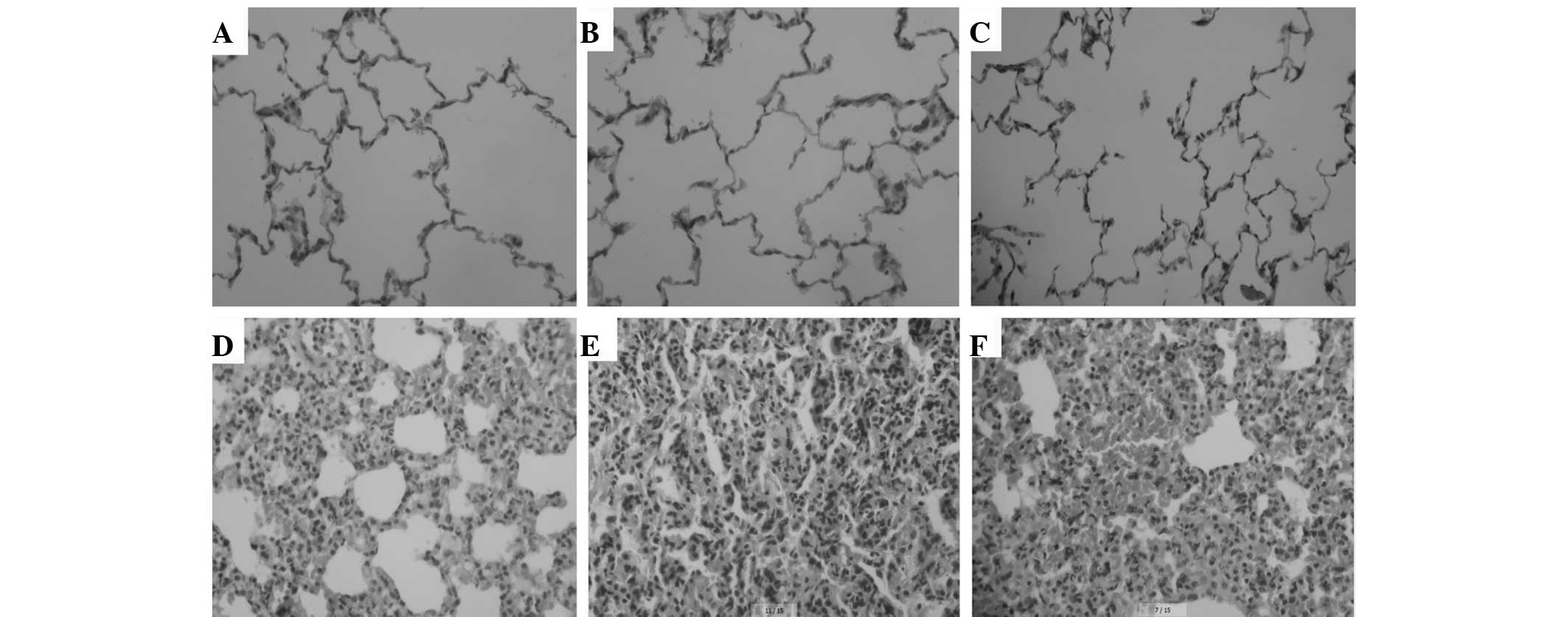
Histological analysis and
pathomorphological scores
Optical microscopic observation indicated the
presence of intact alveoli structure, clean alveolar spaces, no
alveolar interstititum, few inflammatory cell infiltrations and
rare light alveolar wall thickening in groups A, B and C. However,
in LPS-induced groups D, E and F, marked diffuse pulmonary edema of
varying degrees, capillarectasia and hyperaemia were observed. In
addition, margination and emigration of leucocytes widened alveolar
spaces were also present, particularly in groups E and F (Fig. 2).
Pathomorphological scores were investigated and the
results are shown in Table II. No
significant differences were observed among groups A, B and C
(P>0.05) and in comparison with these, pathomorphological scores
were significantly increased in LPS-induced ALI models (groups D, E
and F). However, compared with group F, pathomorphological scores
in group D were significantly decreased (P<0.05) due to
post-conditioning with 2.4% Sevo. Further analysis indicated that
pathomorphological scores in group E were also significantly higher
compared with those in group D, indicating that the PI3K inhibitor
LY294002 eliminated the protective effects of Sevo.
 | Table IIPathomorphological lung injury score
in each group. |
Table II
Pathomorphological lung injury score
in each group.
| Group (n=8) | Congestion of
alveolar septae | Intra-alveolar cell
infiltrates | Alveolar
hemorrhage | Total score |
|---|
| A | 0.13±0.35 | 0 | 0 | 0.15±0.35 |
| B | 0.25±0.46 | 0 | 0 | 0.25±0.46 |
| C | 0.25±0.46 | 0 | 0 | 0.25±0.46 |
| D | 2.13±0.64 | 2.13±0.83 | 2.13±0.64 | 6.38±1.60a |
| E | 2.88±0.35 | 2.88±0.35 | 2.75±0.46 | 8.50±1.07a,b |
| F | 2.75±0.46 | 2.88±0.35 | 2.63±0.52 | 8.13±0.64a,b |
Protein expression of pPI3K and pAkt
The protein expression levels of pPI3K and pAkt in
each group were detected by western blot analysis, using total PI3K
and Akt as an internal standard. The results are shown in Figs. 3 and 4. There was limited pPI3K and pAkt
expression in groups A, B and C, but a significant increase in
group F. However, pPI3K and pAkt were further increased in group D
following Sevo post-conditioning compared with group F. In
addition, LY294002 pretreatment in group E significantly decreased
pPI3K and pAkt expression compared with group D.
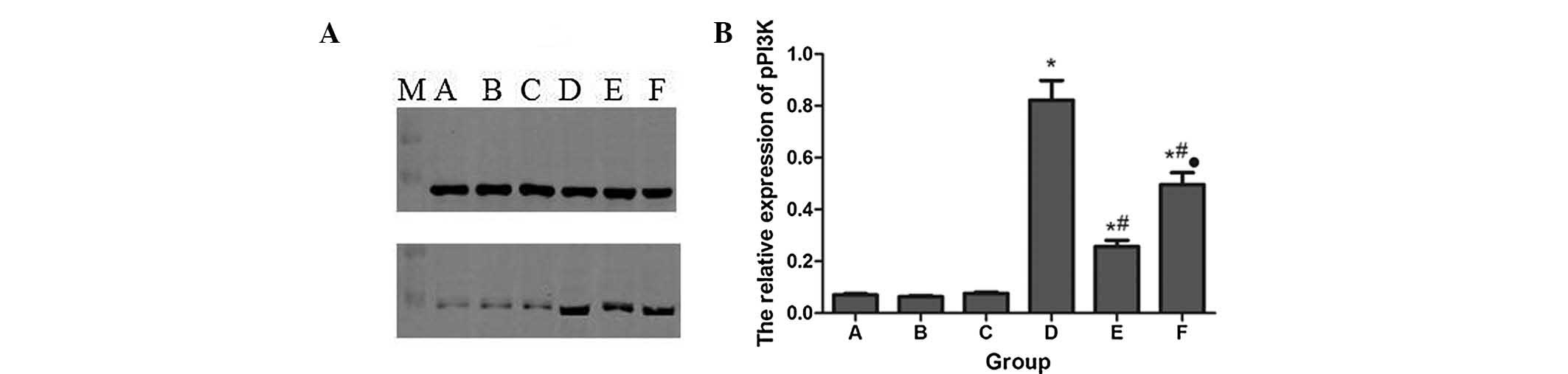 | Figure 3Protein expression levels of pPI3K in
each group. (A) Western blot; (B) Quantification of A in a
histogram. *P<0.05, compared with group A.
#P<0.05, compared with group D.
•P<0.05, compared with group E. A, control; B, 2.4%
Sevo; C, LY294002 (phosphatidylinositol 3-kinase inhibitor); D, LPS
+ 2.4% Sevo; E, LY294002 + LPS + 2.4% Sevo; F, LPS; M, marker;
pPI3K, phosphorylated phosphatidylinositol 3-kinase. |
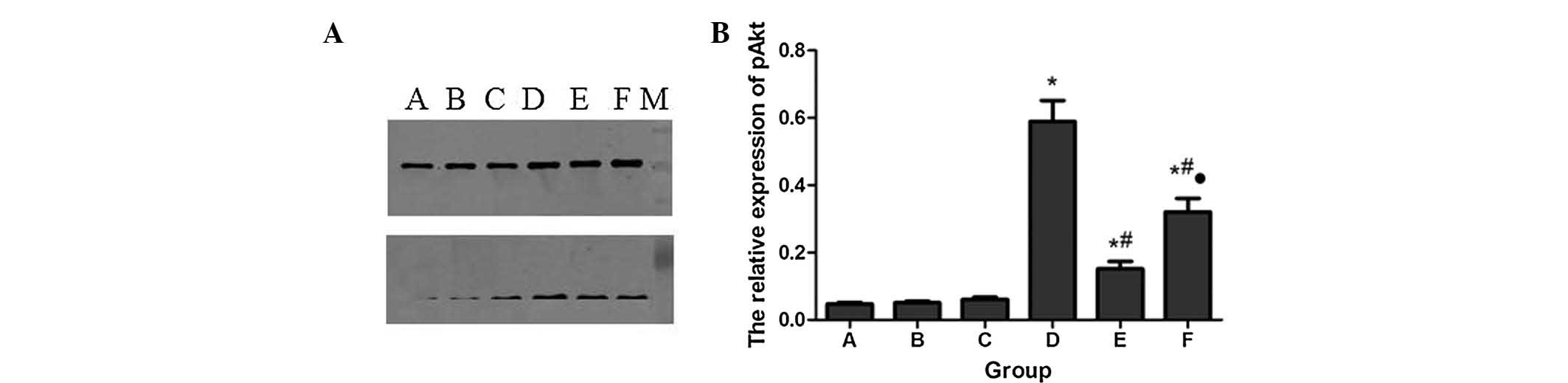 | Figure 4Protein expression levels of pAkt in
each group. (A) Western blot; (B) Quantification of A in a
histogram. *P<0.05, compared with group A.
#P<0.05, compared with group D.
•P<0.05, compared with group E. A, control; B, 2.4%
Sevo; C, LY294002 (phosphatidylinositol 3-kinase inhibitor); D, LPS
+ 2.4% Sevo; E, LY294002 + LPS + 2.4% Sevo; F, LPS; M, marker;
pAKT, phospho-Akt. |
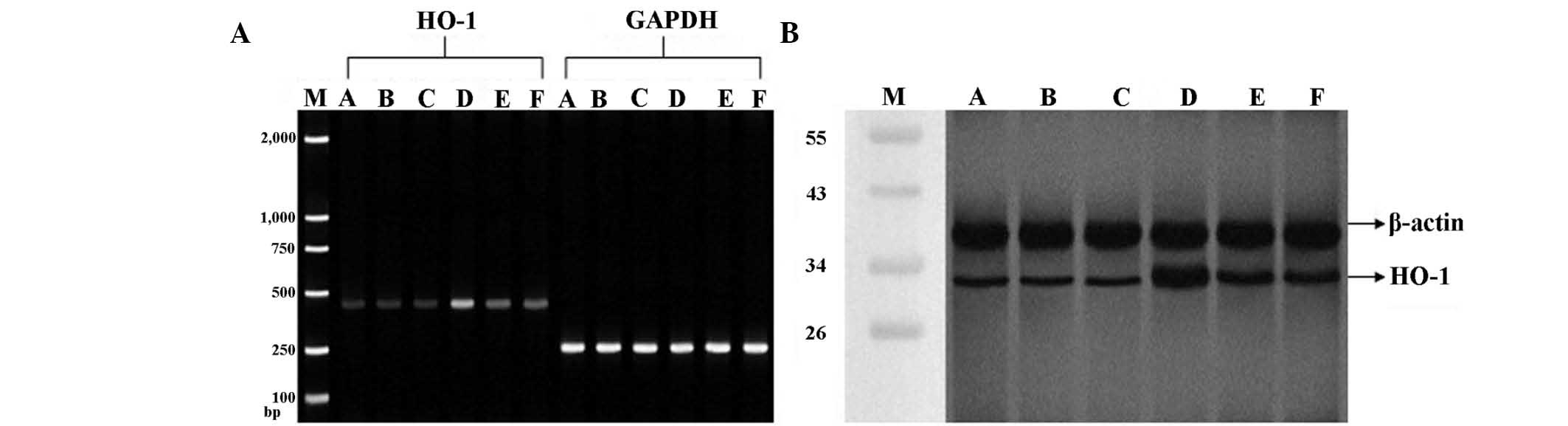
mRNA and protein expression of HO-1
As shown in Fig. 5,
no significant differences were observed in mRNA and protein levels
of HO-1 between groups A, B and C. LPS injection (groups D, E and
F) induced a significant increase in mRNA and protein expression of
HO-1 as compared with group A. A subsequent 2.4% Sevo inhalation
(group D) significantly increased HO-1 expression compared with
group F. Notably, HO-1 expression was significantly decreased in
group E compared with group D, indicating that PI3K may be involved
in the regulation of HO-1 expression.
Discussion
Inflammatory cells and inflammatory mediators have
been well-characterized and are known to contribute to the overall
pathogenesis of ALI. Infiltration with inflammatory cell has been
found by histological analysis. Intercellular adhesion molecule
(ICAM-1) is an inflammatory biomarker that is involved in the
adhesion of monocytes to endothelial cells (ECs) and HO-1 has been
reported to inhibit the expression of adhesion molecules in
vascular ECs (18,19).
At present, the expression of the HO-1 gene is
primarily regulated at the transcriptional level, although there
are variations between species (20). The human HO-1 gene is localized on
chromosome 22q12 and consists of five exons and four introns,
spanning 14-kb (21,22). A promoter sequence is located ~28
base pairs upstream from the starting site of transcription, which
contains a number of enhancer and transcriptional regulatory
elements, including the antioxidant response element (ARE), heat
shock element and hypoxic response element. These transcriptional
regulatory elements may specifically bind to a series of oxidative
stress-responsive transcription factors (23), including nuclear factor-κB
(24), activator protein-1
(25) and nuclear factor erythroid
2-related factor (Nrf2) (26),
suggesting a potential role of these factors in modulating HO-1
induction.
Complex regulatory regions indicate the possibility
that several signal transduction pathways may be involved in HO-1
regulation, including mitogen-activated protein kinase, protein
kinase C and tyrosine protein kinase (27,28).
A recent study indicated that the PI3K/Akt pathway also has a role
in HO-1 regulation. For example, Chen et al (29) found that 4-ketopinoresinol, a novel
naturally occurring ARE activator, induces activation of the
Nrf2/HO-1 axis and protects against oxidative stress-induced cell
injury via activation of PI3K/Akt signaling. PI3K, a lipid kinase,
can be activated to generate the lipid second messenger PIP3 and
subsequently activates its main downstream effector, the
serine/threonine kinase Akt, which phosphorylates a variety of
substrates that function to suppress apoptosis and promote
progression through the cell cycle (30). A previous study found that Sevo
post-conditioning activates the PI3K/Akt pathway to protect
isolated rat hearts against I/R injury with decreased cardiomyocyte
apoptosis (31). However, such
cardioprotective effects were entirely eliminated by LY294002
(32). Similarly,
post-conditioning with Sevo reduced nerve cell apoptosis,
upregulated B-cell lymphoma 2 (Bcl-2) and downregulated P53 and
Bcl-2-associated X protein. Wortmannin, a PI3K inhibitor, prevented
pAkt from increasing, indicating that this neuroprotective effect
may be partly due to the activation of the PI3K/Akt pathway and Akt
phosphorylation (33).
In the present study, low levels of PI3K and pAkt
were identified in the saline control groups A, B and C; however,
expression significantly increased in the LPS-injection group. The
activity of SOD was reduced, the activities of MDA and MPO were
increased, and the mRNA and protein expression of HO-1 were all
increased. This indicates that LPS elicited a marked oxidant stress
response and activation of a protection mechanism.
However, Sevo post-conditioning reversed the oxidant
damage status by increasing the SOD activity and decreasing MDA and
MPO activity. In addition, treatment with Sevo also induced
increasing HO-1 expression as well as PI3K and Akt phosphorylation.
To confirm that the upregulation of HO-1 expression was mediated by
the PI3K/Akt pathway, the PI3K inhibitor LY294002 was introduced.
The results showed that LY294002 inhibited PI3K and Akt
phosphorylation, but also HO-1 expression at the mRNA and protein
levels, indicating that the PI3K/Akt pathway may be involved in
HO-1 expression induced by Sevo.
In conclusion, the present study suggested that the
upregulation of HO-1 expression caused by inhalation of Sevo, which
decreases the release of the inflammatory mediator ICAM-1 in ALI
processes, may proceed via the PI3K/Akt pathway. Numerous advances
in clinical applications of Sevo/HO-1 enhancers are expected in the
coming years based on the present analysis.
Acknowledgements
This study was supported by the Hunan Provincial
Science and Technology Plan Project of China (nos. 06SK3022 and
2013FJ4106) and the High Technology Industry Department,
Development and Reform Commission of Hunan Province, China (no.
2012-1493).
References
|
1
|
Nakao A, Kaczorowski DJ, Zuckerbraun BS,
Lei J, Faleo G, Deguchi K, McCurry KR, Billiar TR and Kanno S:
Galantamine and carbon monoxide protect brain microvascular
endothelial cells by heme oxygenase-1 induction. Biochem Biophys
Res Commun. 367:674–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim HP, Ryter SW and Choi AM: CO as a
cellular signaling molecule. Annu Rev Pharmacol Toxicol.
46:411–449. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mura M, Andrade CF, Han B, Seth R, Zhang
Y, Bai XH, Waddell TK, Hwang D, Keshavjee S and Liu M: Intestinal
ischemia-reperfusion-induced acute lung injury and oncotic cell
death in multiple organs. Shock. 28:227–238. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takemori K, Kobayashi K and Sakamoto A:
Expression of pulmonary vasoactive factors after sevoflurane
anaesthesia in rats: a quantitative real-time polymerase chain
reaction study. Br J Anaesth. 100:190–194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schwer CI, Stoll P, Pietsch U, Stein P,
Laqua J, Goebel U, Hoetzel A and Schmidt R: Up-regulation of heme
oxygenase-1 by sevoflurane is not dependent on Kupffer cells and
associates with ERK1/2 and AP-1 activation in the rat liver. Int J
Biochem Cell Biol. 42:1876–1883. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ye Z, Guo Q, Xia P, Wang N, Wang E and
Yuan Y: Sevoflurane postconditioning involves an up-regulation of
HIF-1alpha and HO-1 expression via PI3K/Akt pathway in a rat model
of focal cerebral ischemia. Brain Res. 1463:63–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Osaki M, Oshimura M and Ito H: PI3K-Akt
pathway: its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pachori AS, Smith A, McDonald P, Zhang L,
Dzau VJ and Melo LG: Heme-oxygenase-1-induced protection against
hypoxia/reoxygenation is dependent on biliverdin reductase and its
interaction with PI3K/Akt pathway. J Mol Cell Cardiol. 43:580–592.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ogborne RM, Rushworth SA and O’Connell MA:
Epigallocatechin activates haem oxygenase-1 expression via protein
kinase Cdelta and Nrf2. Biochem Biophys Res Commun. 373:584–588.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Furukawa Y, Urano T, Minamimura M,
Nakajima M, Okuyama S and Furukawa S: 4-Methylcatechol-induced heme
oxygenase-1 exerts a protective effect against oxidative stress in
cultured neural stem/progenitor cells via PI3 kinase/Akt pathway.
Biomed Res. 31:45–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park SY, Park da J, Kim YH, Kim Y, Kim SG,
Shon KJ, Choi YW and Lee SJ: Upregulation of heme oxygenase-1 via
PI3K/Akt and Nrf-2 signaling pathways mediates the
anti-inflammatory activity of Schisandrin in Porphyromonas
gingivalis LPS-stimulated macrophages. Immunol Lett.
139:93–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun Y, Cui Y and Wang J: Effects of
sevoflurane on endotoxin-induced oxidative stress response of the
lungs in rats. Chin J Anesthesiol. 8:735–737. 2006.(In
Chinese).
|
|
13
|
Crawford MW, Lerman J, Saldivia V and
Carmichael FJ: Hemodynamic and organ blood flow responses to
halothane and sevoflurane anesthesia during spontaneous
ventilation. Anesth Analg. 75:1000–1006. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coimbra R, Melbostad H, Loomis W, Porcides
RD, Wolf P, Tobar M and Hoyt DB: LPS-induced acute lung injury is
attenuated by phosphodiesterase inhibition: effects on
proinflammatory mediators, metalloproteinases, NF-kappaB, and
ICAM-1 expression. J Trauma. 60:115–125. 2006. View Article : Google Scholar
|
|
15
|
Gao JZ, LV FH, Teng QL and Zhang JY: Role
of adiponectin/phosphatidylinositol 3-kinase/protein kinase B
signaling pathway on limb ischemic preconditioning on myocardial
protection. Afr J Biotechnol. 11:10976–10980. 2012.
|
|
16
|
Al-Amran FG, Hadi NR and Hashim AM:
Leukotriene biosynthesis inhibition ameliorates acute lung injury
following hemorrhagic shock in rats. J Cardiothorac Surg. 6:812011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Itoh S, Taketomi A, Harimoto N, et al:
Antineoplastic effects of gamma linolenic acid on hepatocellular
carcinoma cell lines. J Clin Biochem Nutr. 47:81–90. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu AL, Lu CY, Wang TS, Tsai CW, Liu KL,
Cheng YP, Chang HC, Lii CK and Chen HW: Induction of heme oxygenase
1 and inhibition of tumor necrosis factor alpha-induced
intercellular adhesion molecule expression by andrographolide in
EA. hy926 cells. J Agric Food Chem. 58:7641–7648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chao CY, Lii CK, Tsai IT, Li CC, Liu KL,
Tsai CW and Chen HW: Andrographolide inhibits ICAM-1 expression and
NF-κB activation in TNF-α-treated EA. hy926 cells. J Agric Food
Chem. 59:5263–5271. 2011.
|
|
20
|
Kim HR, Kim S, Kim EJ, Park JH, Yang SH,
Jeong ET, Park C, Youn MJ, So HS and Park R: Suppression of
Nrf2-driven heme oxygenase-1 enhances the chemosensitivity of lung
cancer A549 cells toward cisplatin. Lung Cancer. 60:47–56. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Loboda A, Jazwa A, Grochot-Przeczek A,
Rutkowski AJ, Cisowski J, Agarwal A, Jozkowicz A and Dulak J: Heme
oxygenase-1 and the vascular bed: from molecular mechanisms to
therapeutic opportunities. Antioxid Redox Signal. 10:1767–1812.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Funke C, Tomiuk J, Riess O, Berg D and
Soehn AS: Genetic analysis of heme oxygenase-1 (HO-1) in German
Parkinson’s disease patients. J Neural Transm. 116:853–859.
2009.PubMed/NCBI
|
|
23
|
Chung HT, Pae HO and Cha YN: Role of heme
oxygenase-1 in vascular disease. Curr Pharm Des. 14:422–428. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choi HG, Lee DS, Li B, Choi YH, Lee SH and
Kim YC: Santamarin, a sesquiterpene lactone isolated from
Saussurea lappa, represses LPS-induced inflammatory
responses via expression of heme oxygenase-1 in murine macrophage
cells. Int Immunopharmacol. 13:271–279. 2012.PubMed/NCBI
|
|
25
|
Soo Kim H, Young Park S, Kyoung Kim E,
Yeon Ryu E, Hun Kim Y, Park G and Joon Lee S: Acanthopanax
senticosus has a heme oxygenase-1 signaling-dependent effect on
Porphyromonas gingivalis lipopolysaccharide-stimulated
macrophages. J Ethnopharmacol. 142:819–828. 2012.PubMed/NCBI
|
|
26
|
Harada H, Sugimoto R, Watanabe A, et al:
Differential roles for Nrf2 and AP-1 in upregulation of HO-1
expression by arsenite in murine embryonic fibroblasts. Free Radic
Res. 42:297–304. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang L, Kou MC, Weng CY, Hu LW, Wang YJ
and Wu MJ: Arsenic modulates heme oxygenase-1, interleukin-6, and
vascular endothelial growth factor expression in endothelial cells:
roles of ROS, NF-κB, and MAPK pathways. Arch Toxicol. 86:879–896.
2012.PubMed/NCBI
|
|
28
|
Wu Y, Dong Y, Song P and Zou MH:
Activation of the AMP-activated protein kinase (AMPK) by nitrated
lipids in endothelial cells. PLoS One. 7:e310562012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen HH, Chen YT, Huang YW, Tsai HJ and
Kuo CC: 4-Ketopinoresinol, a novel naturally occurring ARE
activator, induces the Nrf2/HO-1 axis and protects against
oxidative stress-induced cell injury via activation of PI3K/AKT
signaling. Free Radic Biol Med. 52:1054–1066. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Graham JR, Hendershott MC, Terragni J and
Cooper GM: mRNA degradation plays a significant role in the program
of gene expression regulated by phosphatidylinositol 3-kinase
signaling. Mol Cell Biol. 30:5295–5305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zheng Z, Yang M, Zhang F, Yu J, Wang J, Ma
L, Zhong Y, Qian L, Chen G, Yu L and Yan M: Gender-related
difference of sevoflurane postconditioning in isolated rat hearts:
focus on phosphatidylinositol-3-kinase/Akt signaling. J Surg Res.
170:e3–e9. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yao YT, Fang NX, Shi CX and Li LH:
Sevoflurane postconditioning protects isolated rat hearts against
ischemia-reperfusion injury. Chin Med J (Engl). 123:1320–1328.
2010.
|
|
33
|
Wang Y, Romigh T, He X, Orloff MS,
Silverman RH, Heston WD and Eng C: Resveratrol regulates the
PTEN/AKT pathway through androgen receptor-dependent and
-independent mechanisms in prostate cancer cell lines. Hum Mol
Genet. 19:4319–4329. 2010. View Article : Google Scholar : PubMed/NCBI
|