Introduction
The prolactin (PRL) family is comprised of multiple
proteins that regulate a plethora of biological functions (1–6). In
rats and mice, the PRL gene family consists of at least 20
well-characterized genes, including placental lactogens, PRL-like
proteins (PLPs), PRL-related proteins, proliferin and
proliferin-related protein (7).
The PRL family genes, located on chromosome 13 in mice and
chromosome 17 in rats, are mainly expressed in the pituitary gland,
uterus and placenta (1–6). These proteins demonstrate a unique
spatio-temporal expression profile and strongly influence various
aspects of gestation (1,7–9).
PLP-J, also termed PLP-I, Prlpj, Prlpi or Prl3c1, is
a protein belonging to the PRL family. PLP-J was first reported in
three independent studies (8,10,11).
Using northern blotting, Toft and Linzer (10) displayed PLP-J expression in RNA
samples isolated from maternal decidua but not in those from the
heart, lung, kidney, liver, muscle, spleen, thymus and testis. They
demonstrated that PLP-J mRNA production is limited to early
gestation, (peaking on day 7, and beginning to decrease on day 9,
with undetectable levels by day 11) using the technique of in
situ hybridization in placental tissue with a PLP-J probe.
Another study demonstrated that the decidual expression of PLP-J is
independent of extra-embryonic or embryonic factors (12).
Alam et al (13) indicated that PLP-J promotes
proliferation of uterine stromal cells but inhibits that of
endothelial cells. PLP-J has also been demonstrated to interact
with heparin-containing molecules, including syndecan-1 which is
expressed in uterine stromal and endothelial cells. The restricted
expression of PLP-J and its unique interaction with basal and
endothelial cells suggest that this protein may be involved in the
development of decidual cells and endometrial vasculature (13).
Preliminary experiments of the present study suggest
that PLP-J may be present in the mouse testis. By characterizing
the expression and cellular localization of PLP-J in relation to
developmental changes in the mouse testis, the current study
attempted to determine whether the testis is a source of PLP-J in
the mouse. Molecular and immunological approaches, including
reverse transcription (RT)-polymerase chain reaction (PCR),
immunofluorescence, in situ hybridization and western
blotting were employed to investigate the mRNA and protein levels
of PLP-J in the mouse testis. In addition, the effects of PLP-J on
testosterone production, cell proliferation, and apoptosis were
studied in the murine TM3 Leydig cell line.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM)/F-12 and
fetal bovine serum (FBS) were purchased from HyClone Laboratories,
Inc., (Logan, UT, USA). The SYBR® PrimeScript™ RT-PCR
kit (Perfect Real Time) was purchased from Takara Biotechnology
(Dalian, China). The Bicinchoninic Acid (BCA) Protein Assay kit,
Protein Extraction kit, BeyoECL Plus Western Blotting Detection
kit, and Annexin V-Fluorescein Isothiocyanate (FITC) Apoptosis
Detection kit were purchased from Beyotime Institute of
Biotechnology (Jiangsu, China). Testosterone Immunoassay kits were
obtained from USCNK (Wuhan, China). Antibody for 3β-HSD1 (sc-30821)
was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA,
USA). The In Situ Hybridization Detection kit was purchased
from Haoyang (Tianjing, China). All other chemicals were purchased
from Sangon Biotech (Shanghai, China).
Animals and tissue preparation
A total of 24 male Balb/c mice at 18 days, 4 months,
and 16 months of age (n=8 in each age group) and a New Zealand
white rabbit were obtained from the Chongqing Medical University
Animal Center (Chongqing, China). Mice were sacrificed by
intraperitoneal injection of 10% chloral hydrate solution at the
dose of 8 ml/kg. Immediately after euthanasia, the testes were
removed and washed with saline (0.9% NaCl). The left testis was
fixed in a 4% paraformaldehyde solution for in situ
hybridization and immunohistochemistry, while the right testis was
used for protein and RNA extraction. The animal protocols conformed
to those approved by the Chongqing Medical University Animal Care
and Use Committee.
Cell culture
TM3 Leydig cells were obtained from The Cell Bank of
the Chinese Academy of Sciences (Shanghai, China)) and 293FT cells
were from Invitrogen (Carlsbad, CA, USA). TM3 Leydig cells were
cultured in F12/DMEM supplemented with 10% FBS. 293FT cells were
cultured in DMEM containing 10% FBS. All cells were placed in an
incubator containing 95% air and 5% CO2 at 37°C, and the
culture media were replaced every 2 days.
Preparation of the polyclonal
antibody
A polyclonal anti-PLP-J antibody was prepared to
detect PLP-J protein expression. Based on the amino acid sequence
of PLP-J (NP_038794.1; http://www.ncbi.nlm.nih.gov/protein/7305407), the
following peptide fragments were designed: RYDRKSNEEI, IQPGIEENNE,
KTNEDLLK, KMYKILD, VLTHLGSYDGMM, RELRSSKKSK and FFYCLRKDTK. These
fragments were joined, along with connecting residues, to yield the
final sequence:
RYDRKSNEEIGSIQPGIEENNEAKTNEDLLKKMYKILDGSVLTHLGSYDGMMGSRELRSSKKSKFFYCLRKDTK.
The amino acid sequence was converted to a nucleotide sequence,
which was generated via gene synthesis following incorporation of
KpnI and HindIII sites, and cloned into pET-30a
(Novagen, Madison, WI, USA) via these two sites. The resulting
plasmid was used to transform BL21-competent cells prior to the
selection of positive clones. Protein production was facilitated by
isopropyl β-D-1-thiogalactopyranoside induction.
To immunize a single New Zealand white rabbit, 200
μg of the recombinant protein was subcutaneously injected followed
by three boosters of 100 μg recombinant protein at 21 days, 42 days
and 63 days. Subsequent to the last booster, a blood sample was
obtained from an ear vein for ELISA analysis to determine the
titer. If the desired level (titer of antibody >12,800) was
reached, blood was drawn from the carotid artery and centrifuged at
5,000 × g for 10 min to collect antiserum, which was subjected to
protein G affinity purification. The purity of the antiserum was
determined by western blot analysis. The antiserum was divided into
aliquots, and the pre-immune serum was used as a control.
Quantitative RT-PCR
The expression of PLP-J mRNA in testis tissues was
assessed by RT-PCR. The primer designs were based on the sequences
of mouse PLP-J (NM_013766; http://www.ncbi.nlm.nih.gov/nuccore/7305406). The
primer sequences were as follows: Forward,
5′-ACCAAGATGTGCCAAACCATTTCTA-3′; and reverse,
5′-CAGCTCTTGTCATCATCCCATCA-3′. The predicted size of the RT-PCR
product was 163 bp. For mouse β-actin amplification, the primer
sequences were as follows: Forward, 5′-TCGTGCGTGACATCAAAGAG-3′; and
reverse, 5′-CAAGAAGGAAGGCTGGAAAA-3′. The length of the RT-PCR
product was 177 bp.
Briefly, total RNA from the mouse testis was
extracted using TRIzol reagent according to the manufacturer’s
instructions. First-strand cDNA was synthesized using the
SYBR® PrimeScript™ RT-PCR kit (Perfect Real Time) in the
thermal cycler (CFX96; Bio-Rad, Hercules, CA, USA). Each RT-PCR
reaction was performed in triplicate in a total volume of 20 μl
reaction mixture [2 μl cDNA, 6.8 μl H2O, 10 μl
SYBR® Premix Ex Taq™, 0.6 μl of each of the forward and
reverse primers (10 μM)]. The PCR program consisted of 1 min at
95°C, followed by 40 cycles of 10 sec at 95°C and 30 sec at 60°C.
Data were analyzed according to the 2−ΔΔCt method
(14). The expression level of
PLP-J mRNA was normalized to the concurrent measurement of β-actin
mRNA.
Probe synthesis and in situ
hybridization
In concordance with the gene sequence of murine
PLP-J, a probe with the sequence 5′-GGCAGTCACATATACCCACAGGAACAT-3′
was designed in the current study, which was then synthesized at
Sangon Biotech. To identify the cell type expressing PLP-J mRNA,
in situ hybridization was performed on 5-μm tissue sections.
Paraffin-embedded tissues were dewaxed and blocked in
H2O2 solution for 20 min at room temperature
to prevent endogenous catalase activity. After rinsing with 0.01 M
phosphate-buffered saline (PBS) 3 times, acetylation solution was
added dropwise until it covered the surface of the tissues, which
were then incubated at room temperature for 10–30 min. The
specimens were washed with PBS 3 times and rinsed once with 0.2X
saline sodium citrate (SSC) buffer. Prehybridization buffer was
added dropwise until it covered the tissues, which were then
incubated at 37°C for 2 h. The samples were rinsed 3 times with
0.2X SSC for 5 min. Hybridization buffer was added dropwise until
it covered the tissues, which were incubated at 37°C for 8 h and
then washed with SSC. Mouse Anti-Digoxin-Biotin Antibody solution
was added and the samples incubated at 37°C for 45 min. The
specimens were rinsed 3 times with 0.01 M PBS for 5 min. A working
solution of high-performance streptavidin peroxidase complex (3 ml)
was added to the specimens, incubated at 37°C for 45 min, and then
washed with PBS. The slides were stained with 3,3′-diaminobenzidine
and counter-stained with hematoxylin, which stained the nuclei
blue. The slides were then observed with a BX51 microscope
(Olympus, Tokyo, Japan).
Immunofluorescent staining of PLP-J
To examine whether PLP-J is expressed in Leydig
cells, the cellular colocalization of PLP-J with 3β-hydroxysteroid
dehydrogenase (HSD) 1 was examined in the testes of adult mice
using a double immunofluorescence staining method. Briefly, the
sections were incubated for 48 h at 4°C with a mixture of rabbit
anti-PLP-J IgG and goat anti-3β-HSD1 IgG. Subsequently, the
sections were rinsed in 0.01 M PBS (pH 7.4) and then incubated for
24 h at 4°C with a mixture of FITC-conjugated anti-rabbit IgG and
tetramethyl rhodamine isothiocyanate-conjugated anti-goat IgG
(ZSGB-Bio, Beijing, China). The tissues were then examined under
the BX51 microscope.
For immunofluorescent staining of PLP-J in TM3
cells, the TM3 cells were fixed with 4% paraformaldehyde in PBS for
15 min at room temperature, followed by permeabilization in PBS
containing 0.5% Triton X-100 at room temperature for 10 min. The
cells were then immersed in blocking solution for 1 h. The cells
were immunolabeled with rabbit anti-PLP-J primary antibody
overnight at 4°C. Following 3 washes with PBS, the cells were
incubated with FITC-conjugated anti-rabbit IgG at room temperature
for 1 h. The slides were then observed and analyzed using a
TE-2000U fluorescence microscope (Nikon, Tokyo, Japan).
Western blotting
In order to isolate protein, tissues or cells were
homogenized and lysed with radioimmunoprecipitation assay buffer.
Insoluble material was removed by centrifugation at 12,000 × g for
10 min at 4°C, and the supernatant was collected. The protein
concentration was determined using the BCA assay. For western
blotting, samples were subjected to SDS-PAGE and then transferred
to a polyvinylidine fluoride membrane, followed by incubation with
anti-PLP-J or anti-β-actin polyclonal antibody. Membranes were
washed briefly in Tris-buffered saline with Tween 20 and incubated
with secondary antibodies. The antigen-antibody complexes were then
detected using the BeyoECL kit according to the manufacturer’s
instructions.
Lentiviral-mediated overexpression of
PLP-J in TM3 cells
To investigate the biological activities of PLP-J
in vitro, a PLP-J-expressing lentivirus was generated, which
was transfected into TM3 cells to induce upregulation of PLP-J
expression. The PLP-J sequence was obtained from Genbank
(NM_013766.2; http://www.ncbi.nlm.nih.gov/nuccore/NM_013766.2),
synthesized, and then cloned into the LV5 vector (GenePharma Co.,
Ltd., Shanghai, China). Lentiviruses were then produced in 293T
cells, titrated, and used to transfect the TM3 cell line.
Testosterone measurement
To analyze the effect of PLP-J overexpression on
testosterone production in TM3 cells, TM3-N cells (transfected with
control lentivirus) and TM3-PLP-J cells (transfected with PLP-J
lentivirus) were cultured for 48 h. The culture supernatants and
cells were collected for testosterone analysis according to the
manufacturer’s instructions.
Flow cytometry for cell apoptosis
analysis
Harvested TM3 cells were centrifuged at 300 × g for
10 min and the supernatant was removed. They were then washed once
with 1X binding buffer. The cells were resuspended in 200 μl
binding buffer prior to incubation with 5 μl Annexin V and 10 μl
propidium iodide (PI) at room temperature for 15 min. Flow
cytometric analysis was conducted using a FACSVantage SE flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
Flow cytometric analysis of the cell
cycle
TM3 cells were trypsinized and centrifuged at 300 ×
g for 10 min. Following rinsing with PBS, the cells were fixed in
70% ethanol. TM3 cells were then centrifuged for 5 min and washed
with 1X PBS. Next, the cells were incubated for 30 min in Hank’s
Balanced Salt Solution containing PI (10 μg/ml) and RNase (100
μg/ml) at room temperature, followed by analysis using the
FACSVantage SE flow cytometer.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Significant differences were evaluated using one-way analysis of
variance with the Newman-Keuls method. P<0.05 was considered to
indicate a statistically significant difference.
Results
Preparation and validation of anti-PLP-J
polyclonal antibodies
To evaluate the production of PLP-J, polyclonal
anti-PLP-J antibodies were prepared. New Zealand white rabbits were
immunized 4 times with a recombinant protein containing multiple
PLP-J epitopes, and the resulting antiserum was shown by ELISA to
have a titer of 1:25,600. Subsequent protein G affinity
purification yielded an antibody concentration of 1.66 mg/ml, as
revealed by UV spectroscopy. To verify the specificity of the
anti-PLP-J antibody, 293FT cells were transfected with
PLP-J-expressing lentivirus or control lentivirus and analyzed via
western blotting using the polyclonal anti-PLP-J antibody. The
results demonstrated that 293FT cells transfected with the PLP-J
lentivirus produced a specific band with the expected size, which
was absent in 293FT cells transfected with control virus,
indicating that the prepared anti-PLP-J antibody specifically
recognized its antigen (Fig. 1)
and was suitable for subsequent experiments.
Localization of PLP-J mRNA and protein in
murine testis and TM3 cells
The expression of PLP-J was first analyzed by
RT-PCR, using RNA extracted from the testes of the mice. A single
amplification product of 163 bp was sequenced, which matched
perfectly with the PLP-J nucleotide sequence (data not shown),
indicating the presence of PLP-J mRNA in the testes.
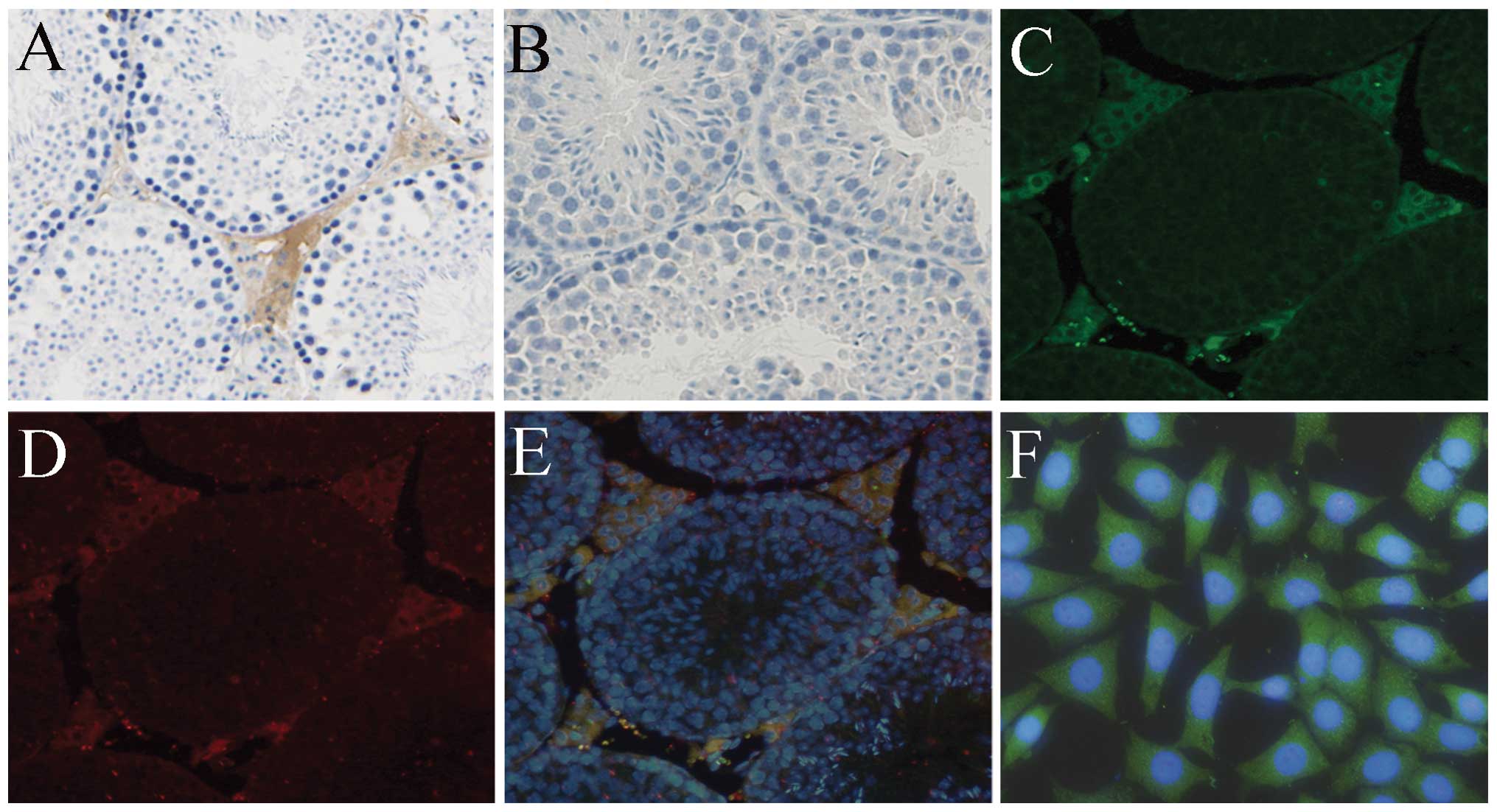
In situ hybridization was used to elucidate
the spatial distribution of PLP-J in the mouse testis. Positive
signals (brownish-yellow) were detected in the interstitial tissues
of the testis, but not in Sertoli and sperm cells (Fig. 2A). No signal was observed using the
sense cRNA PLP-J probe (Fig.
2B).
The localization of PLP-J in the mouse testis was
further studied by immunofluorescence using sections prepared from
testes of adult mice. PLP-J and 3β-HSD1 were colocalized in the
cytoplasm of Leydig cells (Fig.
2C–E). As 3β-HSD1 is known to be expressed in Leydig cells,
their colocalization indicated that PLP-J was also present in the
Leydig cells of testis interstitial areas. PLP-J immunofluorescence
of TM3 Leydig cells further revealed that this protein was
localized within the cytoplasm of Leydig cells (Fig. 2F).
PLP-J expression levels during
development
Using RT-PCR and western blotting, PLP-J expression
levels in the testes of male mice aged 18 days, 4 months, and 16
months were evaluated (Fig. 3).
The RT-PCR results indicated that PLP-J was expressed at all the
time points that were examined. PLP-J expression levels were
significantly higher in testes of 4-month-old mice compared with
levels in 18-day-old mice. In 16-month-old mice, PLP-J expression
levels were lower than those observed in 4-month-old mice, but
still significantly higher than levels observed in 18-day-old mice
(Fig. 3A). Western blotting was
then employed to further clarify the developmental changes in PLP-J
protein expression using the polyclonal anti-PLP-J antibody.
Similar to the observed mRNA expression pattern, expression levels
of PLP-J protein were age-dependent (Fig. 3B and C).
Lentiviral-mediated PLP-J overexpression
efficiently upregulates PLP-J expression in TM3 cells
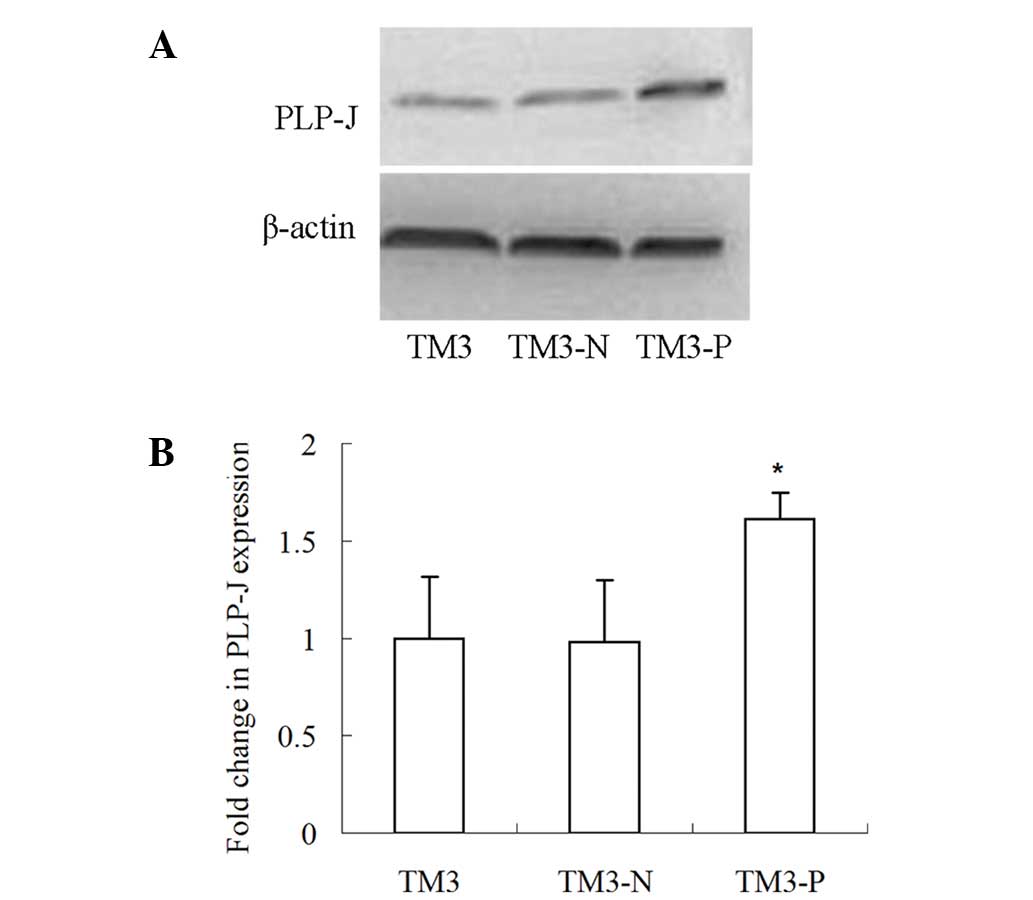
To investigate the function of PLP-J in TM3 cells,
its expression in these cells was examined and it was discovered
that the PLP-J expression levels were relatively low low (data not
shown).. Thus, to accommodate the functional study, its expression
was upregulated. A PLP-J-expressing lentivirus was concentrated via
centrifugation to reach a titer of 1×109 TU/ml, which
was able to significantly boost PLP-J expression in TM3 cells
(P<0.05; Fig. 4), further
corroborating the specificity of the antibody.
PLP-J overexpression does not affect
basal testosterone production
The basal testosterone secretion level in cultured
TM3 Leydig cells was not significantly different between TM3,
TM3-N, and TM3-P cells (P>0.05; Fig. 5). This result implies that PLP-J is
not directly involved in testosterone production in
vitro.
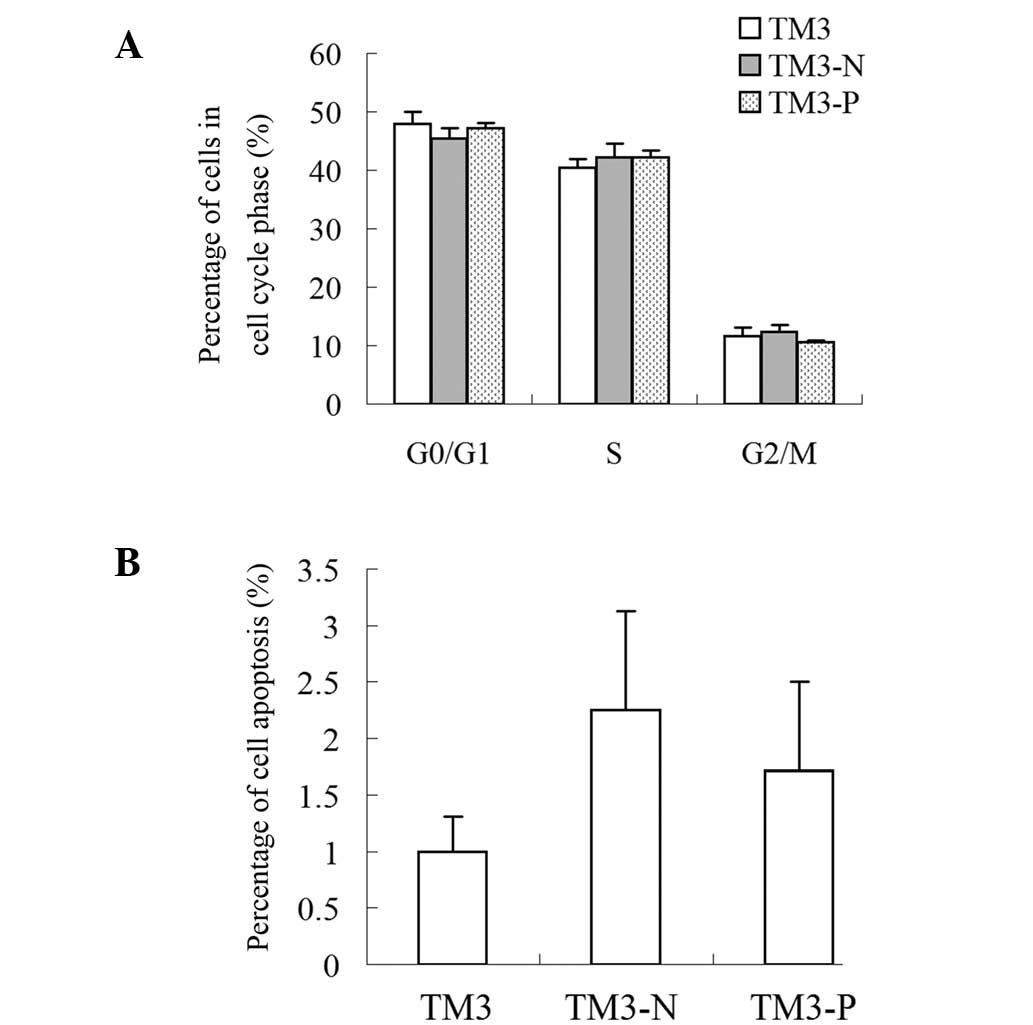
PLP-J overexpression does not affect cell
cycle progression and apoptosis in TM3 cells in vitro
The flow cytometry results demonstrated that PLP-J
overexpression did not significantly affect cell cycle progression
(Fig. 6A) or apoptosis (Fig. 6B).
Discussion
In female rats, the gestation process involves
multiple physiological events that require numerous growth hormones
and cytokines (15,16). These studies initially reported
that PLP-J was a hormone of pregnancy, and its expression was only
detected in the decidua of pregnant mice and not in other tissues
or organs. Therefore, previous studies on this gene have focused
solely on regulatory roles for PLP-J in female gestation.
To the best of our knowledge, the data presented in
the current study are the first to demonstrate that PLP-J is also
expressed in the postnatal mouse testis. This conclusion is based
on the results of molecular and immunological experiments. Primers
specific for PLP-J successfully amplified a gene fragment from the
mouse testis, and its sequence matched perfectly with the PLP-J
gene. In situ hybridization further revealed that PLP-J was
expressed in the Leydig cells but not in Sertoli or germ cells.
Expression levels of the PLP-J protein were examined by
immunofluorescence and western blotting, which was facilitated by
our production of a polyclonal anti-PLP-J antibody. The
immunofluorescence results indicated that PLP-J and 3β-HSD1 were
colocalized in Leydig cells of the testis, indicating that PLP-J is
expressed in Leydig cells. Using TM3 Leydig cells, it was observed
that PLP-J was localized in the cytoplasm.
To adapt to the physiological functions of different
developmental stages, the testis exhibits a stage-specific gene
expression profile (17–19). Therefore, the temporal expression
pattern of PLP-J in the mouse testis during development was further
explored in the current study. The results revealed that expression
of the PLP-J protein and mRNA displayed similar patterns at
different developmental stages: They were low in the 18-day-old
mice and highest in the 4-month-old animals; and the levels in the
16-month-old mice were lower than those in the 4-month-old mice,
but remained significantly higher than those in the 18-day-old
mice.
Together, the characterization of PLP-J expression
in the male mouse testis and the evaluation of its developmental
expression pattern in the present study suggest that this protein
has specific roles in male reproductive function.
In males, Leydig cells are the main producers of
testosterone and also synthesize cytokines to support the
development of Sertoli and germ cells. Therefore, this cell type is
indispensable for maintaining normal reproductive activity
(20).
A decrease in serum testosterone production is often
associated with infertility, osteoporosis, diminished energy and
muscle strength, poor cognition, reduced sexual behavior and
occurrence of depression (21–25).
Testosterone production by Leydig cells is regulated by complex
interplay between multiple molecules including those involved in
endocrine and paracrine signaling, and proper regulation is
critical for optimum reproductive capacity. As a result, a number
of Leydig cell-specific genes have been identified as important
regulators of reproductive processes (26–30).
As PLP-J is specifically expressed in Leydig cells of the testis
and its expression peaks in the adult, similar to the expression
pattern of testosterone, we hypothesized that PLP-J is also
involved in testosterone biogenesis.
Since PLP-J exhibited low expression levels in TM3
cells, a strategy of PLP-J overexpression was adopted in the
present study to analyze its function. Overexpression of PLP-J in
TM3 Leydig cells by lentiviral transfection significantly increased
the PLP-J protein levels. However, ELISA results revealed that
overexpression of PLP-J did not influence basic testosterone
production in TM3 cells, suggesting that this protein is not
directly involved in testosterone biogenesis in vitro.
Nevertheless, whether PLP-J participates in testosterone synthesis
through other mechanisms remains to be examined.
In addition, experiments investigating the cell
cycle and apoptosis in the present study revealed that PLP-J
overexpression did not affect these two processes. Thus, the
precise biological roles of this protein in the testis remain to be
elucidated.
In summary, the current study demonstrated that
PLP-J is expressed in the testis and that its mRNA and protein are
expressed in an age-dependent manner. The present investigation of
PLP-J function further revealed that PLP-J is not directly involved
in testosterone production in vitro. To the best of our
knowledge, the current study is the first to display that PLP-J is
expressed in the male reproductive system. Expression of PLP-J in
Leydig cells of the testis indicates a role for it in the male
reproductive system, in addition to previously reported functions
within the female reproductive system. Thus, the results may aid
further elucidation of the mechanisms of the male reproductive
system.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (30901062 and 81270687) and the Science
and Technology Commission Foundation of Yuzhong District
(20110505).
References
|
1
|
Mallon AM, Wilming L, Weekes J, et al:
Organization and evolution of a gene-rich region of the mouse
genome: a 12.7-Mb region deleted in the Del(13)Svea36H mouse.
Genome Res. 14:1888–1901. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soares MJ: The prolactin and growth
hormone families: pregnancy-specific hormones/cytokines at the
maternal-fetal interface. Reprod Biol Endocrinol. 2:512004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wiemers DO, Shao LJ, Ain R, Dai G and
Soares MJ: The mouse prolactin gene family locus. Endocrinology.
144:313–325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simmons DG, Rawn S, Davies A, Hughes M and
Cross JC: Spatial and temporal expression of the 23 murine
Prolactin/Placental Lactogen-related genes is not associated with
their position in the locus. BMC Genomics. 9:3522008. View Article : Google Scholar
|
|
5
|
Wit JM, Drayer NM, Jansen M, et al: Total
deficiency of growth hormone and prolactin, and partial deficiency
of thyroid stimulating hormone in two Dutch families: a new variant
of hereditary pituitary deficiency. Horm Res. 32:170–177. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Featherstone K, White MR and Davis JR: The
prolactin gene: a paradigm of tissue-specific gene regulation with
complex temporal transcription dynamics. J Neuroendocrinol.
24:977–990. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ho-Chen JK, Bustamante JJ and Soares MJ:
Prolactin-like protein-f subfamily of placental hormones/cytokines:
responsiveness to maternal hypoxia. Endocrinology. 148:559–565.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ishibashi K and Imai M: Identification of
four new members of the rat prolactin/growth hormone gene family.
Biochem Biophys Res Commun. 262:575–578. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ain R, Dai G, Dunmore JH, Godwin AR and
Soares MJ: A prolactin family paralog regulates reproductive
adaptations to a physiological stressor. Proc Natl Acad Sci USA.
101:16543–16548. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Toft DJ and Linzer DI: Prolactin
(PRL)-like protein J, a novel member of the PRL/growth hormone
family, is exclusively expressed in maternal decidua.
Endocrinology. 140:5095–5101. 1999.PubMed/NCBI
|
|
11
|
Hiraoka Y, Ogawa M, Sakai Y, et al: PLP-I:
a novel prolactin-like gene in rodents. Biochim Biophys Acta.
1447:291–297. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dai G, Wang D, Liu B, et al: Three novel
paralogs of the rodent prolactin gene family. J Endocrinol.
166:63–75. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alam SM, Konno T, Sahgal N, Lu L and
Soares MJ: Decidual cells produce a heparin-binding prolactin
family cytokine with putative intrauterine regulatory actions. J
Biol Chem. 283:18957–18968. 2008. View Article : Google Scholar
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
15
|
Kimura F, Takakura K, Takebayashi K, et
al: Messenger ribonucleic acid for the mouse decidual prolactin is
present and induced during in vitro decidualization of
endometrial stromal cells. Gynecol Endocrinol. 15:426–432. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Knox K, Leuenberger D, Penn AA and Baker
JC: Global hormone profiling of murine placenta reveals Secretin
expression. Placenta. 32:811–816. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Johnston DS, Olivas E, DiCandeloro P and
Wright WW: Stage-specific changes in GDNF expression by rat Sertoli
cells: a possible regulator of the replication and differentiation
of stem spermatogonia. Biol Reprod. 85:763–769. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Johnston DS, Wright WW, Dicandeloro P,
Wilson E, Kopf GS and Jelinsky SA: Stage-specific gene expression
is a fundamental characteristic of rat spermatogenic cells and
Sertoli cells. Proc Natl Acad Sci USA. 105:8315–8320. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pang AL, Johnson W, Ravindranath N, Dym M,
Rennert OM and Chan WY: Expression profiling of purified male germ
cells: stage-specific expression patterns related to meiosis and
postmeiotic development. Physiol Genomics. 24:75–85. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Midzak AS, Chen H, Papadopoulos V and
Zirkin BR: Leydig cell aging and the mechanisms of reduced
testosterone synthesis. Mol Cell Endocrinol. 299:23–31. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zirkin BR and Tenover JL: Aging and
declining testosterone: past, present, and hopes for the future. J
Androl. 33:1111–1118. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X and Stocco DM: The decline in
testosterone biosynthesis during male aging: a consequence of
multiple alterations. Mol Cell Endocrinol. 238:1–7. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Emmelot-Vonk MH, Verhaar HJ, Nakhai Pour
HR, et al: Effect of testosterone supplementation on functional
mobility, cognition, and other parameters in older men: a
randomized controlled trial. JAMA. 299:39–52. 2008.
|
|
24
|
Travison TG, Morley JE, Araujo AB,
O’Donnell AB and McKinlay JB: The relationship between libido and
testosterone levels in aging men. J Clin Endocrinol Metab.
91:2509–2513. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Walker WH: Molecular mechanisms of
testosterone action in spermatogenesis. Steroids. 74:602–607. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahn SW, Gang GT, Kim YD, et al: Insulin
directly regulates steroidogenesis via induction of the orphan
nuclear receptor DAX-1 in testicular Leydig cells. J Biol Chem.
288:15937–15946. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matzkin ME, Yamashita S and Ascoli M: The
ERK1/2 pathway regulates testosterone synthesis by coordinately
regulating the expression of steroidogenic genes in Leydig cells.
Mol Cell Endocrinol. 370:130–137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mishra J, Gautam M, Dadhich R, Kowtharapu
BS and Majumdar SS: Peritubular cells may modulate Leydig
cell-mediated testosterone production through a nonclassic pathway.
Fertil Steril. 98:1308–1317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martin LJ and Tremblay JJ: Nuclear
receptors in Leydig cell gene expression and function. Biol Reprod.
83:3–14. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao L, Hao J, Hu J, et al: Expression of
proliferin-related protein in testis and the biological
significance in testosterone production. Mol Cell Endocrinol.
343:25–31. 2011. View Article : Google Scholar : PubMed/NCBI
|