Introduction
As a novel therapeutic strategy to prevent or
reverse ventricular remodeling, heart failure, arrhythmias and
myocardial infarction, mesenchymal stem cell (MSC)-based cell
therapy replaces endogenous myocardial repair as an improved
approach with marked potential (1–3).
However, this approach has a number limitations that restrict its
application, including low efficiency of MSCs in colonization,
survival and differentiation towards myocardial tissue, and
diminished donor cell-function in an ischemia microenvironment
following transplantation (4–6).
Therefore, it is crucial that studies focus on devising a mechanism
to increase the survival of cells following transplantation to
areas of ischemia tissue.
The activated inflammatory response and cytokine
elaboration following myocardial infarction together contribute to
cardiac remodeling and eventual host outcome (7). Cytokines are released immediately
following ischemia in order to modulate tissue repair and
adaptation. Previous studies revealed that MSCs treated with
inflammatory mediators activate a series of pathophysiological
processes, including cell survival, cell migration, cell adhesion,
chemokine release, induction of angiogenesis and modulation of
immune responses (8–10). Several studies have demonstrated
that pretreatment of MSCs with cytokines, which were released by
the myocardium following ischemic injury, increased MSC-mediated
cardioprotection following acute myocardial infarction (AMI)
(11,12). Interleukin (IL)-1β and tumor
necrosis factor (TNF)-α are not constitutively expressed in normal
myocardium; however, their levels markedly increase in the infarct
and non-infarct areas following AMI (13).
Cell adhesion molecules and their ligands,
extracellular matrix components, chemokines and specialized bone
marrow niches all have roles in the precise regulation of MSC
adhesion to endothelial cells (14). It was reported that VCAM-1 together
with its ligand very late antigens-4 (VLA-4) was able to bind to
stromal or endothelial cells, which subsequently facilitated stem
cell homing (14–16). Furthermore, blockage of VCAM-1 or
VLA-4 markedly reduced stem cell migration and adhesion ability
(15,17,18).
Several investigations have also demonstrated that MSCs treated
with appropriate cytokines affect the paracrine of cells and then
improve cardioprotection (19–21).
In the present study, MSCs with two representative
inflammatory cytokines were stimulated alone or in combination to
examine the effect on the expression of VCAM-1 in MSCs and the
cardiac protective efficiency of cell-transplantation therapy in a
rat model of AMI.
Materials and Methods
Animals
Male Sprague-Dawley (SD) rats were purchased from
the Experimental Animal Center of Anhui Province (Anhui, China).
The rats received a 12 h light and dark cycle everyday, and were
kept at 20–25°C and 40–70% humidity. In addition, the rats were fed
standard laboratory rodents feed ad libitum. All animals
used in the present study received the appropriate care according
to the Guide for the Care and Use of Laboratory Animals (NIH
publication no. 85-23, revised 1996). The present study was
approved by the ethics committee of the Experimental Animal Center
of Anhui Province.
MSCs isolation and culture
MSCs were isolated from bone marrow of male SD rats
(4–6 weeks old) following the standard procedure with certain
modifications (22). In brief,
bone marrow cells were collected from the bilateral femurs and
tibias by removing the epiphyses, flushing the cavity with
Dulbecco’s modified Eagle’s medium (DMEM) and centrifuging the
suspension for 10 min at 300 × g. The cell pellet was then
resuspended and cultured in 25 cm2 culture flasks with
complete media containing 10% fetal bovine serum (HyClone, Logan,
UT, USA), at 37°C, 90% humidity and 5% CO2. Non-adherent
cells in the suspension were discarded following 48 h and fresh
complete medium was added and replaced every 3–4 days thereafter.
At 90% confluence, the cells were trypsinized (0.25% trypsin) and
passaged at 1:3 ratios. Cells were identified by flow cytometry as
described previously (23). MSCs
between passages three and four were used for the following
experiments.
Stimulation of MSCs
MSCs were stimulated for 24 h with IL-1β (PeproTech,
Rocky Hill, NJ, USA; 10 or 20 ng/ml), TNF-α (PeproTech; 10 or 20
ng/ml) or IL-1β (10 ng/ml) combined with TNF-α (10 ng/ml). In the
meantime, cells in the control group were incubated in parallel
without stimulation. Control and treated cells were used for
subsequent experiments. Each experiment was repeated at least three
times.
Flow cytometry
The control and treated MSCs were harvested and
adjusted to a cell density of 106/ml, then resuspended
in 100 μl phosphate-buffered saline (PBS; 1×106 cells).
The cells were then incubated with phycoerythrin (PE)-VCAM-1 (BD
Biosciences, Franklin Lakes, NJ, USA) antibody at room temperature
for 30 min in the dark. Following this, the cells were washed twice
with PBS and dispersed to make a single cell suspension in 400 μl
PBS. Labeled cells were assayed using a flow cytometer (BD
FACSCalibur; BD Biosciences) and analyzed with FCS4 software
(version 1.2.4.1; De Novo Software, Los Angeles, CA, USA). At least
10,000 events were analyzed for each sample.
In vitro adhesion assays
To analyze the MSC adhesion capacity, MSCs
(5×104cells/well) were seeded in collagen-coated 24-well
plates in 250 μl complete medium and incubated for 20 min as
previously described (8,24). The wells were gently washed twice
with PBS to remove the non-adherent cells and the adherent cells
were counted in six random fields per well under a microscope
(magnification, ×100). The quantity of cells adhered to the plate
reflected the relative adhesion ability of the MSCs.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted by using the TRIzol reagent
(Invitrogen Life Technologies, Carlsbad, CA, USA), cDNA was
obtained using a RevertAid First Strand cDNA Synthesis kit (Thermo
Fischer Scientific) and then an amplification reaction was
performed according to the manufacturer’s instructions. The
gene-specific primers were designed using Primer Premier 5 software
(Premier, Canada) based on cDNA sequences from Genebank and they
were as follows: VCAM-1 forward, 5′-CCA GCG AGG GTC TAC CA-3′ and
reverse, 5′-ACA GGG CTC AGC GTC AG-3′; β-actin forward, 5′-GTG GGC
GGC CCT AGG CAC CA-3′ and reverse, 5′-CTC TTT AAT GTC ACG CAC
GAT-3′. A Biometra T-Gradient thermal cycler (Biometra, Göttingen,
Germany) was used for PCR. The PCR conditions were as follows:
denaturation at 94°C for 30 sec, annealing at 60°C (VCAM-1)/51°C
(β-actin) for 30 sec and extension at 72°C for 40 sec for 35
cycles. The PCR products were electrophoresed on a 1.0% agarose gel
stained with 0.5 μg/ml ethidium bromide. The electrophoresis gel
containing the PCR products was scanned using the UVP gel imaging
system (JD-801; Jieda, Nanjing, Jiangsu, China). The expression of
VCAM-1 mRNA was normalized to the expression of β-actin mRNA.
Western blot analysis
Western blot analysis of cell lysates was performed
as previously described (25),
proteins were denatured in Laemmli sample buffer (Beyotime
Institute of Biotechnology, Shanghai, China) for 5 min at 95°C,
samples were separated on 10% SDS-PAGE and transferred to a
polyvinylidene difluoride (PVDF) membrane. Membranes were blocked
in 5% non-fat dried milk in Tris-buffered saline containing 0.05%
Tween-20 (TBST; Sigma, St. Louis, MO, USA) for 2 h prior to
incubation with anti-VCAM-1 (1:1,000; Bioworld Technology, Inc.,
Minneapolis, MN, USA) overnight at 4°C and then conjugated with a
secondary antibody, anti-rabbit Immunoglobulin G-horseradish
peroxidase (Beyotime Institute of Biotechnology), for 1 h at room
temperature. Membranes were washed three times in TBST and positive
bands were detected by the enhanced chemiluminescence kit (Thermo
Fischer Scientific). All the protein bands were scanned using Chemi
Imager 5500 V2.03 software (Alpha Innotech, San Leandro, CA, USA).
Protein band intensities were then analyzed by computerized image
analysis system (Gel-Pro analyzer 4 software; Media Cybernetics,
Rockville, MD, USA) and equal protein was normalized to
β-actin.
Rat model of AMI and cell
transplantation
Myocardial infarction was produced in male SD rats
(weighing, 180–220 g) as previously described (26,27).
First, a left thoracotomy was performed through the fourth
intercostal space to expose the rat heart. Then, the left anterior
descending coronary artery (LAD) was ligated with a 6-0 polyester
suture. Successful ligation was confirmed by the typical myocardial
infarction waves in electrocardiography recordings. The cells were
harvested for 1 h prior to transplantation. The infarcted hearts
(n=8) received intramyocardial injections of 100 μl control or
treated MSCs (1×106 cells). The injections were
performed at four different sites in the free wall of the left
ventricles.
Assessment of cardiac function
Left ventricular (LV) function was assessed in
anesthetized animals four weeks following transplantation of MSCs
using two-dimensional echocardiography equipped with a 12-MHz probe
(Philips Healthcare, Woerden, Netherlands). The animals were placed
on a warming pad in the supine or lateral position. The greatest LV
diameter of the internal end-diastole (LVID,d) and internal
end-systole (LVID,s) was measured from the long axis view. The LV
ejection fraction (LVEF,%) was calculated as
[(LVID,d)3-(LVID,s)3]/(LVID,d)3 × 100. All measurements were
averaged on at least three consecutive cardiac cycles and analyzed
by an observer blinded to the treatments received by the
animals.
Histology
Animals were euthanized prior to isolation and
sectioning of their hearts into two transverse slices through the
infarct area. The hearts were fixed in 10% formaldehyde prior to
being embedded in paraffin. Sections (3 μm) were stained with
Masson’s trichrome according to the manufacturer’s instructions
(Maixin, Fuzhou, Fujian, China). Images of each slide were captured
with an Olympus BX41 microscope (Tokyo, Japan). Image-Pro Plus 6.0
(Media Cybernetics, Rockville, MD, USA) was used to evaluate the
percentage of myocardial infarction area which exhibited collagen
deposition. The percentage of collagen deposition area was
calculated as: (Fibrotic area/total LV area) × 100.
Statistical analysis
All values are expressed as the mean ± standard
deviation (SD). Statistical analysis of the results between two
groups was performed using a Student’s t-test. Differences among
the groups were determined by a one-way analysis of variance. The
analysis was performed using Prism 5.0 software (GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference between values.
Results
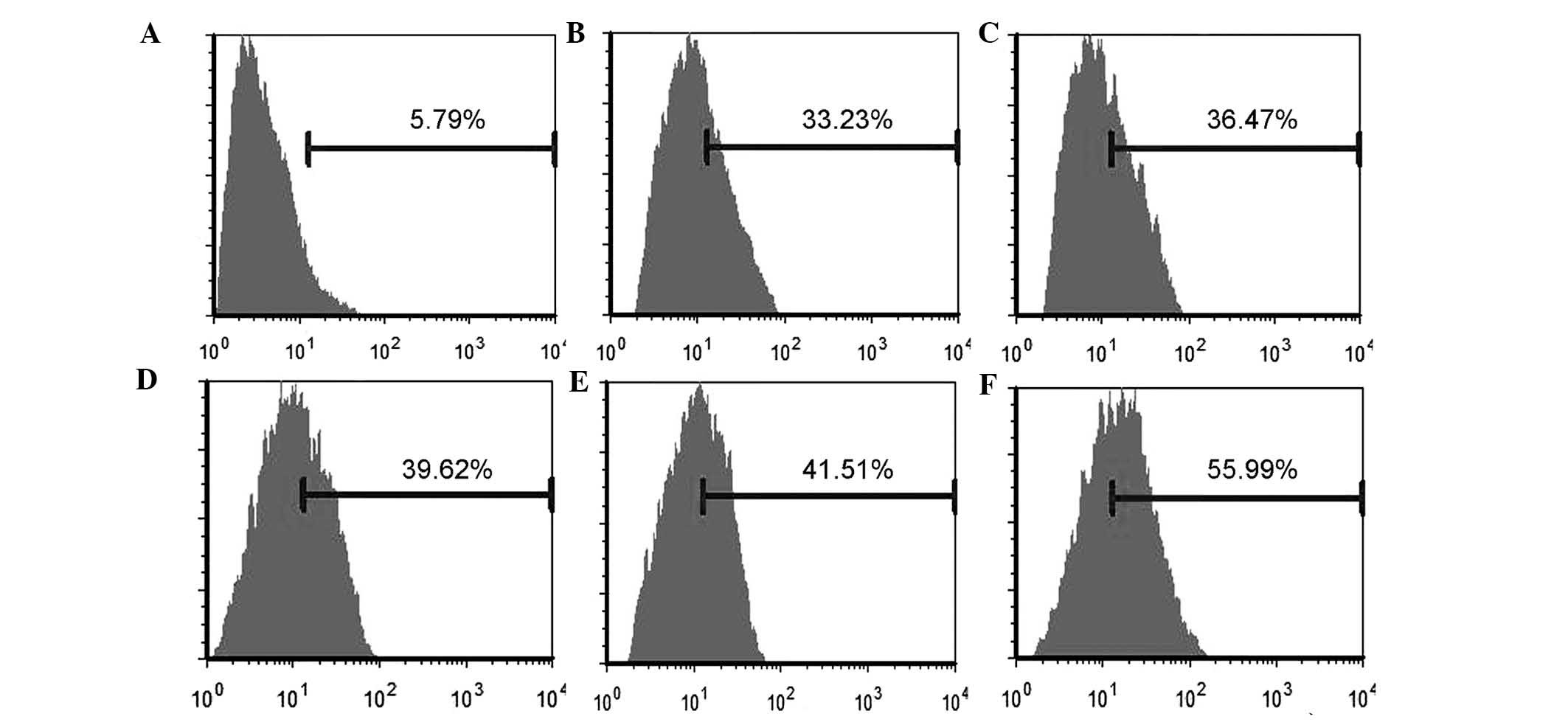
Flow cytometric analysis
Following the collection and immunostaining of the
cells with antibodies, fluorescence was measured by flow cytometry.
The results demonstrated the expression of cell surface markers,
and that the majority of the cells were positive for CD29 and CD90,
while they were negative for CD34. PE-VCAM-1 fluorescence
intensities of treated MSCs were markedly increased and had
statistical significance when compared with the control group
(P<0.01). The expression of the VCAM-1 was moderately elevated
with increasing concentration of the cytokines, but this difference
was not statistically significant (P>0.05). However,
co-treatment with the two factors markedly increased the VCAM-1
expression of MSCs as compared with the cells treated with one
factor only (P<0.01; Fig.
1).
IL-1β and TNF-α enhance MSC adhesion in
vitro
Pre-treatment with IL-1β and TNF-α significantly
increased the MSC adhesion ability in vitro (P<0.01) with
responses similar to those of the VCAM-1 expression. Incubation in
20 ng/ml cytokine moderately increased the number of adhered cells
as compared with the 10 ng/ml treatment group; however, this
difference was not statistically significant (P>0.05). There
were only 8.4±2.3 cells adhered to the plate in the control group,
but when treated with IL-1β, the number of MSCs adhered to the
plate increased to 28.0±5.2 in the 10 ng/ml group and 30.4±3.4 in
20 ng/ml group. Similarly, in the TNF-α stimulation groups, the
number of MSCs increased from 47.1±4.3 in the 10 ng/ml group to
49.7±6.2 in the 20 ng/ml group. By contrast, the number of adherent
cells in the combined cytokine treatment group was significantly
increased compared with the single cytokine groups (P<0.01). In
the combined cytokine group, the number of MSCs adhered to the
plates increased to 68.8±5.8 when treated with TNF-α (10 ng/ml) and
IL-1β (10 ng/ml) (Fig. 2).
IL-1β and TNF-α upregulate the gene
expression of VCAM-1
qPCR was used to detect VCAM-1 mRNA levels of MSCs.
Following subtraction of the background, VCAM-1 mRNA levels were
compared among the different groups relative to the β-actin mRNA
levels. Incubation with IL-1β (10 or 20 ng/ml) induced the mRNA
expression of VCAM-1 to 0.27±0.03 and 0.29±0.03, respectively,
compared with 0.09±0.01 in the untreated control group. Stimulation
with TNF-α (10 or 20 ng/ml) induced the transcription of VCAM-1 to
~0.33±0.03 and 0.36±0.04, respectively. By contrast, exposure of
MSCs to IL-1β (10 ng/ml) and TNF-α (10 ng/ml) resulted in a marked
increase in mRNA synthesis with a value of 0.52±0.05 relative to
the level of β-actin mRNA (Fig.
3).
 | Figure 3mRNA levels of VCAM-1. (A)
Electrophoresis gel of polymerase chain reaction products. (B)
Ratio of VCAM-1 mRNA/β-actin mRNA. #P<0.01, compared
with the control group; *P<0.05, compared with the
combination group; &P>0.05, compared between the
10 ng/ml and 20 ng/ml groups. I10/20, treated with 10/20 ng/ml
IL-1β; T10/20, treated with 10/20 ng/ml TNF-α; I10+T10, treated
with 10 ng/ml IL-1β and TNF-α each; C, control; IL-1β,
interleukin-1β; TNF-α, tumor necrosis factor-α; VCAM-1, vascular
cell adhesion molecule-1. |
Protein expression of VCAM-1
To further confirm the above results, the protein
expression of VCAM-1 was quantified by measuring protein bands
which were transferred to a PVDF membrane. In concordance with the
flow cytometry results, adhesion experiments and mRNA data, the
western blot analysis demonstrated that stimulation with IL-1β and
TNF-α induced an evident increase in the VCAM-1 protein expression
levels. IL-1β alone (10 ng/ml, 2.4±0.2-fold; 20 ng/ml,
2.7±0.3-fold), TNF-α alone (10 ng/ml, 3.1±0.2-fold; 20 ng/ml,
3.2±0.4-fold) and combination of the two cytokines (4.8±0.6-fold)
markedly increased the protein expression of VCAM-1 in MSCs in
vitro (Fig. 4).
 | Figure 4Western blot analysis demonstrating
that IL-1β and TNF-α significantly increased the expression of
VCAM-1. The protein expression was normalized to β-actin.
#P<0.01, compared with the control group; *P<0.05,
compared with the combination group; &P>0.05,
compared between the 10 ng/ml and 20 ng/ml groups. I10/20, treated
with 10/20 ng/ml IL-1β; T10/20, treated with 10/20 ng/ml TNF-α;
I10+T10, treated with 10 ng/ml IL-1β and TNF-α each; C, control;
IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α; VCAM-1,
vascular cell adhesion molecule-1. |
Measurement of heart function
To examine the therapeutic efficacy of treated MSCs
in MI in vivo, the cells were transplanted into the border
region between the infarcted and normal area of rat hearts
following coronary ligation. At the end of the fourth week
following surgery, nine of the rats had not survived the
experiment, three of them in the control group, two in the IL-1β
(10 ng/ml) group and the other four were one for each group. The
LVID,d and LVID,s of the heart was measured and then the LVEF was
calculated (Table I). The LVEFs of
the stimulation groups were evidently improved as compared with the
control group (28.6±1.5%) and the specific measurement results were
as follows: The LVEFs in the 10 and 20 ng/ml IL-1β groups were
33.7±2.1 and 34.8±1.7%, respectively, in the 10 and 20 ng/ml TNF-α
groups they were 40.9±2.2 and 43.0±2.1%, respectively, and in the
co-treatment group, the LVEF was 49.9±2.4%.
 | Table IEffects on heart function four weeks
following cell implantation. |
Table I
Effects on heart function four weeks
following cell implantation.
| Group | LVID,d (mm) | LVID,s (mm) | LVEF (%) |
|---|
| C (n=5) | 10.1±1.0 | 9.1±0.9 | 28.6±1.5* |
| I10 (n=6) | 9.0±0.6 | 7.9±0.6 | 33.7±2.1*#& |
| I20 (n=7) | 9.0±0.7 | 7.9±0.6 | 34.8±1.7*#& |
| T10 (n=7) | 8.4±0.4 | 7.0±0.4 | 40.9±2.2*#$ |
| T20 (n=7) | 8.1±0.6 | 6.7±0.5 | 43.0±2.1*#$ |
| I10+T10 (n=7) | 8.0±0.4 | 6.3±0.4 | 49.9±2.4# |
Histological changes
To further verify the myocardial protection effect
in vivo, the cardiac slices were stained with Masson’s
trichrome. The non-infarcted left ventricular appeared red, while
the infarcted myocardium replaced with fibroblasts and collagen
appeared blue. The measurements revealed that the myocardial
infarct size in both the IL-1β (10 ng/ml, 23.11±1.64; 20 ng/ml,
21.61±1.94%) and TNF-α (10 ng/ml, 17.71±1.85%; 20 ng/ml,
16.23±1.85%) groups was notably reduced compared with the control
group (32.44±2.74%; P<0.01). Furthermore, the infarct size was
even more reduced in the cytokine combination treatment group
(8.37±1.60%). However, there was no significant difference between
the infarct size in the hearts of the 20 ng/ml and 10 ng/ml
cytokine stimulation groups (P>0.05; Fig. 5).
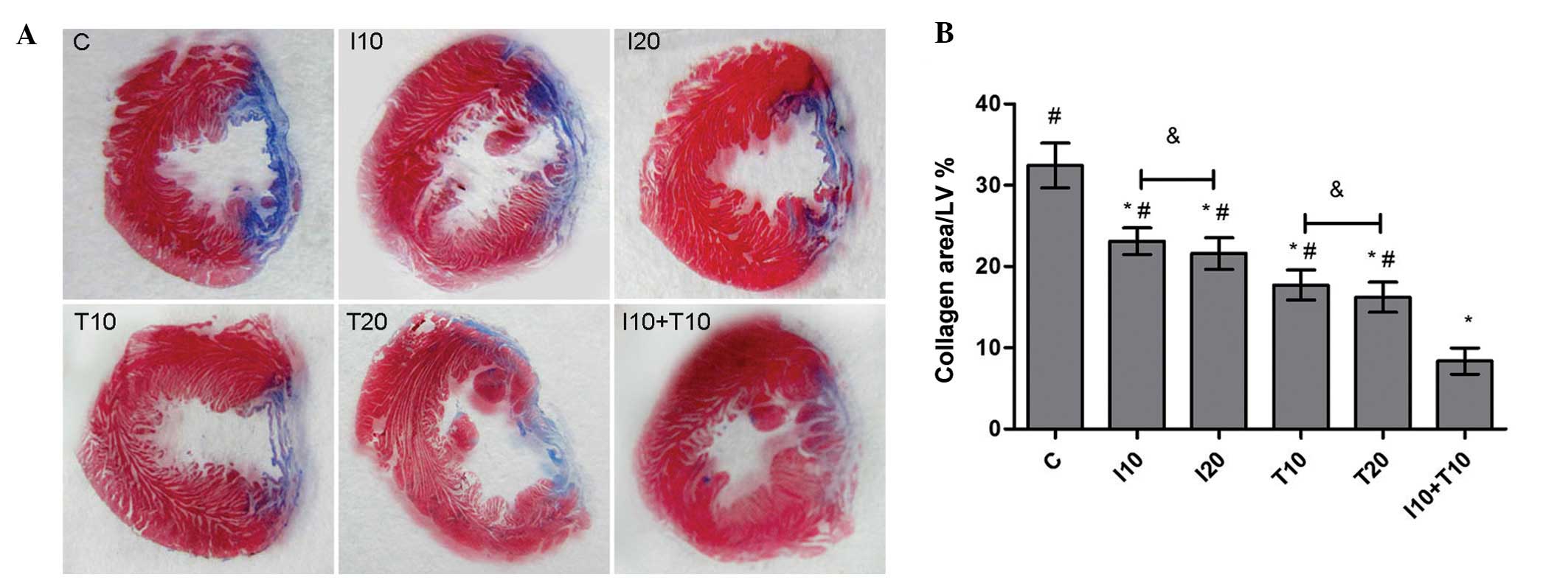 | Figure 5Histological analysis. (A)
Representative images of the whole LV for Masson’s trichome
staining in the different groups. (B) Ratios of blue area in the
whole left ventricular wall. #P<0.01, compared with
the control group; *P<0.05, compared with the
combination group; &P>0.05, compared between the
different doses (10 or 20 ng/ml). LV, left ventricule; I10/20,
treated with 10/20 ng/ml IL-1β; T10/20, treated with 10/20 ng/ml
TNF-α; I10+T10, treated with 10 ng/ml IL-1β and TNF-α each; C,
control; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α. |
Discussion
In the present study, it was identified that IL-1β
and TNF-α stimulation significantly elevated the VCAM-1 secretion
and adhesion ability of MSCs, and the combination of these two
cytokines potentiated this effect. Furthermore, intramyocardial
injection with MSCs which were pretreated with IL-1β and TNF-α
markedly improved the myocardial function and decreased the
collagen deposition in infarcted myocardium in rats. Cytokine
concentrations of 10 and 20 ng/ml were selected as the appropriate
stimulation concentrations, as these concentrations were previously
shown to activate paracrine signaling without changing surface
makers or the viability of MSCs (28,29).
Previous studies have indicated that homing of
circulating stem cells within the myocardium is possibly the first
step of the myocardial regeneration process. This step requires
adhesion of stem cells to the cardiac microvascular endothelium
(15). Adhesion molecules are cell
surface proteins that mediate the inter-communication between
cells, or between the cells and the extracellular matrix (ECM).
Several investigations have demonstrated that VCAM-1 has a key role
in MSC-mediated adhesion and immunosuppression (15,24).
VCAM-1 is also important in the adhesion and migration of
leukocytes through brain microvascular endothelial cells via
binding to the α4β1 and α4β7 integrins (30,31).
In previous studies, the adhesion of MSCs to endothelial cells was
significantly eliminated following incubation with monoclonal
blocking antibodies against VCAM-1. By contrast, it had only a weak
and non-significant effect when the ICAM-1 antibody was added
(15,24). In the present study, using an
adhesion assay, the crucial role of VCAM-1 on MSCs adhesion was
confirmed. Following the addition of cytokines, the quantity of
cell adhesion to the plate markedly increased along with the
upregulation of the adhesion molecule.
A number of investigators have suggested that
intramyocardial MSC transplantation recruits a number of
inflammatory factors which contribute to cardiac remodeling
(32,33). Several studies have demonstrated
that the cardioprotective effect of MSCs may be regulated by
mediators which are secreted by stem cells. Together with these
mediators, stem cells promoted tissue repair and elicited other
beneficial effects (34–36). Tsoyi et al (37) and Ward et al (38) reported that PI3K participated in
the regulation of VCAM-1 expression and in intracellular signal
transduction of the cell migration, which were induced by TNF-α in
human endothelial cells. Other studies suggested that IL-1β induced
MSC migration and adhesion through NF-κb (8). Since stem cells are consistently
exposed to the inflammatory environment following implantation to
ischemic areas, these inflammatory cytokines are critical for MSC
behavior. Investigating the response of MSCs to an inflammatory
environment will be undoubtedly valuable for improving
transplantation efficiency.
Following the above rationale, MSCs were cultured in
the presence of two typical inflammatory mediators, IL-1β and
TNF-α, and administered to rats following experimentally-induced
myocardial ischemic injury. The expression of VCAM-1 and the
cardiac function of the left ventricular region were then assessed.
It was identified that the expression of the adhesion molecule
significantly increased following treatment with either of the
cytokines, as did the cardiac function of the rats. As a number of
investigations have demonstrated that the effect of inflammatory
cytokines activating stem cell paracrine exhibited a dose-dependent
trend (2,29), the dosage of the cytokines was
doubled. Stimulation of MSCs with 20 ng/ml IL-1β or TNF-α did
elevate the VCAM-1 protein expression and the quantity of
plate-adhered cells compared with the 10 ng/ml-treated group;
however, the difference was not statistically significant. Of note,
the combination of the two cytokines induced more beneficial
effects than the doubled dose alone. It is necessary to lower the
dosage of the cytokines, but maintain a high level of VCAM-1 and
the adhesion ability of the MSCs, as well as to avoid unwanted
adversary effects to the MSCs or heart during the treatment.
There are a number limitations to be addressed in
the present study. Due to the persistence of inflammatory factors
in the myocardium during infarction, it remains unclear whether the
complicated microenvironment would affect the treated MSCs.
Furthermore, the present study did not investigate the mechanism
underlying the effects of the combination of the two cytokines.
Further studies are required to examine the underlying mechanisms
and other biological behaviors of MSCs in the inflammatory
environment, so as to fully elucidate their potential of cell-based
therapies for MI.
Acknowledgments
This study was supported by the Research Grants
Council of Anhui Province (no. 11040606M155) and by the School of
Life Sciences, University of Science and Technology of China.
References
|
1
|
Schulman IH and Hare JM: Key developments
in stem cell therapy in cardiology. Regen Med. 7(6 Suppl): 17–24.
2012. View Article : Google Scholar
|
|
2
|
Luo Y, Wang Y, Poynter JA, et al:
Pretreating mesenchymal stem cells with interleukin-1β and
transforming growth factor-β synergistically increases vascular
endothelial growth factor production and improves mesenchymal stem
cell-mediated myocardial protection after acute ischemia. Surgery.
151:353–363. 2012.PubMed/NCBI
|
|
3
|
Zheng Z, Leng Y, Zhou C, Ma Z, Zhong Z,
Shi XM and Zhang W: Effects of matrix metalloproteinase-1 on the
myogenic differentiation of bone marrow-derived mesenchymal stem
cells in vitro. Biochem Biophys Res Commun. 428:309–314. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Herrmann JL, Weil BR, Abarbanell AM, Wang
Y, Poynter JA, Manukyan MC and Meldrum DR: IL-6 and TGF-α
costimulate mesenchymal stem cell vascular endothelial growth
factor production by ERK-, JNK-, and PI3K-mediated mechanisms.
Shock. 35:512–516. 2011.
|
|
5
|
Cui X, Wang H, Guo H, Wang C, Ao H, Liu X
and Tan YZ: Transplantation of mesenchymal stem cells
preconditioned with diazoxide, a mitochondrial ATP-sensitive
potassium channel opener, promotes repair of myocardial infarction
in rats. Tohoku J Exp Med. 220:139–147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang M, Methot D, Poppa V, Fujio Y, Walsh
K and Murry CE: Cardiomyocyte grafting for cardiac repair: graft
cell death and anti-death strategies. J Mol Cell Cardiol.
33:907–921. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nian M, Lee P, Khaper N and Liu P:
Inflammatory cytokines and postmyocardial infarction remodeling.
Circ Res. 94:1543–1553. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carrero R, Cerrada I, Lledó E, et al: IL1β
induces mesenchymal stem cells migration and leucocyte chemotaxis
through NF-κB. Stem Cell Rev. 8:905–916. 2012.
|
|
9
|
Fan H, Zhao G, Liu L, et al: Pre-treatment
with IL-1β enhances the efficacy of MSC transplantation in
DSS-induced colitis. Cell Mol Immunol. 9:473–481. 2012.
|
|
10
|
Heo SC, Jeon ES, Lee IH, Kim HS, Kim MB
and Kim JH: Tumor necrosis factor-α-activated human adipose
tissue-derived mesenchymal stem cells accelerate cutaneous wound
healing through paracrine mechanisms. J Invest Dermatol.
131:1559–1567. 2011.
|
|
11
|
Kim YS, Park HJ, Hong MH, et al: TNF-alpha
enhances engraftment of mesenchymal stem cells into infarcted
myocardium. Front Biosci (Landmark Ed). 14:2845–2856. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herrmann JL, Abarbanell AM, Weil BR, et
al: Postinfarct intramyocardial injection of mesenchymal stem cells
pretreated with TGF-alpha improves acute myocardial function. Am J
Physiol Regul Integr Comp Physiol. 299:R371–R378. 2010. View Article : Google Scholar
|
|
13
|
Deten A, Volz HC, Briest W and Zimmer HG:
Cardiac cytokine expression is upregulated in the acute phase after
myocardial infarction. Experimental studies in rats. Cardiovasc
Res. 55:329–340. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chute JP: Stem cell homing. Curr Opin
Hematol. 13:399–406. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Segers VF, Van Riet I, Andries LJ, et al:
Mesenchymal stem cell adhesion to cardiac microvascular
endothelium: activators and mechanisms. Am J Physiol Heart Circ
Physiol. 290:H1370–H1377. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Balzer EM and Konstantopoulos K:
Intercellular adhesion: mechanisms for growth and metastasis of
epithelial cancers. Wiley Interdiscip Rev Syst Biol Med. 4:171–181.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bonig H, Priestley GV and Papayannopoulou
T: Hierarchy of molecular-pathway usage in bone marrow homing and
its shift by cytokines. Blood. 107:79–86. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu Y, Cheng P, Xue YX and Liu YH: Glioma
cells promote the expression of vascular cell adhesion molecule-1
on bone marrow-derived mesenchymal stem cells: a possible mechanism
for their tropism toward gliomas. J Mol Neurosci. 48:127–135. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Crisostomo PR, Wang M, Markel TA,
Novotny NM and Meldrum DR: TGF-alpha increases human mesenchymal
stem cell-secreted VEGF by MEK- and PI3-K- but not JNK- or
ERK-dependent mechanisms. Am J Physiol Regul Integr Comp Physiol.
295:R1115–R1123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Herrmann JL, Abarbanell AM, Wang Y, Weil
BR, Poynter JA, Manukyan MC and Meldrum DR: Transforming growth
factor-α enhances stem cell-mediated postischemic myocardial
protection. Ann Thorac Surg. 92:1719–1725. 2011.
|
|
21
|
Böcker W, Docheva D, Prall WC, et al:
IKK-2 is required for TNF-alpha-induced invasion and proliferation
of human mesenchymal stem cells. J Mol Med (Berl). 86:1183–1192.
2008.PubMed/NCBI
|
|
22
|
Tropel P, Noël D, Platet N, Legrand P,
Benabid AL and Berger F: Isolation and characterisation of
mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res.
295:395–406. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang A, Shen F, Lang Y and Wang J:
Marrow-derived MSCs and atorvastatin improve cardiac function in
rat model of AMI. Int J Cardiol. 150:28–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren G, Roberts AI and Shi Y: Adhesion
molecules: key players in mesenchymal stem cell-mediated
immunosuppression. Cell Adh Migr. 5:20–22. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim HJ, Lee SI, Lee DH, Smith D, Jo H,
Schellhorn HE and Boo YC: Ascorbic acid synthesis due to
L-gulono-1,4-lactone oxidase expression enhances NO production in
endothelial cells. Biochem Biophys Res Commun. 345:1657–1662. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niagara MI, Haider HKh, Jiang S and Ashraf
M: Pharmacologically preconditioned skeletal myoblasts are
resistant to oxidative stress and promote angiomyogenesis via
release of paracrine factors in the infarcted heart. Circ Res.
100:545–555. 2007. View Article : Google Scholar
|
|
27
|
Mias C, Lairez O, Trouche E, et al:
Mensenchymal stem cells promote matrix metalloproteinase secretion
by cardiac fibroblasts and reduce cardiacventricular fibrosis after
myocardial infarction. Stem Cells. 27:2734–2743. 2009. View Article : Google Scholar
|
|
28
|
Xiao Q, Wang SK, Tian H, et al: TNF-α
increases bone marrow mesenchymal stem cell migration to ischemic
tissues. Cell Biochem Biophys. 62:409–414. 2012.
|
|
29
|
Thankamony SP and Sackstein R: Enforced
hematopoietic cell E- and L-selectin ligand (HCELL) expression
primes transendothelial migration of human mesenchymal stem cells.
Proc Natl Acad Sci USA. 108:2258–2263. 2011. View Article : Google Scholar
|
|
30
|
Hyun YM, Chung HL, McGrath JL, Waugh RE
and Kim M: Activated integrin VLA-4 localizes to the lamellipodia
and mediates T cell migration on VCAM-1. J Immunol. 183:359–369.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yilmaz G and Granger DN: Leukocyte
recruitment and ischemic brain injury. Neuromolecular Med.
12:193–204. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Armiñán A, Gandía C, García-Verdugo JM, et
al: Mesenchymal stem cells provide better results than
hematopoietic precursors for the treatment of myocardial
infarction. J Am Coll Cardiol. 55:2244–2253. 2010.PubMed/NCBI
|
|
33
|
Kawada H, Fujita J, Kinjo K, et al:
Nonhematopoietic mesenchymal stem cells can be mobilized and
differentiate into cardiomyocytes after myocardial infarction.
Blood. 104:3581–3587. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Horwitz EM and Prather WR: Cytokines as
the major mechanism of mesenchymal stem cell clinical activity:
expanding the spectrum of cell therapy. Isr Med Assoc J.
11:209–211. 2009.PubMed/NCBI
|
|
35
|
Shake JG, Gruber PJ, Baumgartner WA, et
al: Mesenchymal stem cell implantation in a swine myocardial
infarct model: engraftment and functional effects. Ann Thorac Surg.
73:1919–1925. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mangi AA, Noiseux N, Kong D, et al:
Mesenchymal stem cells modified with Akt prevent remodeling and
restore performance of infarcted hearts. Nat Med. 9:1195–1201.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsoyi K, Jang HJ, Nizamutdinova IT, et al:
PTEN differentially regulates expressions of ICAM-1 and VCAM-1
through PI3K/Akt/GSK-3β/GATA-6 signaling pathways in
TNF-α-activated human endothelial cells. Atherosclerosis.
213:115–121. 2010.PubMed/NCBI
|
|
38
|
Ward SG, Westwick J and Harris S: Sat-Nav
for T cells: role of PI3K isoforms and lipid phosphatases in
migration of T lymphocytes. Immunol Lett. 138:15–18. 2011.
View Article : Google Scholar : PubMed/NCBI
|