Introduction
Hair cells (HCs) transform sound and balance signals
into electrical impulses in the cochlear and vestibular end organs.
By contrast to vertebrates that are able to spontaneously
regenerate new hair and supporting cells (1,2),
there is no effective way to stimulate their regeneration in
mammals once hair cells have been damaged by noise, ototoxic drugs
or aging, which hampers the treatment of sensorin, a neural hearing
impairment that is caused by hair cell loss.
Atoh1 is a basic helix-loop-helix transcription
factor that is crucial in hair cell formation (3,4).
Knockout of atoh1 in mice results in the absence of
differentiated hair cells and supporting cells, while Atoh1
overexpression in cultured explants or in vivo induces
ectopic hair cell-like cell (EHCLC) formation (3,5–14).
Studies in a novel atoh1 ‘self-terminating’
mouse model have suggested that Atoh1 expression level and duration
is crucial for inner and outer hair cell differentiation in
vivo (15). Therefore, we
aimed to investigate how Atoh1 affects EHCLC formation and whether
Atoh1 expression defines the fate of LER cells as either ectopic,
newly formed hair cells or nonsensory epithelial cells. In the
present study, cultured explants were infected with several virus
titers and EHCLC expression was detected in the LER at different
time points. It was identified that the formation of EHCLCs was
Atoh1 dependent, as no EHCLCs formed upon infection by GFP alone.
Following LER infection with an appropriate titer
(EGFP-atoh1) for Atoh1 expression (1.6×109
PFU/ml), EHCLC production was detected as early as 2.5 days and the
number of EHCLCs increased with time. Higher
Ad5-EGFP-atoh1 titers induced increased Atoh1
expression and a larger quantity of hair cell-like cells appeared
at earlier time points compared with lower titers. Lower
Ad5-EGFP-atoh1 titers induced less Atoh1
expression and required a greater duration for EHCLC formation.
Extremely low Ad5-EGFP-atoh1 titers induced
only weak Atoh1 expression and no formation of EHCLCs. Therefore,
Atoh1 expression levels define the fate of LER cells as either
EHCLCs or nonsensory epithelial cells, and greater Atoh1 expression
decreases the time required for EHCLC formation in the LER. These
data define an appropriate Ad5-EGFP-atoh1
titer range for ectopic hair cell formation and which will act as
an important guideline for future studies.
Materials and methods
Cultures of postnatal rat cochleae and
atoh1 gene infection
This study was approved by the Institutional Animal
Care and Animal Ethics Committee of Fudan University (Xuhui,
Shanghai, China). One-day-old postnatal (P1) SD rats were used for
the experiments and were purchased from Slaccas Experimental Animal
Company (Xuhui, Shanghai, China). The rats were sacrificed by
CO2 asphyxiation. The cochlear explants culture was
prepared as described previously (11,12).
The final concentrations of the Ad5-EGFP-atoh1
vector were 0.1×108, 0.4×108,
0.8×108, 1.6×108 and 2.4×108
PFU/ml in serum-free DMEM/F12. The control group (Ad5-EGFP)
included corresponding titers. The viruses used were as described
previously (6,7,11,12).
Tissue preparation and
immunofluorescence
The cochlear explants were fixed with 4%
paraformaldehyde for 30 min and then treated with 0.1% Triton X-100
plus 10% donkey serum for 30 min. Following this, the explants were
incubated with the following primary antibodies for 24 h at 4°C;
myosin7A (1:100; Proteus Biosciences Inc., Ramona, CA, USA),
myosin7A (1:200; Developmental Studies Hybridoma Bank, Iowa City,
IA, USA), p27kip1 (1:100; Cell Signaling Technology, Inc., CA, USA)
and Sox2 (1:300, Santa Cruz Biotechnology, Inc, Santa Cruz, CA,
USA). The preparation was washed 3–5 times in PBS and then
incubated with secondary antibodies for 2 h at 37°C in the dark.
The secondary antibodies included donkey anti-mouse/rabbit Alexa
Fluor 555 (1:1,000) and/or donkey anti-mouse/rabbit/goat (H+L)
Alexa Fluor 647 (1:1,000; Molecular Probes, Invitrogen Life
Technologies, Carlsbad, CA, USA). The specimens were visualized
with a Zeiss LSM 510 confocal laser-scanning microscope (Carl
Zeiss, Oberkochen, Germany) and only one image was captured by the
microscope.
Cell counting and statistical
analysis
Only cells at the LER region of the mid-basal turns
were counted. Using random samples, the cells in 200 μm segments
along the length of the cochlea were counted. Each group had at
least five different cochlear explants and each explant was sampled
at five areas. Ectopic hair cells were counted 3, 5, 7, 9 and 11
days post-infection. All the cell count was precisely performed by
manually analyzing the confocal images. The values are expressed as
the mean ± standard error and using a one-way ANOVA statistical
test when appropriate. P<0.05 was considered to indicate a
statistically significant difference.
Results
Ad5 vector transfection efficiency in the
LER
The
Ad5-EGFP/Ad5-EGFP-atoh1 transfection
efficiency in the LER (outside of the outer hair cells) was
determined by infection with different virus titers (Fig. 1). At a titer of 0.16×108
PFU/ml, only 8±2% of LER cells were GFP positive with weak green
fluorescence (Fig. 1J). At a titer
of 0.4×108 PFU/ml, 27±4% of LER cells were GFP positive
with moderate green fluorescence (Fig.
1A and 1D and 1G). At 0.8×108 PFU/ml, 91±7% of LER
cells were GFP positive with moderate-to-strong green fluorescence
(Fig. 1B and 1E and 1H). At
1.6×108 PFU/ml, 94±9% of LER cells were GFP positive
with strong green fluorescence (Fig.
1C and 1F and 1I). However, when 2.4×108 PFU/ml was
used, the cultured explants disintegrated (Fig. 1K). Higher viral infection
efficiency was observed with increasing titer, because the
transfection efficiency of 0.4×108 PFU/ml was
significantly higher than that of 0.16×108 PFU/ml (n=5,
P<0.05) and that of 0.8×108 PFU/ml was significantly
higher than that of 0.4×108 PFU/ml (n=5, P<0.05),
whereas the transfection efficiency of 1.6×108 PFU/ml
was similar to 0.8×108 PFU/ml (n=5, P>0.05). However,
the fluorescence intensity at 1.6×108 PFU/ml was higher
than at 0.8×108 PFU/ml. Therefore, it was concluded that
the most effective virus titer was 1.6×108 PFU/ml.
 | Figure 1Different Ad5 vector infection rates
in the LER at different titers. (A and D) At 0.4×108
PFU/ml, a number of LER cells were GFP positive with moderate green
fluorescence. (B and E) At 0.8×108 PFU/ml, the majority
of LER cells were GFP positive with moderate to strong green
fluorescence. (C and F) Following infection at 1.6×108
PFU/ml, the majority of LER cells were GFP positive with strong
green fluorescence. (G–I) Magnified views of (D), (E) and (F). (J)
Following infection at 0.16×108 PFU/ml, sporadic cells
were GFP positive with weak green fluorescence. (K) Following
infection at 2.4×108 PFU/ml, the virus damaged the
cochlear explants. (L) Histogram of the
Ad5-EGFP-atoh1/Ad5-EGFP infection rate (x-axis, virus titer;
y-axis, number of GFP/number of DAPI). Green, GFP; blue, myosin7A;
purple, DAPI. Scale: A–F, 100 μm; G–J, 50 μm. White brackets
indicate the sensory epithelium. LER, lesser epithelial ridge; Ad5,
human adenovirus serotype 5. |
Formation of new EHCLCs at the LER is
Atoh1 dependent
Following Ad-EGFP infection of the cultured
explants (Fig. 2A), the LER cells
presented robust EGFP fluorescence, however no myosin7A-positive
cells were observed. The LER cells were unable to differentiate
into hair cells. Following Ad5-EGFP-atoh1
infection, the LER was the target (Fig. 1B). Consistent with previous studies
(11,14), Ad5-EGFP-atoh1
infection resulted in the induction of myosin7A-positive cells in
the LER regions (Fig. 2B),
suggesting that these newly formed hair cells were Atoh1
overexpression dependent. Many of the EGFP-positive cells were
myosin7A negative, despite having been infected with
Ad5-EGFP-atoh1. To determine whether the Atoh1
expression level or duration led to this phenomenon, different
Ad5-EGFP-atoh1 titers were utilized, and the
quantities and percentages of new hair cells were detected at
different time points in the following study.
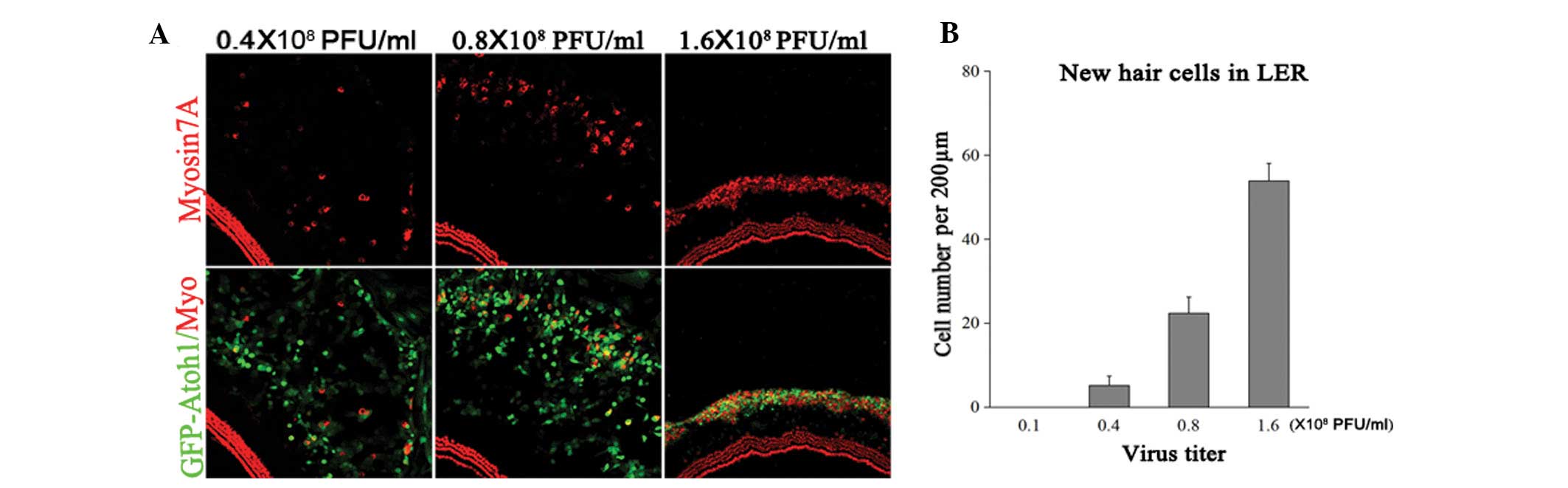
EHCLC formation requires a certain Atoh1
expression level
To address whether new EHCLC formation depends on
Atoh1 expression, cultured explants were treated with
Ad-EGFP-atoh1 at four different titers:
0.16×108, 0.4×108, 0.8×108 and
1.6×108 PFU/ml. The samples were fixed at five days
following viral infection (DVI) and the numbers of EGFP-myosin7A
double-positive cells and EGFP-positive cells were counted in the
LER (Fig. 3). At
0.16×108 PFU/ml Ad5-EGFP-atoh1, no
myosin7A-positive cells were detected, implying that low Atoh1
expression was unable to induce hair-cell-like cell formation
(n=5). At 0.4×108 PFU/ml
Ad5-EGFP-atoh1, there were 5±2 myosin7A-EGFP
double-positive cells per 200 μm in the LER (4±3% of all
EGFP-positive cells). At 0.8×108 PFU/ml
Ad5-EGFP-atoh1, there were 22±4 myosin7A-EGFP
double-positive cells per 200 μm in the LER (14±5% of all
EGFP-positive cells). At 1.6×108 PFU/ml
Ad5-EGFP-atoh1, there were 54±4 myosin7A-EGFP
double-positive cells per 200 μm in LER (57±13% of all
EGFP-positive cells; n=5). The number of EHCLCs in the higher virus
titer groups was significantly greater compared with the lower
virus titer groups (Fig. 2B).
Furthermore, the LER to hair cell-like cell conversion rate was
significantly enhanced in the higher than in the lower virus titer
group. These data demonstrate that increasing the virus titer
increased Atoh1 expression and this subsequently increased the
myosin7A-positive cell number in the LER. If Atoh1 expression was
too low, few LER cells converted to hair-cell-like cells. Thus,
EHCLCs production was dependent on specific Atoh1 expression
levels.
Higher Atoh1 expression reduces the
duration of EHCLC formation in the LER
The number of Atoh1-induced EHCLCs increased with
time. When applied to cultured explants with 0.16×108
PFU/ml Ad5-EGFP-atoh1, no myosin-positive cells were
detected even at 11 DVI. At 0.4×108 PFU/ml
Ad5-EGFP-atoh1, 5±2 EHCLCs per 200 μm were detected as early
as 5 DVI in the LER, which increased to 12±2 at 7 DVI, 11±2 at 9
DVI and 11±1 at 11 DVI (Fig. 4A and
D). At 0.8×108 PFU/ml Ad5-EGFP-atoh1, 14±1
EHCLCs per 200 μm were detected as early as 3 DVI in the LER, which
increased at 22±4 on 5 DVI, 29±7 at 7 DVI, 31±2 at 9 DVI and 31±1
at 11 DVI (Fig. 4B and D). At
1.6×108 PFU/ml Ad5-EGFP-atoh1, we detected
myosin7A-positive cells as early as 60 h following atoh1
infection, 31±2 EHCLCs per 200 μm were detected at 3 DVI in the
LER, which increased to 54±4 on 5 DVI, 70±5 on 7 DVI, 67±6 on 9 DVI
and 67±2 on 11 DVI (Fig. 4C and
D). Therefore, at a low titer (0.4×108 PFU/ml) with
low Atoh1 expression, EHCLC formation required a longer time (5
days). At a higher titer (1.6×108 PFU/ml) with high
Atoh1 expression, however, EHCLC formation required only 2.5 days.
EHCLCs increased with time but remained constant from 7–11 days in
all groups (Fig. 4D). In
conclusion, the Atoh1 expression level critically affected the time
required for EHCLC formation.
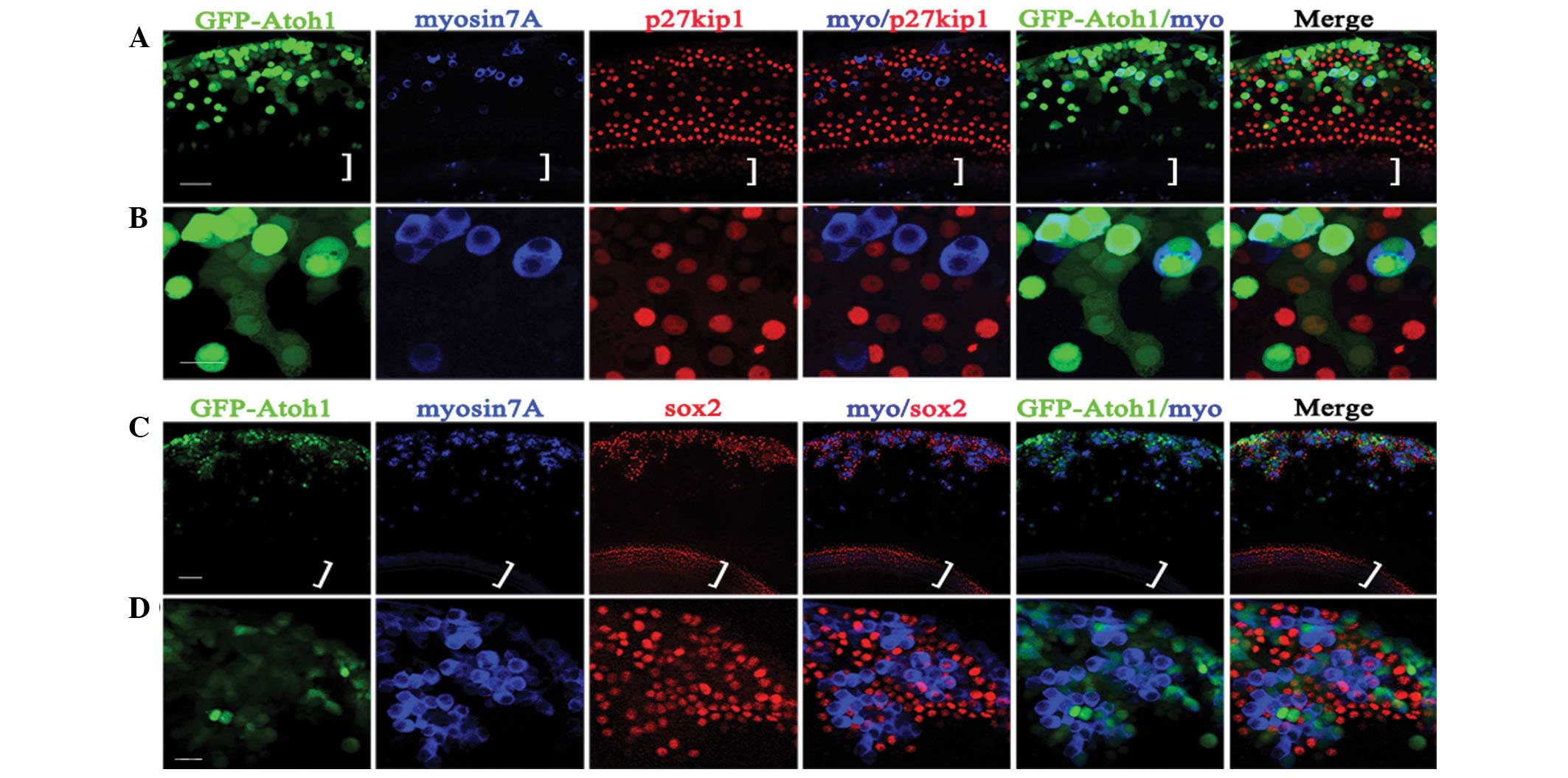
Atoh1 expression defines the fate of LER
cells
The data indicated that the number of Atoh1-induced
EHCLCs increased with time but this effect ceased at 7–11 days,
regardless of the titer (Fig. 4D).
Despite infection of cultured cochlear explants with
Ad-EGFP-atoh1 at 1.6×108 PFU/ml,
only ~71% of infected cells in the LER were able to
transdifferentiate into hair cell-like cells. Following
Ad-EGFP-atoh1 infection, a number of the infected LER cells
(EGFP positive) transformed to hair-cell-like cells (myosin7A
positive) with an oblong or round shape, whereas other cells
remained nonsensory epithelial cells (p27kip1) with a polygonal,
flat shape (Fig. 5A and B).
Furthermore, many myosin7A-positive cells clustered in the LER with
sox2-positive cells surrounding them, indicating that
hair-cell-like cells may induce supporting cell formation (Fig. 5C and D).
Discussion
The human Ad5 vector, encoding both Atoh1 and the
reporter gene EGFP, is a useful tool for new hair cell production
due to its high transfection efficiency, low level of target tissue
damage and ease of control (6–12,14).
The viral titration in the present study indicated that appropriate
titers induce optimal Atoh1 expression. At titers
>2.4×108 PFU/ml, cochlear cultured explants may be
severely damaged. At titers <0.16×108 PFU/ml,
although weak Atoh1 expression was observed, it was not sufficient
to generate ectopic hair cell formation. Our data indicate that
0.4–1.6×108 PFU/ml Ad5-EGFP-atoh1
is an efficient and safe titer range for hair-cell-like cell
formation in cultured cochlear explants.
At titers of 0.16–1.6×108 PFU/ml, higher
infection efficiency and expression levels were observed (Fig. 1). GFP alone did not induce EHCLCs
expression, whereas Ad5-EGFP-atoh1 did induce
robust EHCLCs formation. Thus, EHCLCs formation in the LER was
Atoh1 expression dependent. At titers <0.1×108
PFU/ml, although a number of weakly GFP-positive cells were
identified, no myosin7A-positive cells were detected, even at 11
DVI. At titers of 0.4×108 PFU/ml
Ad5-EGFP-atoh1, only ~4% of
atoh1-infected cells converted to hair-cell-like cells by 5
DVI in the LER. At titers of 1.6×108 PFU/ml, ~54% of
atoh1-infected cells converted to hair cell-like cells.
These data suggest that hair cell formation requires a certain
level of Atoh1, with higher expression inducing more hair cell-like
cell formation.
At 0.4–1.6×108 PFU/ml
Ad5-EGFP-atoh1, EHCLC formation increased with
time. At 0.4×108 PFU/ml, EHCLC formation required 5
days. However, at 1.6×108 PFU/ml, ectopic hair cell
formation only required 2.5 days. Thus, greater Atoh1 expression
shortens the time required for EHCLC formation. Furthermore, the
number of EHCLCs increased with time but then ceased increasing at
7 DVI for all titers. Even at 1.6×108 PFU/ml, only ~71%
of Ad5-EGFP-atoh1-infected cells in the LER
transdifferentiated into hair cell-like cells. The fate of the
non-differentiated cells may help explain this phenomenon.
The data from the present study further revealed
that hair cell formation requires a certain Atoh1 expression level;
if it was too low, the LER did not convert into hair cell-like
cells. A number of Ad5-EGFP-atoh1-infected LER
cells (EGFP positive) had already converted to hair cell-like cells
(myosin7A positive) with an oblong or round shape at 3 DVI, while
the other LER cells remained nonsensory epithelial cells (p27kip1
positive) with a polygonal, flat shape (Fig. 5A and B). At 3 DVI, the majority of
myosin7A-positive cells exhibited a strong green fluorescence and
p27kip1-positive cells appeared to have weak or no green
fluorescence. However, numerous myosin7A-positive cells were
observed clustered in the LER with sox2-positive cells surrounding
them (Fig. 5C and D), indicating
that hair cell-like cells induce supporting cell formation, which
has also been previously reported (10). Therefore, the majority of
Ad5-EGFP-atoh1-infected LER cells (with
sufficient Atoh1 expression) converted into hair cells and induced
the surrounding nonsensory epithelial cells to transform into
supporting cells.
In the present study, an appropriate virus titer
range for infecting cultured cochlear explants was examined,
providing highly efficient infection and conversion rates but
reducing the infection side effects. Atoh1 expression is critical
to hair cell formation, as it defines the fate of LER cells as
either hair cell-like cells or nonsensory epithelial cells. The
present study provides an important guideline for future
investigations to develop novel gene therapy strategies in the
treatment of deafness.
Acknowledgements
This study was supported by the following: Major
State Basic Research Development Program of China (973 Program)
(2011CB504500, 2011CB504506) to H. L.; NSFC grant 81028003/H1305 to
P. C and F. C.; Key Basic Research Project of Shanghai Committee of
Science and Technology (no. 10JC1402500) to F. C.; 81200740/H1304
to J. Y.; Innovation Program of Shanghai Committee of Science and
Technology (no. 11411952300) to F. C.
References
|
1
|
Corwin JT and Cotanche DA: Regeneration of
sensory hair cells after acoustic trauma. Science. 240:1772–1774.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ryals BM and Rubel EW: Hair cell
regeneration after acoustic trauma in adult Coturnix quail.
Science. 240:1774–1776. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bermingham NA, Hassan BA, Price SD,
Vollrath MA, Ben-Arie N, Eatock RA, et al: Math1: an essential gene
for the generation of inner ear hair cells. Science. 284:1837–1841.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen P, Johnson JE, Zoghbi HY and Segil N:
The role of Math1 in inner ear development: Uncoupling the
establishment of the sensory primordium from hair cell fate
determination. Development. 129:2495–2505. 2002.PubMed/NCBI
|
|
5
|
Gubbels SP, Woessner DW, Mitchell JC,
Ricci AJ and Brigande V: Functional auditory hair cells produced in
the mammalian cochlea by in utero gene transfer. Nature.
455:537–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han Z, Yang JM, Chi FL, Cong N, Huang YB,
Gao Z, et al: Survival and fate of transplanted embryonic neural
stem cells by Atoh1 gene transfer in guinea pigs cochlea.
Neuroreport. 21:490–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang Y, Chi F, Han Z, Yang J, Gao W and
Li Y: New ectopic vestibular hair cell-like cells induced by Math1
gene transfer in postnatal rats. Brain Res. 1276:31–38. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Izumikawa M, Minoda R, Kawamoto K,
Abrashkin KA, Swiderski DL, Dolan DF, et al: Auditory hair cell
replacement and hearing improvement by Atoh1 gene therapy in deaf
mammals. Nat Med. 11:271–276. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kawamoto K, Ishimoto S, Minoda R, Brough
DE and Raphael Y: Math1 gene transfer generates new cochlear hair
cells in mature guinea pigs in vivo. J Neurosci. 23:4395–4400.
2003.PubMed/NCBI
|
|
10
|
Woods C, Montcouquiol M and Kelley MW:
Math1 regulates development of the sensory epithelium in the
mammalian cochlea. Nat Neurosci. 7:1310–1318. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang J, Bouvron S, Lv P, Chi F and Yamoah
EN: Functional features of trans-differentiated hair cells mediated
by Atoh1 reveals a primordial mechanism. J Neurosci. 32:3712–3725.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang J, Cong N, Han Z, Huang Y and Chi F:
Ectopic hair cell-like cell induction by Math1 mainly involves
direct transdifferentiation in neonatal mammalian cochlea. Neurosci
Lett. 549:7–11. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang SM, Chen W, Guo WW, Jia S, Sun JH,
Liu HZ, et al: Regeneration of stereocilia of hair cells by forced
Atoh1 expression in the adult mammalian cochlea. PLoS One.
7:e463552012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng JL and Gao WQ: Overexpression of
Math1 induces robust production of extra hair cells in postnatal
rat inner ears. Nat Neurosci. 3:580–586. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pan N, Jahan I, Kersigo J, Duncan JS,
Kopecky B and Fritzsch B: A novel Atoh1 ‘self-terminating’ mouse
model reveals the necessity of proper Atoh1 level and duration for
hair cell differentiation and viability. PLoS One.
7:e303582012.
|